Abstract
The FULFIL study evaluated once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100 µg/62.5 µg/25 µg versus twice-daily budesonide/formoterol (BUD/FOR) 400 µg/12 µg in patients with symptomatic COPD at risk of exacerbations. FULFIL demonstrated clinically meaningful and statistically significant improvements at Week 24 in trough forced expiratory volume in 1 second (FEV1), St George’s Respiratory Questionnaire (SGRQ) Total scores and reduced exacerbation frequency. Predefined analyses were performed to evaluate treatment effects in a subgroup of patients recruited in China (China subgroup; FF/UMEC/VI, n = 32; BUD/FOR, n = 29). Analyses included treatment by region (China versus non-China) to allow estimated treatment effects in patients from China to be compared with those of the non-China subgroup and the overall FULFIL intent-to-treat (ITT) population. In the China subgroup at Week 24: the mean change from baseline in trough FEV1 was 125 mL (95% confidence interval [CI] 36, 214) for FF/UMEC/VI and -70 mL (95% CI -163, 23) BUD/FOR (between-treatment difference: 195 mL [95% CI 67, 323]; p = 0.003) and in SGRQ Total score was -5.6 units (95% CI -10.5, -0.7) and -0.3 units (95% CI -5.4, 4.7), respectively (between-treatment difference: -5.3 [95% CI -12.3, 1.7]; p = 0.140). Fewer moderate/severe exacerbations occurred with FF/UMEC/VI than BUD/FOR (16% and 28%, respectively). The overall incidence of adverse events was similar between arms (FF/UMEC/VI: 38%; BUD/FOR: 31%). This prespecified subgroup analysis of patients recruited in China to FULFIL demonstrated comparable efficacy and safety to that observed in the non-China and in the overall ITT populations, for FF/UMEC/VI versus BUD/FOR.
ClinicalTrials.gov Identifier::
Introduction
Chronic obstructive pulmonary disease (COPD), which is characterized by persistent and progressive airflow limitation, is a leading cause of morbidity and mortality worldwide (Citation1). The estimated overall prevalence of COPD in China is 8.2%, with tobacco smoking, the use of biomass fuels and genetic susceptibility being the major risk factors (Citation2, Citation3). Guidelines recommend triple pharmacologic therapy with a long-acting muscarinic antagonist (LAMA) plus an inhaled corticosteroid (ICS) and a long-acting β2-agonist (LABA) for patients with advanced COPD with persistent symptoms and risk of exacerbations (Citation1). However, there are limited randomized controlled data comparing triple therapy with ICS/LABA (Citation4).
FULFIL (Lung FUnction and quality of LiFe assessment in COPD with closed trIpLe therapy) compared once-daily single inhaler triple therapy (fluticasone furoate/umeclidinium/vilanterol [FF/UMEC/VI] 100 µg/62.5 µg/25 µg using the ELLIPTA® inhaler) with twice-daily ICS/LABA therapy (budesonide/formoterol [BUD/FOR] 400 µg/12 µg using the Turbuhaler®) in patients with symptomatic COPD, who are at risk of exacerbations (Citation5). FULFIL demonstrated clinically meaningful and statistically significant improvements at Week 24 in lung function (as measured by trough forced expiratory volume in 1 second [FEV1]) and St George’s Respiratory Questionnaire (SGRQ) Total score (co-primary endpoints), as well as significantly reducing exacerbation frequency, with once-daily FF/UMEC/VI versus twice-daily BUD/FOR. These benefits were also observed in a subset of patients who received blinded treatment for up to 52 weeks (Citation5). Once-daily single inhaler triple therapy offers further benefits as it can eliminate the need to deliver medications using multiple inhalers several times per day and reduce the likelihood of inhaler use errors (Citation6–8). Here we report the findings of a predefined analysis of patients in a China subgroup of the FULFIL study who were recruited in China.
Methods
Study design
FULFIL was a phase III, randomized, double-blind, double-dummy, parallel-group, multicenter study (GSK study CTT116853; ClinicalTrials.gov NCT02345161). The study design has been reported in detail previously (Citation5). Briefly, patients were randomized to receive 24 weeks of once-daily FF/UMEC/VI 100 µg/62.5 µg/25 µg inhalation powder delivered using a single ELLIPTA inhaler or twice-daily BUD/FOR 400 µg/12 µg delivered using the Turbuhaler. The co-primary objectives of FULFIL were to evaluate the effects of once-daily single inhaler triple therapy (FF/UMEC/VI) on lung function and health-related quality of life, compared with twice-daily therapy (BUD/FOR) at 24 weeks.
The FULFIL protocol was approved by applicable ethics committees or institutional review boards, and the study was conducted in accordance with the International Council for Harmonisation on Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and applicable country-specific regulations. Written informed consent was obtained from all patients.
Patients
FULFIL enrolled patients with COPD aged ≥40 years defined as being in Gold Initiative for Chronic Obstructive Lung Disease group D: i.e. with (Citation1) FEV1 <50% and COPD Assessment Test™ (CAT) score ≥10, or (Citation2) FEV1 ≥50% to <80% and a CAT score ≥10, and either ≥2 moderate exacerbations in the past year or ≥1 severe exacerbation in the past year (Citation1). Patients were required to be receiving daily maintenance therapy for COPD for ≥3 months prior to screening, and to remain on this therapy up to the time of randomization. Exclusion criteria included a current diagnosis of asthma causing symptoms, unresolved pneumonia, or an unresolved COPD exacerbation. The analysis reported here was performed to evaluate treatment effects in a subgroup of patients enrolled in China.
Efficacy assessments and endpoints
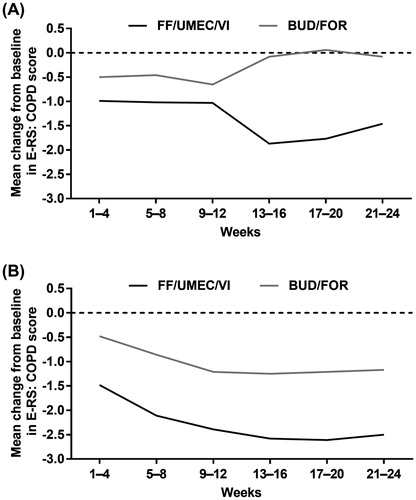
Spirometry was performed at the screening visit and at each scheduled clinic visit during the treatment period (Day 1 [randomization visit] and Weeks 2, 4, 12, and 24) using standardized equipment according to American Thoracic Society–European Respiratory Society guidelines (Citation9). The SGRQ for COPD patients was completed by patients using an eDiary at baseline and at Weeks 4 and 24. COPD exacerbations were identified based on symptoms reported by patients in the eDiary and were confirmed by the investigator. The Evaluating Respiratory Symptoms in COPD (E-RS: COPD; formerly EXACT RS) instrument was completed each evening by patients using the eDiary; E-RS: COPD scores were averaged over 4-weekly periods.
The co-primary efficacy endpoints were change from baseline in FEV1 and change from baseline in SGRQ Total score at Week 24. Secondary and other efficacy endpoints analyzed in the China subgroup included: the proportion of patients with a clinically meaningful change from baseline in trough FEV1 (≥100 mL) at Week 24; the proportion of responders according to SGRQ Total score at Week 24 (response was defined as a ≥ 4-unit decrease from baseline in SGRQ Total score); annual rate of on-treatment moderate and/or severe COPD exacerbations; and change from baseline in E-RS: COPD score over 24 weeks.
Safety assessments
The incidence of adverse events (AEs), serious AEs (SAEs), pneumonia, major adverse cardiovascular events (MACE), bone fractures, and other AEs of special interest (AESIs; prespecified AEs associated with ICS, LAMA or LABA use) were recorded. Investigators were responsible for detecting, documenting, and reporting any AEs at study visits, and patients were provided with an eDiary worksheet to record any medications used or AEs between visits. AEs, electrocardiogram (ECG) measurements, 24-hour Holter monitoring, vital signs, hematology and clinical chemistry parameters were recorded at each study visit. AEs and SAEs, including substantial changes in laboratory test values and abnormal safety assessments, were defined per the original FULFIL study criteria (Citation5).
Statistical analyses in the China subgroup
Analyses of treatment effects in the China subgroup included evaluation by region (China versus non-China), which allowed treatment effects to be estimated in the China and non-China subgroups separately. The results of the China subgroup were compared with the results of the non-China subgroup (i.e. rest-of-world data) and with the results of the intent-to-treat (ITT) population in the overall FULFIL analysis. No multiplicity adjustment was performed for these China subgroup analyses, and there was no formal hypothesis testing; all the summaries, analyses or comparisons performed were for descriptive purposes only. SAS Version 9.3 was used to conduct the statistical analyses.
Results
Patients
A total of 1810 patients were included in the ITT population in the overall FULFIL study (FF/UMEC/VI, n = 911; BUD/FOR, n = 899); 61 patients were from China (FF/UMEC/VI, n = 32; BUD/FOR, n = 29), of whom 57 (93%) completed study treatment. Reasons for study treatment discontinuation in the China subgroup were AEs (n = 1) and treatment burden (n = 2) in the FF/UMEC/VI group, and lack of efficacy (n = 1) in the BUD/FOR group.
Patients from China were less likely to be female, current smokers, have cardiovascular risk factors, or have a history of COPD exacerbations within in the previous 12 months, compared with the non-China subgroup (). The China subgroup also had a lower baseline post-bronchodilator FEV1 and percent predicted FEV1 versus the non-China subgroup ().
Table 1. Patient characteristics at baseline in the China and non-China subgroups.
Lung function
In the China subgroup, clinically meaningful improvements from baseline in trough FEV1 were observed at all time points over the 24-week treatment period with FF/UMEC/VI (). The least squares (LS) mean change from baseline in trough FEV1 at Week 24 was 125 mL (95% confidence interval [CI] 36, 214) and -70 mL (95% CI -163, 23) in the FF/UMEC/VI and BUD/FOR groups, respectively (between-treatment difference: 195 mL [95% CI 67, 323]; p = 0.003) (). In the non-China subgroup, the LS mean change from baseline in trough FEV1 at Week 24 was 142 mL (95% CI 125, 159) and -27 mL (95% CI -45, -10) with FF/UMEC/VI and BUD/FOR, respectively (between-treatment difference: 170 mL [95% CI 145, 194]; p < 0.001) (). A similar difference was observed in the overall FULFIL ITT population (171 mL (95% CI 148, 194; p < 0.001). The proportion of patients who achieved an increase of ≥100 mL from baseline in trough FEV1 at Week 24 was larger with FF/UMEC/VI versus BUD/FOR in both the China and non-China subgroups (47% versus 3% and 51% versus 22%, respectively) (). Similarly, in the overall FULFIL ITT population, a ≥ 100 mL increase from baseline in trough FEV1 was observed in a larger proportion of patients with FF/UMEC/VI (51%) versus BUD/FOR (21%) at Week 24 (Citation5).
Figure 1. Mean change from baseline in trough FEV1 at 24 weeks in the (A) China and (B) non-China subgroups. BUD, budesonide; CI, confidence interval; FOR, formoterol; FF, fluticasone furoate; LS, least squares; UMEC, umeclidinium; VI, vilanterol.
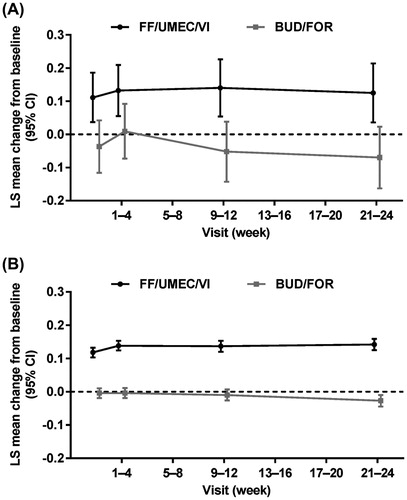
Table 2. Trough FEV1 and SGRQ Total score responses in the China and non-China subgroups.
Health-related quality of life
In the China subgroup, there was a clinically meaningful improvement from baseline in SGRQ Total score at Week 24 with FF/UMEC/VI (mean change from baseline: -5.6 units [95% CI -10.5, -0.7]), but not with BUD/FOR (mean change from baseline: -0.3 units [95% CI -5.4, 4.7]; between-treatment difference: -5.3 [95% CI -12.3, 1.7]; p = 0.140) (). In the non-China subgroup, the mean change from baseline in SGRQ Total score at Week 24 was -6.6 (95% CI -7.5, -5.6) and -4.5 (95% CI -5.4, -3.5) with FF/UMEC/VI and BUD/FOR, respectively (between-treatment difference: -2.1 [95% CI -3.4, -0.8]; p = 0.002) (). A larger proportion of patients in the FF/UMEC/VI group versus the BUD/FOR group experienced a clinically meaningful improvement from baseline in SGRQ Total score in both the China and non-China subgroups (44% versus 31% and 50% versus 42%, respectively) ().
In the overall FULFIL ITT analysis, there was a difference of -2.2 (95% CI -3.5, -1.0; p < 0.001) in change from baseline in SGRQ Total score at Week 24 for FF/UMEC/VI versus BUD/FOR. A larger proportion of patients in the FF/UMEC/VI group (50%) experienced a clinically meaningful improvement from baseline in SGRQ Total score at Week 24, compared with the BUD/FOR group (41%) (Citation5).
COPD exacerbations
In the China subgroup, the incidence of moderate/severe COPD exacerbations over the 24-week treatment period was 16% and 28% in the FF/UMEC/VI and BUD/FOR groups, respectively. In the FF/UMEC/VI group, no patients experienced exacerbations that required hospitalization; in the BUD/FOR group, two exacerbations (14%) required hospitalization (). In the non-China subgroup, the incidence of moderate/severe COPD exacerbations over the 24-week treatment period was 10% and 14% in the FF/UMEC/VI and BUD/FOR groups, respectively. Of the exacerbations of all severities experienced by the non-China subgroup during the study, 13 (11%) in the FF/UMEC/VI group and 23 (14%) in the BUD/FOR group required hospitalization ().
Table 3. Summary of exacerbation frequency in the China and non-China subgroups.
In the overall FULFIL ITT analysis, the incidence of moderate/severe COPD exacerbations was 10% and 14% in the FF/UMEC/VI and BUD/FOR groups, respectively; among all exacerbations, 13 (10%) and 25 (14%) required hospitalization in the FF/UMEC/VI and BUD/FOR groups, respectively (Citation5).
E-RS: COPD score
During each 4-week period of the study, there was a greater improvement from baseline in E-RS: COPD score with FF/UMEC/VI than with BUD/FOR in both the China and non-China subgroups (). These results were broadly consistent with the results of the overall ITT population in the FULFIL trial (Citation5).
Safety
In the China subgroup, on-treatment AEs up to Week 24 occurred in 12/32 (38%) patients in the FF/UMEC/VI group and 9/29 (31%) patients in the BUD/FOR group, and on-treatment SAEs occurred in 3/32 (9%) in the FF/UMEC/VI group and 2/29 (7%) in the BUD/FOR group (). The most common on-treatment AEs were upper respiratory tract infection (n = 5 [16%] and n = 4 [14%] for FF/UMEC/VI and BUD/FOR, respectively) and nasopharyngitis (n = 4 [13%] and n = 1 [3%] for FF/UMEC/VI and BUD/FOR, respectively). One patient (3%) who received FF/UMEC/VI permanently discontinued study treatment due to an AE (pneumonia); there were no fatal SAEs in either treatment arm. There was a higher incidence of pneumonia AESI in the FF/UMEC/VI group (2/32 [6%]) than in the BUD/FOR group (0/29). AE incidence was similar in the non-China subgroup; the most common on-treatment AEs in this group were nasopharyngitis (n = 60 [7%] and n = 42 [5%] for FF/UMEC/VI and BUD/FOR, respectively) and headache (n = 44 [5%] and n = 53 [6%] for FF/UMEC/VI and BUD/FOR, respectively). Further details are shown in .
Table 4. Summary of safety findings in the China and non-China subgroups.
These safety findings were similar to the safety profile observed for FF/UMEC/VI and BUD/FOR in the overall ITT population in the FULFIL trial (Citation5).
Discussion
We performed a predefined subgroup analysis involving 61 patients from China who were enrolled in the FULFIL study. Clinically meaningful improvements in trough FEV1 and SGRQ Total score were demonstrated with FF/UMEC/VI compared with BUD/FOR in patients from China at Week 24, as well as a lower incidence of moderate/severe exacerbations with FF/UMEC/VI versus BUD/FOR. The results from the China subgroup were comparable with the results from the non-China subgroup (i.e. the rest-of-world population), despite some differences in the baseline characteristics between these subgroups. The safety profile of FF/UMEC/VI in this analysis was consistent with the known profiles of the component drugs and no new safety signals were observed with FF/UMEC/VI in the China or non-China subgroups. Yet, there were some differences in the results from the China subgroup that should be noted. Patients who received BUD/FOR in the China subgroup experienced a greater deterioration in trough FEV1 and a smaller improvement in SGRQ Total score over the 24-week treatment period than patients who received BUD/FOR in the non-China subgroup, perhaps due to differences in baseline FEV1. There was a higher incidence of pneumonia AESI in patients who received FF/UMEC/VI in the China subgroup (6%) compared with patients who received FF/UMEC/VI in the non-China subgroup (2%). Within the China subgroup, there was also a higher incidence of pneumonia AESI with FF/UMEC/VI compared with BUD/FOR. Given the small number of patients in the China subgroup (n = 61), it is not possible to determine whether these differences are significant.
This is the first report of once-daily triple therapy (FF/UMEC/VI) for COPD in patients from China, and the lung function, health-related quality of life, exacerbation frequency, and safety findings are broadly consistent with the overall results of the FULFIL ITT population (Citation5). Previously, FF/VI and UMEC/VI combinations therapies have been assessed for COPD in patients from China and showed similar efficacy and safety profiles compared with other ethnic populations (Citation10, Citation11). Findings from other studies support the benefits of triple therapy for patients with COPD from a broad range of countries. Two randomized, 3-month studies demonstrated clinically relevant improvements in lung function with UMEC plus FF/VI versus placebo plus FF/VI in patients with moderate-to-very severe COPD (Citation12). A post-hoc analysis of these studies and a further two trials showed that UMEC plus ICS/LABA also improved health status and reduced the exacerbation rate compared with placebo plus ICS/LABA (Citation4). The TRILOGY study showed improvements in lung function and exacerbation rate with single inhaler triple therapy versus ICS/LABA (Citation13). However, unlike the FULFIL study, the TRIOLOGY study excluded patients who were already on triple therapy for COPD. A recent prespecified post-hoc analysis of the FULFIL study showed that regardless of COPD medication or disease severity at study entry, patients receiving FF/UMEC/VI demonstrated improvements in FEV1 and SGRQ compared with BUD/FOR (Citation5, Citation14).
The FULFIL study was designed to be as inclusive as possible, allowing patients with COPD and significant cardiovascular disease to be enrolled, to ensure the study population closely reflected a real-world population of patients with COPD. Another strength of this analysis is that the patients from China were enrolled in a global study, and so the data for the China and non-China subgroups were all collected in the same time frame. A limitation of this China subgroup analysis is that, while it showed overall concordance with the ITT population, the sample size was limited. Furthermore, the relatively small number of patients enrolled from China limited our ability to confirm safety findings in the China subgroup.
Conclusion
This predefined China subgroup analysis from the FULFIL study demonstrated comparable efficacy and safety with FF/UMEC/VI versus BUD/FOR to that observed in the rest-of-world population and the overall FULFIL ITT analysis. Our findings provide further evidence for the clinical value of triple therapy with FF/UMEC/VI in patients from China with COPD.
Acknowledgments
We would like to thank the patients for participating in this study, the study investigators and their staff, and the FULFIL study team.
Medical writing support in the form of development of the draft outline and manuscript drafts in consultation with the authors, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, referencing and graphic services was provided by Thomas Burton, BMBS and Louise Kelly, BSc, of Gardiner-Caldwell Communications, Macclesfield, UK, and was funded by GSK.
ELLIPTA is owned by or licensed to the GSK group of companies; Turbuhaler is a registered trademark of AstraZeneca.
Disclosure statement
Jinping Zheng: participating in advisory board activities for GSK
Nanshan Zhong: no conflicts of interest
Changzheng Wang: no conflicts of interest
Yijiang Huang: no conflicts of interest
Ping Chen: no conflicts of interest
Limin Wang: no conflicts of interest
Fuxin Hui: no conflicts of interest
Li Zhao: no conflicts of interest
Haoyan Wang: no conflicts of interest
Linda Luo: employee of GSK
Xin Duo: employee of GSK and holds stock options/shares
Aik Han Goh: employee of GSK and holds stock options/shares
David A Lipson: employee of GSK and holds stock options/shares
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global strategy for the diagnosis, management and prevention of COPD. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/.
- Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760.
- Gao J, Prasad N. Chronic obstructive pulmonary disease in China: the potential role of indacaterol. J Thorac Dis. 2013;5(4):549–558.
- Siler TM, Kerwin E, Tombs L, Fahy WA, Naya I. Triple therapy of umeclidinium + inhaled corticosteroids/long-acting beta2 agonists for patients with COPD: pooled results of randomized placebo-controlled trials. Pulm Ther. 2016;2:43–58.
- Lipson DA, Barnacle H, Birk R, Brealey N, Locantore N, Lomas DA, et al. FULFIL Trial: Once-daily triple therapy in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446.
- Chrystyn H, van der Palen J, Sharma R, Barnes N, Delafont B, Mahajan A, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22.
- Horsley MG, Bailie GR. Risk factors for inadequate use of pressurized aerosol inhalers. J Clin Pharm Ther. 1998;13(2):139–143.
- De Blaquiere P, Christensen DB, Carter WB, Martin TR. Use and misuse of metered-dose inhalers by patients with chronic lung disease. A controlled, randomized trial of two instruction methods. Am Rev Respir Dis. 1989;140(4):910–916.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al.; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338.
- Zheng J, de Guia T, Wang-Jairaj J, Newlands AH, Wang C, Crim C, et al. Efficacy and safety of fluticasone furoate/vilanterol (50/25 mcg; 100/25 mcg; 200/25 mcg) in Asian patients with chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Current Med Res Opin. 2015;31(6):1191–1200.
- Zheng J, Zhong N, Newlands A, Church A, Goh A. Efficacy and safety of once-daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo controlled study. Int J COPD. 2015:10 1753–1767.
- Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studies. Respir Med. 2015;109(9):1155–1163.
- Singh D, Papi A, Corradi M, Pavlišová I, Montagna I, Francisco C, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973.
- Lipson D, Barnacle H, Birk R, Brealey N, Zhu C-Q, Dransfield M, Lomas D. Single-inhaler triple therapy in advanced COPD patients: prior medication and disease severity FULFIL subanalyses. Presented at the ERS International Congress, Milan, Italy, 9–13 September 2017.