Abstract
The aim of this study was to analyze whether FeNO levels in acute exacerbation of COPD (AECOPD) with hospital admission have better diagnostic value than eosinophilia in blood, and to evaluate its usefulness in predicting a better clinical response. An observational prospective study of patients with AECOPD was carried out. FeNO determinations were made on arrival at the emergency room (ER), at discharge and during stability 3–6 months after discharge. Co-morbidities, bronchodilators, inhaled (IGC) and systemic (SGC) glucocorticoids, eosinophils, systemic inflammation markers (procalcitonin, C-reactive protein), eosinophil cationic protein, and total IgE were collected. Fifty consecutive patients (92% men, mean age 75 ± 6 years) were included in this study. Phenotypes were 26% Asthma-COPD Overlap Syndrome (ACOS), 42% chronic bronchitis (CB) and 32% emphysema. ACOS patients showed significantly higher levels of FeNO (73 ppb) and eosinophils (508 cells/mm3) than the rest (CB: 23 ppb, 184 cells/mm3, emphysema: 27 ppb, 159 cells/mm3; p < 0.05). A significant correlation between FeNO levels measured in ER and eosinophils was observed (r = 0.7; p < 0.001), but not at discharge or in stable phase. No significant association was found with parameters of systemic inflammation and mean stay. In conclusion, the determination of FeNO in AECOPD does not offer advantages over the evaluation of eosinophilia. These parameters rise at arrival in ER, descend at discharge, and remain unchanged in the stable phase. Both present similar diagnostic utility and are able to better identify the ACOS phenotype, which helps select a population that could benefit from a glucocorticoids therapy.
Introduction
The central pathogenic mechanism of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) is related with airway inflammation, constituting a heterogeneous disease in the course of its evolution. In recent years, this characteristic has led to the development of classifications based on clinical phenotypes: chronic bronchitis (CB), emphysema and mixed pattern or Asthma-COPD Overlap Syndrome (ACOS) (Citation1, Citation2). The differences in airway inflammation are based on different cell profiles. In “non-ACOS” COPD, there is a predominance of infiltrates with CD8+ lymphocytes, macrophages and neutrophils, whereas some patients with mixed phenotype may present characteristics of asthma with a predominance of eosinophils and CD4– T helper 2 (Th2) lymphocytes (Citation3–5), which could be measured indirectly with the fraction of exhaled nitric oxide (FeNO) produced by epithelial cells in response to interleukins such as IL4 and IL13 (Citation6–11). Other markers (Citation7–10) may have the same significance and are easy to determine, like eosinophilia in peripheral blood (PB), or other substitutes like periostin (Citation11, Citation12). Several studies have demonstrated that FeNO in COPD patients is associated with a greater presence of eosinophils in sputum, higher degree of bronchial hyperresponsiveness and better response to therapy with inhaled glucocorticoids (IGCs) (Citation13–15).
This background suggests that the determination of these parameters in AECOPD could better adjust the phenotypic classification of these patients and guide therapeutic management. We proposed analyzing whether FeNO in the initial phase of severe AECOPD requiring hospitalization has a better phenotypic diagnostic value, and whether it is capable of being a better predictor of exacerbations and length of hospital stay, compared to eosinophilia in blood. Similarly, we have assessed the association of FeNO with eosinophilia, levels of eosinophil cationic protein (ECP) and inflammatory markers, lung function, hospitalization and mean hospital stay.
Materials and methods
This is a prospective observational study that included patients with a prior diagnosis of COPD who were treated consecutively in the Emergency Room (ER) for AECOPD and required hospitalization. The inclusion criteria were: (Citation1) previous diagnosis of COPD in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation2); (Citation2) age over 40; (Citation3) smoking habit of more than 10 pack-years. Exclusion criteria included: (Citation1) lack of cooperation to measure FeNO; (Citation2) allergic rhinitis or asthma; (Citation3) pneumonia, bronchiectasis or pulmonary fibrosis; (Citation4) chronic kidney disease with glomerular filtration rate lower than 30%; (Citation5) hepatopathy with liver cirrhosis or portal hypertension; (Citation6) acute heart failure due to left ventricular failure (Citation16–19).
The patients were classified by clinical phenotype referenced in their medical files and based on recognized clinical guidelines (Citation1). AECOPD was defined as an increase in dyspnea, cough or changes in expectoration that modified the baseline situation of the patient (Citation1). The severity of the obstruction was classified according to GOLD criteria (Citation2). Categorizes airflow limitation into stages if FEV1/FVC <0.70:GOLD 1-mild if FEV1 ≥ 80% predicted, GOLD 2-moderade if 50% ≤FEV1 < 80% and GOLD 3 –4 severe if FEV1 < 50%. FeNO was measured three different times: in the ER, the day of hospital discharge from the Pulmonology Department, and during stable phase (3–6 months after discharge). Data were collected for demographic (age, sex) and clinical variables: weight, body mass index, smoking habit and pack-years, presence of comorbidities, bronchodilators, inhaled (IGC) and/or systemic(SGC) glucocorticoids in the 24 h prior to FeNO determination, and dosage expressed as equivalent dose of prednisone. In the ER, arterial blood gas parameters were determined at baseline situation; lab work determined total eosinophils and percentage versus total leukocytes, C-reactive protein (CRP), procalcitonin (PCT), ECP and total IgE. The day of hospital discharge, eosinophils, FeNO, CRP and spirometry results were collected again. The number of AECOPD in the year prior to inclusion was recorded, and patients with ≥2 exacerbations/year were considered exacerbators (Citation1, Citation2). The treatment received was indicated by the physician responsible for the patient, and the FeNO level was not shown so as not to influence therapeutic changes. All patients were discharged when they met standard clinical criteria for hospital discharge. The research protocol was approved by the hospital Ethics Committee, and all subjects gave their written informed consent.
FeNO determination
The test was carried out with a portable NIOX MINO device, performing the analysis and electrochemical reaction with the single-breath method, starting from total lung capacity (TLC), in accordance with the recommendations of the American Thoracic Society (ATS) (Citation20–22). The time required for the correct exhalation was 10 s with a pressure of 10–20 cm of water and with a necessary flow maintained at 50 mL/s.
Statistical analysis
A descriptive analysis was conducted of the quantitative variables, expressed as mean and standard deviation or median and interquartile range, according to the distribution type. The normality of the distributions was analyzed with the Kolmogorov–Smirnov test. The comparisons among different groups in terms of eosinophilia and FeNO were conducted with ANOVA, Chi-squared for qualitative variables and the Student’s t-test for quantitative variables. A p value <0.05 was considered statistically significant. ROC curves were used to calculate the sensitivity and specificity of FeNO and eosinophil levels, presenting area under the curve (AUC) values and 95% confidence intervals (CI) to predict the ACOS and exacerbator phenotype. The calculations were made with the IBM SPSS program, version 21.0 (SPSS, Chicago, IL).
Results
Initially, 67 patients were assessed on the ER. However, seven were excluded because they did not meet the inclusion criteria, six due to lack of cooperation during the technique and four because the patient data was lost. The remaining 50 patients underwent assessment and appropriate follow-up. The majority were senior male patients (median age 75) with a BMI of more than 30 kg/m2 in 16 cases (32%) and smoking history of 49 pack-years, five of whom were still active smokers (11%) with a median consumption of 5 cig/day (Citation4–20) ( ). Severe obstruction was detected in an extensive majority of cases (68%), whereas the obstruction was moderate in 32% and mild in only 2%. Regarding the distribution by phenotypes, we found 13 patients (26%) with ACOS, 21 (42%) CB and 16 (32%) emphysema. In all cases of emphysema, thoracic computed tomography was also used to verify the phenotype by imaging techniques.
Table 1. General characteristics of the study population, including clinical, analytical and functional variables.
The mean FeNO measurement time from the moment the patient was admitted to the ER was 5 h (Citation3–10). At the time of the initial determination and even in the study, 29 (58%) patients had already received SGC in the previous 12–24 h and double bronchodilation with anticholinergics (LAMA) in 88% and long-acting beta-adrenergic agonists (LABA) in 90% of cases. The rest had irregular treatment with short-acting bronchodilators. ICG were administered in 79% of cases. The initial FeNO in patients with SGC was 29 ppb, and in those without SGC 49 ppb (p = 0.03). The number of total eosinophils was 225 (2.5%) cells/mm3 with SGC and (3.2%) cells/mm3 without SGC (p = 0.03). shows the variables related with inflammatory parameters in peripheral blood (PB), arterial blood gas test and spirometry. Respiratory failure was observed in 38 (78%) patients, 18 (36%) of whom had hypercapnia with pCO2 >45 mmHg. The percentages of patients with eosinophilia over 2% and 3% were 22 (44%) and 15 (30%), respectively. Mean hospital stay was 4.9 days, with no differences regarding the severity of airflow obstruction, FeNO level or eosinophilia. FeNO levels in the ER were higher than at hospital discharge and in the stable phase (). A significant correlation was found between the FeNO and eosinophilia levels measured in ER, both in absolute values as well as percentage (r = 0.7; p < 0.001), but this was not maintained at patient discharge (r = 0.2; p = 0.2), or when considering the previous administration of SGC (r = 0.3; p = 0.1) ( and ). Additionally, no correlation was found between initial FeNO levels and levels of leukocytes, CRP, PCT, IgE, ECP, PaO2, PaCO2 or FEV1. Eosinophilia in blood and FeNO levels were higher in the ACOS phenotype than in the rest, both in the initial determination as well as at discharge and in stable phase (, ). However, no differences were found in IgE, ECP, CRP or PCT among phenotypes. We compared what was happening with different variables by the groups established with FeNO cut-off points measured in the 50th percentile (P50) and 2% eosinophils. The results are shown in , and no statistically significant differences were found in most of the variables evaluated.
Figure 1. FeNO levels at different moments of determination.
*Differences with p < 0.001 between emergency room and stable phase.
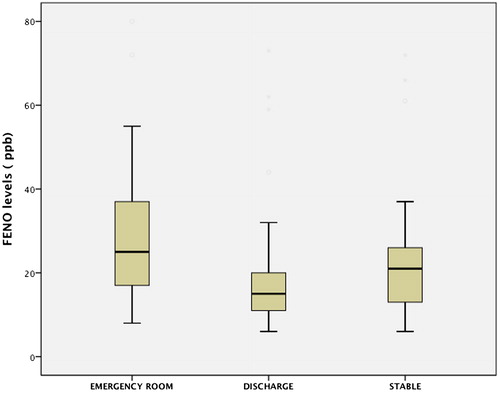
Figure 2. Correlation of FeNO levels with eosinophilia in peripheral blood in the emergency room (ER).
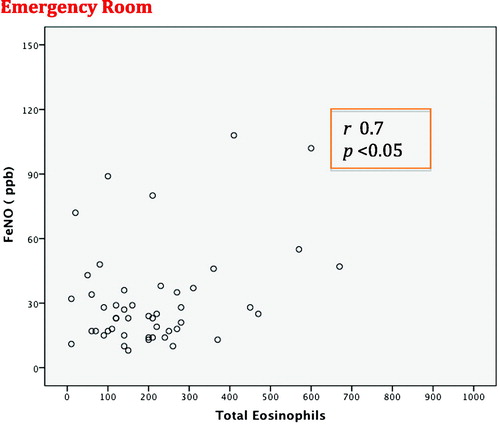
Figure 4. Distribution of FeNO levels according to clinical phenotypes (chronic bronchitis, emphysema, ACOS).
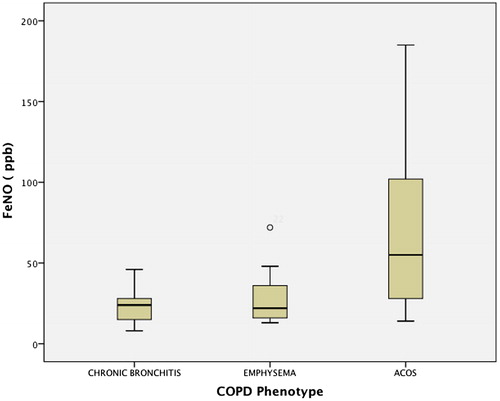
Table 2. FeNO levels, eosinophils and mean hospital stay of COPD exacerbations according to clinical phenotype.
Table 3. Values of the variables studied according to cut-off points of P50 (25 ppb) for FeNO and 2% eosinophils.
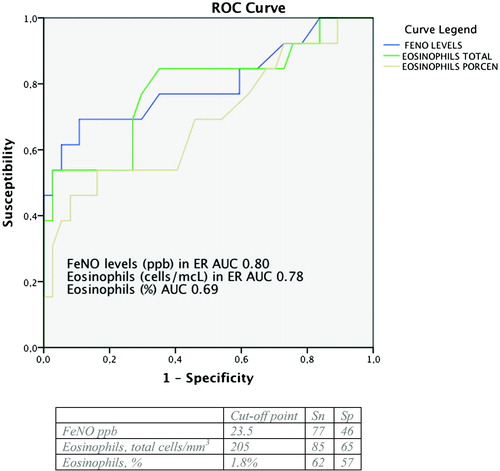
In order to find the cut-off points for FeNO and eosinophilia (total and percentage) measured in the ER that best discriminate the existence of the ACOS phenotype, ROC curves were generated with an AUC of 0.80 (95% CI: 0.63–0.96) for total eosinophils, 0.78 (95% CI: 0.62–0.95) for FeNO levels and 0.68 (95% CI: 0.51–0.87) for eosinophil percentage. They could therefore be considered acceptable markers with discrimination capacity to identify this phenotype (). Nonetheless, when similar calculations were made for the exacerbator phenotype, these variables did not have such a discrimination capacity, with an AUC for FeNO of 0.56 (95% CI: 0.4–0.73) and for ECP of 0.58 (95% CI: 0.42–0.74).
Discussion
The main findings of our study are, first of all, that FeNO has the same clinical significance in terms of utility for phenotype assessment as the determination of eosinophilia in AECOPD, with significantly higher levels detected in the ACOS phenotype compared to the emphysema and CB phenotypes. These two parameters are high upon arrival at the hospital but are lower at discharge and maintain similar values in the clinical stability phase 3–6 months after discharge (). Other studies show FeNO and eosinophil assessments during exacerbations, but very few have evaluated both parameters as in our study. Agustí et al. (Citation23) assessed 17 patients and compared them with 10 control subjects. The FeNO levels at hospitalization were higher than normal (mean 41 ppb versus 13 ppb; p < 0.001). In spite of therapy with SGC, FeNO levels remained high during recovery (37.9 ppb; p < 0.001) until discharge (41 ppb; p < 0.001). In contrast, when patients were clinically stable several months later, these levels dropped significantly (15.8 ppb; p < 0.001) and were no longer different from the baseline situation. They did not conduct a parallel eosinophilia study, however, nor did they find any relationship between FeNO and any of the variables measured during recovery (blood gas or spirometry parameters). In another study, Soter et al. (Citation13) also assessed the role of FeNO and eosinophils in sputum of 49 patients with AECOPD, observing a positive correlation between FeNO levels and the percentage value of eosinophils in sputum at both hospitalization and discharge. In this study, FeNO was considered as a predictor of eosinophils in sputum with an AUC of 0.89 at a cut-off point of 19 ppb, which provided a sensitivity of 90% and specificity of 74%.
Eosinophils are cells that usually exist in small percentages in peripheral blood and have considerable variability. In our study, we found that 44% of patients had levels higher than 2%, which is higher than other reports (Citation13, Citation20) even though an important percentage (58%) had been treated with SGC in previous hours. As the group of patients who have not received treatment with SGC presented higher levels than those who have been indicated this treatment, this factor could suggest even higher levels prior to the administration of SGC (). In our patients, there seems to be a decrease in FeNO and eosinophil levels caused by the administration of SGC in previous hours. While considering methodological differences, most related publications are generally free from the confusion bias of the systemic use of GC. In their eosinophilia study of the ECLIPSE cohort, Singh et al. (Citation24) used a classification according to total stable leukocyte percentages and did not consider the use of SGC a bias, providing no information about patients who have received this treatment. Their study did not find a significant association with exacerbation rates. In their studies about patients with COPD and the effect of IGC, Siddiqui et al. (Citation25) and Pascoe et al. (Citation26) excluded those who used SGC in the 30 days prior to inclusion. These authors found statistical significance between eosinophilia and number of exacerbations. Vedel-Krogh et al. (Citation27), after analyzing a cohort of the general population of Copenhagen, reported on the use of IGC without mentioning SGC. Although they found an association between eosinophilia and exacerbations, the impact of SGC was not evaluated. Bafadhel et al. (Citation28) assessed the use of SGC prior to inclusion with the effect on eosinophils and minimized the importance of their decrease associated with the use of GC. In their study, the re-hospitalizations were not significantly different between the two groups (eosinophilic vs. non-eosinophilic with 2% cut-off), although the group with more than 2% eosinophils at hospitalization had a shorter mean hospitalization.
In our study, we have not found an association of FeNO and ECP with the number of hospitalizations or differences in average hospitalization of the AECOPD patients (Tables 2 and 3). This could be supported by the effect of the early determination of these parameters and the effect of SGC, which was significant (58% of cases). In a retrospective study of 58 patients, Antus et al. (Citation29) evaluated FeNO levels during AECOPD. The exacerbations, average hospital stay and hospitalization days increased significantly in the group that presented lower FeNO levels. In an observational study, Couillard et al. (Citation30) assessed 167 patients for a year after a serious exacerbation, and 55 presented eosinophilia of more than 2%, which was associated with an increase in re-hospitalizations for another exacerbation or for other causes in the following year. However, mean hospital stay and total hospitalization days were not different, and the authors concluded that eosinophilia could be used as a biomarker in AECOPD to predict re-hospitalizations.
In our study, when we applied a cut-off point of 2% or the P50 for eosinophils and FeNO, respectively, we did not find statistically significant differences between these groups and most of the clinical variables studied (Table 4). With our data, the optimal cut-off points of FeNO and eosinophils that best discriminated the diagnosis of the ACOS phenotype based on ROC curves was 24 ppb and 205 cells/mm3. Soter (Citation13) reported a cut-off point of 19 ppb to predict eosinophilia in sputum >3%, which is likewise a characteristic of the ACOS phenotype. Alcázar-Navarrete et al. (Citation31) also determined FeNO levels in 92 patients, and the best cut-off point to identify ACOS was 19 ppb.In our study, the determination of eosinophils in sputum was unfortunately not possible, as they could have been correlated with these values.
Regarding inflammatory markers in PB, we have found no significant differences in terms of ECP or FeNO levels. In general, there was no evidence of very high levels of CRP or PCT, and patients with a higher grade of eosinophilia and FeNO had a tendency towards lower CRP levels. This could be explained in part by the absence of pneumonic consolidations or bacterial infections. In the study published by Bafadhel et al. (Citation28), the patients with eosinophilic COPD presented lower CRP levels, with no differences regardless of whether they presented pneumonic infiltrate. To date, we have found no other studies that evaluate PCT and FeNO levels in acute AECOPD.
Therefore, we can consider that the determination of FeNO has several disadvantages used in emergency care units. Firstly, its levels can become altered by several clinical conditions, such as acute infections, nutrition, fever, tobacco habit, exercise and especially the administration of GC (Citation21, Citation22). Secondly, FeNO measurements are technically a laborious method in acute AECOPD, requiring patient collaboration and the availability of trained personnel upon the patient’s arrival to the ER. Nevertheless, the determination of eosinophilia can be done at the same time and routinely with the complete blood count that is generally done in all acute patients, although with the same disadvantage that, like FeNO, its levels can be altered by GC. These factors, together with our results, seem to support the idea that the determination of blood eosinophils can be enough, without the need for FeNO determination in ER.
The main limitation of our study would be the small number of patients analyzed, which could attenuate the strength of the conclusions. Another fundamental limitation has been the difficulty to perform the FeNO measurement technique outside the standard setting and 24 h a day, which has influenced the recruitment rhythm. As a counterpoint, and in spite of the difficult logistics, our study is one of the few studies in which the first determination was measured directly in the ER in the context of acute AECOPD.
In conclusion, our study shows that the determination of FeNO in AECOPD offers no advantages over blood eosinophils determination. This, together with the relative difficulty for determining FeNO in the exacerbation, leads us to consider not using its determination routinely. The values are higher at admittance than at patient discharge, and they remain stable beyond the exacerbation. Both present similar diagnostic utility and are able to better identify the ACOS phenotype, which helps select a population that could benefit from a directed treatment with glucocorticoids.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, Riesco, JA Trigueros JA, Piñera P, Simón A, et al. Guía Española de la EPOC (GesEPOC). Tratamiento farmacológico de la EPOC estable. Arch Bronconeumol. 2012;48:247–57.
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, FAbbri LM, Martinez FJ, Nishimura M, et al. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of COPD. Am J Respir Crit Care Med. 2013;187(4):347–65.
- Soler-Cataluña JJ, Cosío B, Izquierdo JL, López-Campos JL, Marín JM, Agüero R, Baloira A, Carrizo S, Esteban C, Galdiz JB, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48:331–7.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–35.
- Cosío BG, Soriano JB, López-Campos JL, Calle-Rubio M, Soler-Cataluña JJ, De-Torres JP, Marin JM, Martinez Gonzalez C, De Lucas P, Mir I, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149:45–52.
- Miravitlles M, Alcázar B, Álvarez FJ, Bazús T, Calle M, Casanova C. What pulmonologists think about the asthma-COPD overlap syndrome (ACOS). Int J Chron Obstruct Pulmon Dis. 2015;10:1321–30.
- Gelb AF, Barnes PJ, George SC, Ricciardolo FL, Zamel Z. Review of exhaled oxide in chronic obstructive pulmonary disease, J Breath Res. 2012;6(4):47–101.
- Kunisaki KM. Exhaled nitric oxide and steroid responses in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(5):523–4.
- Abba AA. Exhaled nitric oxide in diagnosis and management of respiratory diseases. Ann Thorac Med. 2009;4(4):173–81.
- Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, Pavord ID, Ratjen F, Silkoff PE, Taylor DR, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2012;138(3):682–92.
- Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54.
- Wagener AH, De Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, Sterk PJ. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70:115–20.
- Soter S. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation. 2013;36(5):1178–85.
- Singh D, Kolsum U, Brightling CE, Locantore N, Agustí A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–700.
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55.
- Sumino H, Sato K, Sakamaki T, Masuda H, Nakamura T, Kanda T, Nagai R. Decreased basal production of nitric oxide in patients with heart disease. Chest. 1998;2:317–22.
- Kakoki M, Matsumoto A, Nagata D, Kobayakawa N, Kimura K, Momomura S, Hirata Y. Analysis of nitric oxide in the exhaled air of patients with chronic glomerulonephritis. Clin Nephrol. 1999;52:83–90.
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15.
- Malerba M, Radaelli A, Olivini A, Damiani G, Ragnoli B, Montuschi P, Ricciardolo FL. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed Res Int. 2014;2014:271918. doi: 10.1155/2014/271918
- ATS/ERS. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30.
- Kharinotov S, Alving K, Barnes PJ. Exhaled and nasal nitric oxide measurements: recommendations. Eur Respir Soc Task Force Eur Respir J. 1997;10:1683–9.
- Fortuna AM, Feixas T, Casan P. Determinación de óxido nítrico en aire espirado(FENO) mediante un equipo portátil (NIOX-MINOR Aerocrine) en población sana. Arch Bronconeumol. 2007;43(3):176–9.
- Agustí AG, Villaverde JM, Togores B, Bosch M. Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 1999;14(3):523–8.
- Singh D, Kampschulte J, Wedzicha JA, Jones PW, Cohuet G, Corradi M, Higenbottam T, Petruzelli S, Vestbo J. A trial of beclomethasone/formoterol in COPD using EXACT-PRO to measure exacerbations. Eur Respir J. 2013;41(1):12–17.
- Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, Wedzicha JA, Singh D. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–5.
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–42.
- Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in COPD: the Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965–74.
- Bafadhel M, Greening NJ, Harvey-Dunstan TC, Willians JE, Morgan MD, Brightling CE, Hussain SF, Pavord ID, Singh SJ, Steiner MC. Blood eosinophils and outcomes in severe hospitalised exacerbations of COPD. Chest. 2016;150(2):320–8.
- Antus B, Barta I, Horvath I, Csiszer E. Relationship between exhaled nitric oxide and the frequency of severe acute exacerbations in COPD patients. Respirology. 2010;15(3):472–7.
- Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–73.
- Alcázar-Navarrete B, Romero-Palacios PJ, Ruiz-Sancho A, Ruiz-Rodriguez O Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COPD phenotypes. Nitric Oxide. 2016;54:67–72.