Abstract
Pulmonary rehabilitation (PR) may not suit all individuals with chronic obstructive pulmonary disease (COPD) and may not result in increased physical activity. Higher levels of physical activity are associated with reduced mortality and morbidity. The aim of this study was to assess the feasibility of conducting a trial to investigate the effectiveness of a clinician-facilitated physical activity intervention (PAI) versus PR in improving physical activity in patients with COPD referred to PR. In this randomised controlled mixed methods feasibility study, all patients referred to PR who were eligible and willing were assessed at baseline and then randomised to the PAI or to PR. The assessments were repeated post-intervention and at 3-month follow-up. The main outcome was step count measured by Actigraph. Semi-structured interviews were conducted post-intervention. The N = 50 patients; mean (SD) age, 64.1(8.6) years, 24M were recruited and randomised; N = 23 (PAI) and n = 26 (PR): one patient was excluded from the analysis as that person did not meet the GOLD diagnostic criteria. Key feasibility criteria were met; recruitment was 11%, dropouts in PAI were 26% (n = 6) and 50% (n = 13/26) PR. Participants in both groups experienced a range of health benefits from their respective programmes. The PAI appears to be effective in increasing step counts in people with COPD: mean change (standard deviation) [confidence interval] for the PAI group was 972.0(3230.3)[–1080.3 to 3024.4], n = 12 and 4.3(662.7)[-440.9 to 449.5], n = 11 for the PR group. The PAI met all domains of fidelity. This study provides key information to inform a future-randomised controlled trial in physical activity.
Keywords:
Introduction
Globally, pulmonary rehabilitation (PR) is established as a core component in the management of chronic obstructive pulmonary disease (COPD) and has been shown to enhance health related quality of life, reduce dyspnoea and improve exercise capacity (Citation1). There is limited evidence to indicate whether the improved exercise capacity after PR translates into improved daily physical activity levels in COPD (Citation2,Citation3). The majority of PR programmes are supervised outpatient-based, and delivered in a group format (Citation4). Not all patients referred to PR attend for the assessment or enroll in the programme after assessment (Citation5), dropouts from and non-adherence rates with PR are high, emphasising that PR may not suit for all patients with COPD (Citation5,Citation6). Current capacity is unable to reach all those with COPD who would potentially benefit from PR (Citation5,Citation7) and hence there is a need to explore alternative platforms for delivering exercise/physical activity interventions (PAIs) traditionally delivered in context of PR.
Physical activity is fundamental for the prevention of chronic disease and premature mortality (Citation8). Walking represents a form of physical activity that has been shown to be effective in increasing physical activity in clinical populations and is necessary for the activities of daily living (Citation9). Although the studies in COPD have demonstrated the effectiveness of PAIs (Citation10) particularly individualised walking programmes (Citation11,Citation12), these alternative programmes do not seem to be offered within current models of healthcare provision for COPD. Interventions have also included different components, for example, use of the internet to record and facilitate the intervention (Citation13), the use of pedometers (Citation14,Citation15), and various behaviour change strategies (Citation16,Citation17). However, to date, a home-based pedometer-driven walking intervention in comparison to PR has not yet been explored. A home-based pedometer-driven walking intervention may offer an innovative and alternative method of delivering physical activity training that could be provided to large numbers of patients with COPD on an individual basis. Walking could provide for flexibility around life commitments and promote a change in activity levels.
The importance of conducting a feasibility study prior to a full-randomised controlled trial has been emphasised by key funders such as the Medical Research Council and the National Institute for Health Research (NIHR) as well as recent publications (Citation18–21). Mixed methods designs can be used in feasibility studies to allow for a greater understanding of patients’ perceptions of feasibility, for example barriers to participation (Citation22). Therefore, the aim of this study is to assess the feasibility of conducting a trial to investigate the effectiveness of a clinician-facilitated physical PAI (physical activity consultation and a pedometer-based walking programme) versus PR in improving physical activity in patients with COPD referred to PR.
Objectives
To use the NIHR criteria () to assess the feasibility of conducting a trial to compare the effectiveness of PAI versus PR in patients with COPD referred to PR (LIVELY COPD project).
Table 1. National Institute for Health Research Success Criteria for a feasibility trial.a
To explore the views and experience of participants relating to their satisfaction and perceived benefits of a PAI and of PR.
To assess the feasibility and fidelity of delivering a PAI intervention to patients with COPD
Methods
The reporting of this trial adheres to the Template for Intervention Description and Replication (TIDieR) and the Consolidated Standards of Reporting Trials (CONSORT) statement 2010 (Citation23,Citation24) (online supplement eTable 1).
Design
The study design was a multicenter-mixed methods randomised, parallel-group, feasibility study. The study was registered at https://clinicaltrials.gov/. Ethical approval was obtained from the Northern Ireland Research Ethics Committee 13/NI/0014.
Population
Patients with COPD (n = 50) referred for PR to any of the eight sites that provide PR within two Health and Social Care (HSC) Trusts in Northern Ireland were included. All PR sites reported that they were adhering to the BTS guidelines for PR prior to the commencement of and midway through the study (Citation4). Patients with a primary diagnosis of COPD, a good understanding of written English (as reported by the individual patient) and in a stable phase (no change in symptoms or medication in previous 4 weeks) at the time of assessment were included. Spirometry was provided by the PR team and when necessary COPD diagnosis was confirmed with the site PI. Exclusion criteria were inability to safely take part in a walking programme or PR (e.g. unstable angina, neurological, spinal or skeletal dysfunction affecting ability to exercise) as decided by the PR team or inability to comprehend or follow instructions (e.g. dementia).
Recruitment and randomisation
Participants were randomly assigned to two groups using computer-generated block random numbers by a member of team not involved in any other aspect of the study in order to ensure allocation concealment: Group 1 – PAI or Group 2 – PR. The allocation was retained in sealed envelopes which were opened to reveal group allocation only after consent and after the completion of baseline assessment. Patients were stratified according to HSC Trust to help ensure that equal numbers of patients within each Trust were randomised to each group.
As this was a feasibility study, no formal sample size calculation was used. Based on the previous publications, a sample size of 50 was deemed appropriate to achieve the aims/objectives of this study (Citation25). This sample size also reflected a realistic target for the intervention period and one which was anticipated would provide sufficient information on the feasibility to inform future studies.
Interventions
Participants were randomised to either the PAI or the PR.
Physical activity intervention
The PAI intervention was a 12-week clinician-facilitated pedometer-driven walking programme. All participants were provided with an unsealed Yamax Digiwalker CW700 and hence they could record and see their daily step count during the PAI, and a manual with weekly step diary and action and coping plans. Per protocol participants had weekly contact with the interventionist [specifically trained physiotherapist or nurse (details on the training are available in online supplement eTable 2)]; weeks 1–6 were face to face and weeks 7–11 were conducted by telephone. Week 12, the final consultation was delivered face to face as planned. Individual face-to-face consultations were expected to last up to 1 hour and were conducted in an outpatient hospital department and telephone consultations were expected to last about 15–20 minutes and were initiated by the clinician at an agreed time. Consultations were expected to transition from face to face towards telephone-based consultations by about Week 6 anticipating that participants would become more familiar and more confident with the intervention, and also to offer flexibility. Regardless, all components of the consultations were expected to be delivered. The PAI considered the ‘capability’, ‘opportunity’, ‘motivation’ and ‘behaviour’ (COM-B) model of behaviour change (Citation26) and included 20 behaviour-change strategies (Citation27). Each week participants set a step goal based on their previous weeks step count, as well as the results of a self-efficacy walk (how many steps the participant walked in 10 minutes) (Citation9). This step goal was individual to the patient. An example of how the weekly step goal was set is available in online supplement eTable 3. The participants wore pedometers each day during the intervention period for motivation and feedback, and also kept a written step diary which was contained with the intervention manual supplied to them. At each subsequent consultation, the clinicians and participants revisited the daily steps of the previous week and reviewed the step goal to assess if it was met/not met or partially met; barriers to physical activity were identified and strategies developed to overcome these; and specific strategies to increase walking were identified. Action and coping plans were made each week led by the participants, and during these consultations clinicians focused on helping participants to build self-efficacy, encouraging social support, providing disease-specific education; participants were given the Living Well With COPD for PR booklet (Citation28). An outcome goal relating to an activity or function was also set at baseline, for example ‘To be able to walk to the centre of town on my own without fear’. This was reviewed during the intervention; at Consultation 6 and, if it was already met or participants felt it was too difficult, it was revised or amended. The outcome goal was then reviewed at the end to determine whether it was achieved.
Table 2. Baseline demographics and characteristics of participants.
Pulmonary rehabilitation
Pulmonary rehabilitation was delivered by clinicians’ as per usual clinical practice. These programmes were delivered in either hospital or health-centre outpatient departments. The participants attended a supervised exercise class twice a week for 6 weeks and were also given a booklet with exercises and encouraged to perform these independently on a third occasion. Pulmonary rehabilitation also consisted of centre-based disease-specific education, at which time participants could engage in discussion and ask questions. The participants were also given the Living Well With COPD for PR booklet (Citation28). The exercise component usually lasted for 1 hour and PR sites reported that it generally consisted of cardiovascular exercises and lower and upper body strengthening exercises. A diary was used to record the exercises undertaken and the level of breathlessness measured with the BORG scale. Education sessions (30–60 minutes) were delivered at least once weekly.
Data collection
All screening, recruitment, adherence (number of sessions attended) and dropouts as well as the occurrence of adverse events (AEs) were recorded, intervention adherence was set at 75% (Citation29). Demographics (gender, age, height and weight), medical and social details (living arrangements and employment status) and spirometry results were gathered at the baseline assessment. The patients attended four study visits for outcome assessment: baseline assessment was conducted over two appointments 7 days apart (Visits 1 and 2). The participants were assessed again post-intervention (Visit 3) and at 3 months following the end of the intervention (Visit 4). All data was collected by a trained independent assessor not involved in the delivery of either intervention; a physiotherapist and/or a research assistant.
The following outcome measures were collected from all participants: physical activity with the Actigraph® GT3X + accelerometer (Citation30) and a sealed Yamax Digiwalker CW700 (Citation31) pedometer which were worn around the waist for 7 days during all waking hours, as well as the long form of the International Physical Activity Questionnaire (IPAQ) (Citation32); exercise capacity with the Incremental Shuttle Walk Test (ISWT) (Citation33); health status with the COPD Assessment Test (CAT) (Citation34) and EQ5D5L (Citation35); and a modified Global Rating of Change (GROC) Scale (Citation36). Participant’s stage of change (Citation37) was assessed at baseline (Visits 1 and 2).
Patient views
Semi-structured interviews were conducted post-intervention (Visit 3) with all available participants. The semi-structured interview script is available in online supplement eTable 4.
Feasibility and fidelity of the PAI
The participants in the PAI group set a weekly step goal. The step goal and the actual step count achieved by the participant were recorded and analysed to assess whether the participants achieved their goal each week, and the degree of change. Additionally, an outcome goal was set at baseline, and at the post-intervention assessment (Visit 3) participants were asked to report the extent to which they met this goal on a visual analogue scale (VAS) (0–10) with 10 being ‘fully met’. The PAI was considered to be feasible based on whether the participants could achieve their weekly step goal, achieve their overall outcome goal and increase their step count across the intervention.
Fidelity of the PAI was assessed using the checklist published by Borrelli (Citation38). This checklist was developed using the treatment fidelity framework provided by the National Institute of Health (NIH) Behavioral Change Consortium (BCC) (Citation39) which includes five domains of treatment fidelity (Study Design, Training of providers, Delivery of treatment, Receipt of treatment and Enactment of treatment skills). Under each of these domains, there are a number of items with which fidelity is assessed. Further details on the assessment of fidelity are available in online supplement e.
Data analysis
All participant screening and outcome measure data was entered into Statistical Package for Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL). Data entry was independently assessed for accuracy and analysed as per protocol. All continuous variables were checked for normal distribution using the Shapiro–Wilk Test, which confirmed that most of the data were normally distributed; BMI, forced expiratory volume in 1 second (FEV1)% and forced vital capacity (FVC) were not normally distributed. Descriptive statistics were used to summarise the screening, recruitment, adherence and population demographics. Only Actigraph data that contained a minimum of 5 days of 10 hours wear time was used for analysis; and only sealed pedometer data that had a minimum of 5 days of 100–50,000 steps was used for analysis (Citation40). As this was a feasibility study, we were not focused on statistical significance and therefore mean (standard deviation, SD) difference, with 95% confidence interval (CI) was estimated at each follow-up time point for all outcome measures using paired t-tests. Data is presented as mean [(95% CI) or (SD)] and nominal data is presented as percentages.
Qualitative data was analysed using Kings Template analysis (Citation41). A template of pre-defined themes was created using the semi-structured interview schedule as guidance. The transcripts were analysed with the predefined themes, and subthemes were added to ensure that all relevant texts were being captured and coded. All transcripts were checked to ensure that all relevant texts had been coded according to the final template, and two researchers outside the team reviewed three transcripts each.
All unsealed pedometer data relating to weekly step goals and steps achieved was recorded in Microsoft Excel 2010. Mean weekly step goals and mean weekly steps achieved were calculated and plotted graphically so as to demonstrate how these numbers tracked each other over time during the PAI. The mean difference between participants’ first and last recorded mean daily unsealed pedometer step count was also calculated. Finally, participants’ VAS scores for whether they felt they had achieved their outcome goal were also recorded and a mean score was calculated.
Results
Participants
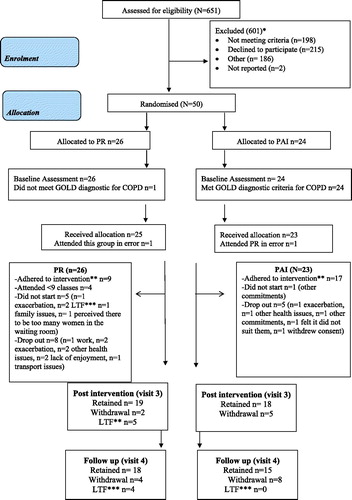
Participants’ flow through the study is shown in . Six hundred and fifty-one patients were screened between 4 April 2014 and 27 July 2015. Of those, eligible (11%) (n = 50/453) were recruited over a 16-month period (for full screening data, see online supplement eTable 5). The N = 50 participants with a mean (SD) age of 64.1(8.6) years, 24M and FEV1 1.4 (0.6) L/min were recruited. The patients were assessed and randomised to the PAI (n = 24) or PR (n = 26). One participant who was randomised to the PAI made a mistake and attended PR. Therefore, n = 27 attended PR and n = 23 attended the PAI. A further n = 1 participant randomised to PR was excluded from the analysis as subsequent information about their diagnosis revealed that the person did not meet the GOLD criteria for COPD (Citation42); therefore, n = 49 have been included in the analysis: n = 23 PAI; n = 26 PR.
Figure 1. CONSORT Flow of participants through the study and adherence to the PAI and PR (Citation24) *reasons for exclusion are in online supplement eTable 5 **Adherence set at 75% (attending 9/12 classes/consultations) (Citation29), ***LTF Lost to follow-up.
Patients’ characteristics are summarised in . This group had complex needs; n = 29 had more than two self-reported comorbidities and were prescribed multiple medications [mean (SD) 7.9 (3.8) which includes their specific respiratory medications]. For further details regarding participants’ characteristics, see online supplement eTable 6.
Intervention adherence
There were 26% (n = 6/23) dropouts/non-starters in the PAI group. The reasons for not starting and dropouts are shown in . The PAI was adhered to (attended 75% sessions) by 17/17 (100%) of those who did not drop out (Citation29). The time taken to compete the intervention was 12.4 weeks, ranging from 10.7 to 16.3 weeks and participants on average completed a mean (SD) 11.8 (0.6) of the 12 planned consultations.
There were 50% (n = 13/26) dropouts/non-starters in the PR group. The reasons for not starting and dropouts are shown in . Pulmonary rehabilitation was adhered to (attended 75% sessions) by 9/13 (70%) of those who did not drop out (Citation29). The participants who adhered to PR attended a mean (SD) of 10.5 (1.2) of the 12 planned classes.
The numbers are too small to fully explore if there were any patterns in the characteristics of dropouts although in both groups it does appear that those who dropped out were younger than completers: in the PAI dropouts had a mean (SD) age of 58.3 (8.9) years and completers 62.6 (7.6) years and in the PR group dropouts had a mean (SD) age of 65.2 (8.1) years and completers 69.1 (7.3) years.
also details the retention rates for participants providing post-intervention (Visit 3) and follow-up (Visit 4) outcome measures: post-intervention n = 18/23 (78.3%) (PAI) and n = 19/26 (73.1%) (PR) and at follow-up n = 15/23 (65.2%) (PAI) and n = 18/26 (69.2%) (PR). These numbers relate to participants providing at least one outcome measure. Some participants did not adhere to their intervention but returned for outcome measure assessment.
Outcome measures
A range of outcome measures were included in this study. The mean (SD) time taken in minutes to administer the study outcome measures across all four visits (3 time points) was <1 hour per visit [59.9 (15.2) minutes]. The number of available outcome measures and reasons for missing data at each time point are available in online supplement eTable 7.
Post-intervention (Visit 3)
The mean (SD) daily step count as recorded by the Actigraph for the PAI group at baseline was 3,305.6 (1,960.2) steps for n = 17 participants, and at post-intervention was 4,768.2 (2,992.2) steps for n = 14 participants; the mean difference (SD) [CI] was 972.0 (3,230.3) [–1080.3 to 3,024.4], n = 12. The mean (SD) daily step count as recorded by the Actigraph for the PR group at baseline was 3,834.6 (2,245.5) steps for n = 23 participants and at post-intervention was 3,476.6 (2,307.9) steps for n = 12 participants; the mean difference (SD) [CI] was 4.3 (662.7) [–440.9 to 449.5], n = 11. The mean (SD) moderate–vigorous physical activity (MVPA) in minutes as recorded by the Actigraph for the PAI at baseline was 14.3 (15.3) for n = 17 participants, and at post-intervention was 24.4 (26.0) for n = 14 participants; the mean difference (SD) [CI] was 6.6 (26.8) [–10.4 to 23.7] minutes, n = 12. The mean (SD) MVPA in minutes as recorded by the Actigraph for the PR group at baseline was 13.9 (15.2) for n = 23 participants and at post-intervention was 12.8 (20.0) for n = 12 participants; the mean difference (SD) [CI] was 0.9 (6.0) [–3.2 to 4.9] minutes, n = 11.
In relation to exercise capacity and quality of life, participants in the PAI had a mean (SD) distance of 253.0 (118.8) m at baseline, n = 23 and 288.1 (107.0) m post-intervention for n = 16 participants; the mean difference (SD) [CI] was –11.9 (90.4) [–60.1 to 36.3] m, n = 16. Participants in the PR group had a mean (SD) distance of 259.2 (140.6) m on the ISWT at baseline, n = 26 and 280 (139.7) m, n = 17 post-intervention; the mean difference (SD) [CI] was –7.6 (69.9) [–43.6 to 28.3] m, n = 16. For the CAT score, participants in the PAI had a mean score of 23.8 (6.9) at baseline, n = 23 and 22.5 (7.0) at post-intervention, n = 17; the mean difference (SD) [CI] was 0.6 (7.7) [–3.3 to 4.6], n = 17. The participants in the PR group had a CAT score of 18.7 (7.3) at baseline for n = 26 and a post-intervention CAT score of 16.6 (5.3), n = 19; the mean difference (SD) [CI] was –0.4 (6.4) [–3.5 to 2.7], n = 19. The full baseline to post-intervention results for all outcome measures are available in online supplement eTable 8.
Follow-up at 3 months (Visit 4)
As recorded by the Actigraph, there appears to be a general trend towards increasing step counts [mean (SD)] across the three time points in the PAI group: baseline step count 3,305.6 (1,960.20, n = 17, post-intervention step count 4,768.2 (2,992.1), n = 18 and 5,332.0 (3,070.7) steps at follow-up, n = 15. In the PR group, there was a decline in step count [mean (SD)] from baseline to post-intervention, and then an increase at follow-up: baseline step count 3,946.2 (2,263.1), n = 24, post-intervention step count 3,476.6 (2,307.9), n = 19, and a step count of 4,984.6 (3,598.0) at follow-up.
Adverse events
There were four related and unexpected AEs; PAI (n = 3): blister on the right heel and big toe, flare up of a knee swelling, reaction to nickel on pedometer due to a nickel allergy; and, PR (n = 1): dizziness when leaving out patient department after an appointment. These AEs were managed by providing advice to the participant for resolution, and no one withdrew based on these AEs.
Qualitative interviews
The N = 32 participants were available to complete the semi-structured interviews; n = 16/23 (69.6%) PAI; n = 16/26 (61.5%) PR. The reasons for not being available for semi-structured are summarised in online supplement eTable 4. Five core themes were identified: (i) Perceived benefits and impact of the PAI/PR on health, (ii) views and satisfaction with content of PAI/PR, (iii) adherence to the PAI/PR, (iv) views about the outcome measures and (v) views about continuing exercise. The participants in both groups enjoyed their respective programmes and experienced a range of benefits across their physical and mental health and also in terms of their social functioning. The participants were generally satisfied with their allocation; participants in the PAI felt that the intervention was tailored specifically to them and the pedometer and step diary were well received. The participants in the PAI were generally satisfied with mix of phone and face-to-face contact. There were mixed views about the duration and frequency of contact; a small number in both the PAI and the PR group felt that they could have engaged in the programme for longer, others in the PR group felt that twice weekly was too intense, given they had other commitments. Adherence to the programmes was explored; participants in both groups encountered a number of barriers to participation including their health, weather, lack of social support as well as time and other commitments. The participants in PR also reported the group setting and a lack of motivation as barriers. However, a number of facilitators were also recorded across the interviews including their own intrinsic motivation, social support and the staff. The pedometer and action and coping plan as well as developing their own strategies to overcome barriers were themed as facilitators for the PAI group. The group setting of PR was a facilitator for some. There were mixed views about the outcome measures; there were some participants who did not mind them, whereas others found them/parts of them burdensome. The majority of participants planned on continuing to engage in exercise/PA with specific plans including continuing to set goals and use the pedometer or join an exercise class. The participants in both groups were generally quite confident that they would continue as the benefits achieved served as motivation.
Feasibility and fidelity of the PAI
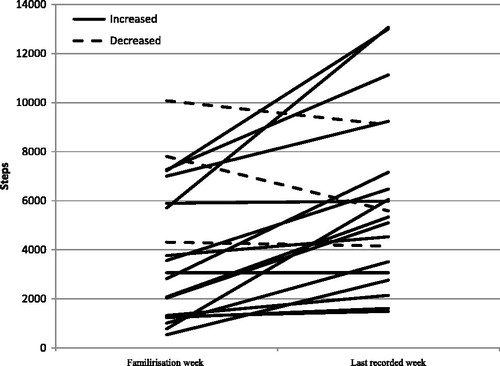
In relation to the achievement of weekly step goal, participants appeared to overachieve their step goals in the first week of the PAI, but as the intervention progressed the step goal and step count achieved aligned more closely (). For those who provided step counts at two time points, most patients (n = 17/20) demonstrated an increase in their step count after the PAI (), n = 13/20 met the minimally clinically important difference (MCID) for step count (600–1,100) (Citation43); step count recorded by the unsealed pedometer improved by a mean (SD) 2,087(2,452) steps between Week 1 and the last step count recorded. After the PAI, participants rated whether they had met their outcome goal set out at the start of the intervention using the VAS scale (0 = not met at all, 10 = fully met). Visual analogue scale scores were available for n = 16/18; n = 1 was unwell and did not travel for outcome measure collection and n = 1 could not remember their outcome goal. Overall, these participants reported achieving their outcome goal; mean (SD) 8.8 (2.9).
Figure 2. Mean daily step count goal compared to the step count achieved across the 12 week PAI [numbers of participants providing step count data at each time point varies due to attendance and withdrawals; Familiarisation Week 1, n = 21; Week 2, n = 18W week 3, n = 19; Week 4, n = 18; Week 5, n = 17; Week 6, n = 18; Week 7, n = 18; Week 8, n = 17; Week 9, n = 17; Week 10, n = 17; Week 11, n = 16; Week 12, n = 3.].
![Figure 2. Mean daily step count goal compared to the step count achieved across the 12 week PAI [numbers of participants providing step count data at each time point varies due to attendance and withdrawals; Familiarisation Week 1, n = 21; Week 2, n = 18W week 3, n = 19; Week 4, n = 18; Week 5, n = 17; Week 6, n = 18; Week 7, n = 18; Week 8, n = 17; Week 9, n = 17; Week 10, n = 17; Week 11, n = 16; Week 12, n = 3.].](/cms/asset/a688d226-f982-4dbe-893b-f3f54b1379ce/icop_a_1486396_f0002_b.jpg)
Figure 3. Difference between the mean daily step count for the familiarisation week and the last available mean daily step count recorded with the unsealed pedometer for all participants who provided a step count at two time points n = 20 in the PAI.
The results were obtained from the assessment of fidelity for the five domains of treatment fidelity, that is (i) Study design: all items under this domain were met except for one (5/6 items were met). (ii) Training of providers: all items under the domain about training of providers were met. (iii) Delivery: a proportion of consultations n = 36/221 (16%) were assessed and in this sample the majority of the components were delivered as intended (n = 43/50). (iv) Receipt. (v) Enactment domains focus on the participants. For receipt, most items were fully received with only a few (n = 3/18) items received on <100% of occasions. For enactment, a few (n = 2/6) items were not fully enacted. Further details on the results of fidelity of the PAI are available in online supplement eTable 4.
Discussion
This feasibility study demonstrates key considerations for conducting a future trial of a PAI versus PR in COPD. The applicable NIHR criteria for the success of a feasibility trial were met and based on the results of this study, including the qualitative data, a future trial is feasible. The PAI was effective for increasing step count, feasible to deliver and had good fidelity. However, before proceeding to larger trial strategies for increasing recruitment, reducing dropouts, improving adherence and for optimising the efficiency of data collection would need to be considered.
Recruitment to this study was generally feasible; we planned to recruit over a period of 14 months and achieved our target number at 16 months. Our recruitment process for this feasibility study was uniquely influenced by opportunities for easy access to programmes within limited study resources; we confined the study to two HSC Trusts and we recruited 11% of those eligible. Recruitment rates can vary across the COPD literature. For example, recruitment rates of 3.9% (103/2,646) in a recent study exploring the feasibility of conventional PR versus a web-based PR (Citation44) and 63.3% 57/90 in a cohort study on PR in COPD (Citation28) have been reported. In research on PAIs in COPD, 18.1% (140/775) were recruited in a study exploring the effects of a short-term (3 months) and a long-term (18 months) exercise program on self-reported disability and physical function in COPD (Citation45) and 89.8% (71/79) in a study exploring the effects of supervised high intensity continuous or interval training with unsupervised self-paced training (Citation46). A large number of patients referred to the PR clinics proved not to be suitable for this study due to, for example, musculoskeletal problems, vascular problems, cardiac issues (198/601, 33%); our criteria helped us to identify these patients and triage their care to an appropriate service, test or procedure prior to further assessment for PR. Not all patients referred for PR were interested in taking part (n = 131/601, 22%), and a small number (44/601, 7%) had COPD but this was not the primary diagnosis and were therefore excluded. This study provides data to estimate the number of sites that would be needed for a larger trial; the estimated sample size for full scale trial is 150 (75 per group) to allow us to detect 1,500 steps between group difference with 80% power, taking into account the current minimally clinical important difference for this population (Citation43). Alternative trial designs could also be considered, for example a non-inferiority trial design or a preference-randomised controlled trial (Citation47,Citation48). Broader inclusion criteria, as well as more PR sites, could improve the recruitment rates. To achieve recruitment targets for a larger trial, we would need to explore the capacity for recruitment at each PR site.
The dropout for the PAI (26%) was lower than the dropout in PR (50%). Although patients who dropped out were younger than completers, this pattern and other differences in important characteristics between dropouts and completers would need to be explored in a larger data set. A number of participants in this study also dropped out of PR for health reasons, patients with COPD can experience frequent exacerbations and often present with a number of comorbidities (Citation5). There were other patient-reported barriers to participation in the PR group that had the potential to be overcome in the PAI; the individualised and flexible nature of the PAI as well as the opportunity for phone contact could have facilitated participation for participants who did not enjoy the PR group setting, had transport difficulties or were restricted due to other commitments. The qualitative component further explored barriers to adherence; the results indicate a need for a more personalised approach and stronger emphasis on identifying each individual’s facilitators to help promote adherence. Furthermore, the dropout rate for PR (50%) was higher than that reported (29%) in a recent PR audit conducted in England and Wales (Citation5). The reasons for this higher rate of dropout are unclear, and the previous studies in PR in the Northern Ireland COPD population have reported dropout rates which are more consistent with the rest of the United Kingdom (between about 10% and 28%) (Citation28,Citation49); therefore, dropout rates from PR could possibly be reduced through the implementation of quality assurance measures prior to a future study.
A high number of participants did not meet the wear time criteria for the Actigraph (Citation40). A future trial could consider less stringent wear time rules to optimise data or consider a utilising a different monitor. The qualitative research findings indicated that a small number of people found the belt uncomfortable and at times cumbersome. Although this was a small number of people, in a study with such a small sample size any loss of data will affect the overall outcome. Although the Actigraph GT3X is considered one of the most valid activity monitors for measuring physical activity in people with COPD (Citation50), a future trial should explore with patients where they are most likely to wear an activity monitor, for example wrist, thigh, ankle or waist. Popular activity monitors such as the Fitbit have been validated in people with COPD and could be considered in a future trial to maximise physical activity data (Citation51). Finally, step count was also assessed with a pedometer which was sealed (to hide the step count data) at baseline and again post-intervention. There were discrepancies between the Actigraph step count data and the pedometer data. Current evidence indicates that these two devices are not interchangeable (Citation52–54). The Actigraph is a more precise measure of physical activity and hence it may be more suitable for data collection as an outcome measure for research (Citation52). The pedometer (unsealed), however, did appear to be a feasible tool for setting and monitoring step counts during the PAI and it provided good motivation to participants.
The PAI appears to be safe to deliver; with few and minor AEs. Recording of achievement of weekly step goals as an indication of feasibility has been reported in other studies (Citation55). Throughout the intervention, the step goals and actual steps achieved were closely matched with most participants achieving their goal each week similar to other studies in clinical populations (Citation9). The greatest improvement was observed in the first week with smaller, more gradual improvements over time; perhaps just wearing the monitor in the first week provided an initial motivation. The pedometer data obtained from participants during the PAI demonstrated (for those who recorded step counts at two time points) a mean increase (2,087) almost double that of the upper end of the MCID for step count in the COPD population (600–1,100) (Citation43). Furthermore, based on the Actigraph data, the MVPA also increased, albeit there is not MCID available for MVPA in COPD. Hence, indicating the potential efficacy of this intervention and potential for use in a future trial. Patients’ selection for such interventions may be important. A recent multicentre-randomised controlled study reported that patients more likely to respond to physical activity coaching interventions were those patients with better preserved functional capacity (Citation56). Some of our patient population were perhaps too frail to benefit maximally from the proposed PAI.
Furthermore, the assessment of fidelity demonstrated that the intervention was delivered as planned. Overall fidelity was good but an improvement could be to ensure that all providers are certified to deliver the intervention, and to assess fidelity regularly throughout the intervention, not at the end as in the present trial. Additionally, our assessment of delivery sought only to assess whether a component was delivered or not, and a scale assessing the quality of delivery of each component could further demonstrate how well the intervention was delivered. The fidelity assessment methods and results will be reported in a future publication.
The estimated time to deliver the PAI to eight patients individually across 12 weeks is 60.8 (34.4) hours. The estimated time to deliver a PR programme to eight patients in a group >6 weeks is 24 hours. The LIVELY PAI appears to takes approximately double the amount of time to deliver to eight patients compared to PR, which would result in increased costs. However, there is a large SD in the predicted length of time to deliver the PAI to eight patients, and the PAI had a higher rate of adherence which has potential for cost-saving implications in the longer term. Finally, we are comparing two different models of treatment for people with COPD and there are opportunities to modify the PAI to reduce costs and bring them more in line with PR. For example, using an online platform linked to the activity monitor whereby the steps are automatically uploaded, so that the interventionist can review these before the consultation, would reduce costs. The number of face-to-face consultations could also be decreased; qualitative data from the current trial demonstrated that some participants felt that they could have transitioned to this earlier. It has been suggested that much of the coaching could be done using a telemedicine approach (Citation56,Citation57) although not all trials were equally successful (Citation58). Furthermore, delivery in a group setting while retaining individual setting of step goals could decrease the time taken to deliver the PAI, delivery of education in a group setting could also be adopted in a future trial. The PAI in this study included management of breathlessness, and advice regarding inhalers and the management of an exacerbation. The patients were also given the LWWCOPD for PR booklet which includes information on the same education topics that are delivered in PR. Additional education and other components could be embedded in a future trial, for example, additional education topics could be added to mirror those included in PR, and/or patients could attend group education sessions.
The underpinning rationale for this study was that PR may not be suitable for all patients with COPD; this may also be true for the PAI, as evidenced by the large standard deviation in step count and MVPA for both groups. also shows that some participants in the PAI were more responsive to this intervention than others. These results suggest that there are patient phenotypes which may be more responsive to a PAI or to PR. The population in this study were of moderate disease severity and according to the CAT scores, their COPD had a severe impact on their quality of life. Characteristics such as disease severity have been reported to have an impact on daily physical activity levels (Citation59) and patients with better preserved functional status are reported to have had better outcomes in a remote telecoaching PAI (Citation56). Furthermore, patients’ preferences for the type of activity may also have an impact on outcome; for example, the results of the qualitative component of this study found that the group setting was both a facilitator and a barrier for participants in the PR group. Booth et al. (Citation60) reported that individuals have clear preferences for the types of activity they wish to engage in. Therefore, patients’ selection in terms of disease severity, functional status and individual preference may be important to consider in a future trial. Further research is required to establish phenotypes and preferences to better stratify patient care and optimise outcome.
Strengths and limitations
A key strength of this study is that it provides important feasibility data regarding screening, recruitment, delivery of the intervention and data analysis for a future trial. The PR was delivered as part of usual care. The results of the PR group in relation to exercise capacity and quality of life are not in line with expected outcomes (Citation1) and may in part be explained by the proportion of non-adherers/dropouts in this group; a future trial should consider ensuring all PR programmes are optimised prior to study implementation through to study completion and quality assurance measures for PR should be included as part of usual care.
A future trial would also need to include a cost effectiveness limb as well as additional data beyond the EQ5D5L to allow for a full-health economic appraisal. Furthermore, in this intervention the measurement of step count alone as an indicator of physical activity, although central to a number of tasks, does not take into account all the components necessary to execute all activities of daily living.
Conclusion
All applicable NIHR criteria for the success of a feasibility study were met with important learning and information regarding recruitment, eligibility, outcome measures and the sample size for a future study identified. The mixed methods design has enriched the data and exploring patients’ views and satisfaction has helped complement and verify the quantitative findings. The LIVELY PAI appears to be effective in improving step counts in people with COPD, feasible to deliver and had good fidelity. This study provides key information to inform a future-randomised controlled trial in physical activity.
Supplemental Material
Download PDF (381.9 KB)Acknowledgments
The authors thank specifically to Dr Denise Cosgrove, Adrian McDonald and Dr. Catherine Hanratty. They also thank their patient representative (JH) for their valuable input to the study.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;64(2):1–173.
- Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. Eur Respir Rev. 2010;19(115):24–9.
- Watz H, Pitta F, Rochester CL, Garcia-Aymerich J, ZuWallack R, Troosters T, Vaes AW, Puhan MA, Jehn M, Polkey MI, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–37.
- Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, Greening NJ, Heslop K, Hull J, Man WD-C, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults: accredited by NICE. Thorax. 2013;68(Suppl 2):ii1–30.
- Steiner MC, Roberts MC, Lowe D, Welham S, Searle L, Skipper E, Welham S, Roberts CM. National chronic obstructive pulmonary disease (COPD) audit programme: clinical audit of pulmonary rehabilitation services in England and Wales 2015. London: Healthcare Quality Improvement Partnership; 2016.
- Jones SE, Green SA, Clark AL, Dickson MJ, Nolan AM, Moloney C, Kon SS, Kamal F, Godden J, Howe C, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax. 2014;69(2):181–2.
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, Spruit MA, Masefield S, Casaburi R, Clini EM, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–86.
- Min-Lee I, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 2001;33(6):S459–S71.
- McDonough SM, Tully MA, Boyd A, O’Connor SR, Kerr DP, O’Neill SM, Delitto A, Bradbury I, Tudor-Locke C, Baxter GD, et al. Pedometer-driven walking for chronic low back pain: a feasibility randomized controlled trial. Clin J Pain. 2013;29(11):972–81.
- Wilson JJ, O’Neill B, Collins EG Bradley J. Interventions to increase physical activity in patients with COPD: a comprehensive review. COPD 2015:12(3):339–354.
- Behnke M, Wewel AR, Kirsten D, Joreres, RA, Magnussen, H. Exercise training raises daily activity in stronger than predicted from exercise capacity in patients with COPD. Resp Med. 2005;99(6):711–17.
- Breyer M, Breyer-Kohansal R, Funk G, Dornhofer N, Spruit MA, Wouters EF, Burghuber OC, Hartl S. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Resp Res. 2010;11:112.
- Moy ML, Janney AW, Nguyen HQ, Matthess KR, Cohen M, Garshick E, Richardson CR. Use of pedometer and Internet-mediated walking program inpatients with chronic obstructive pulmonary disease. J Rehabil Res Dev. 2010;47(5):485–96.
- Hospes G, Bossenbroek L, ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns. 2009;75(2):274–8.
- Altenburg WA, ten Hacken NH, Bossenbroek L, Kerstjens HA, de Greef MH, Wempe JB. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med. 2015;109(1):112–21.
- Tabak M, Vollenbroek-Hutten MM, van der Valk PD, van der Palen J, Hermens HJ. A telerehabilitation intervention for patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot trial. Clin Rehabil. 2013;28(6):582–91.
- Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard J, Haselkorn JK. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil. 2008;89(3):404–12.
- National Institute for Health Research Success Criteria for a feasibility trial [Internet]. Cited 2017 May 5. Available from: http://www.nets.nihr.ac.uk/glossary.
- Craig P, Dieppe P, MacIntyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council [Internet]. 2006. Developing and evaluating complex interventions: new guidance; [cited 2017 May 20]. Available from: www.mrc.ac.uk/complexinterventionsguidance.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1.
- Lancaster G. Pilot and feasibility studies come of age! Pilot Feasibility Stud. 2015;1:1.
- O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, Jansen YJFM, Mills N, Moore G, Donovan JL. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud. 2015;1:32.
- Hoffman TC, Glasziou PP, Milne R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Br Med J. 2014;348:g1687.
- Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32.
- Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol. 2012;65(3):301–8.
- Michie S, Atkins L, West R. The behaviour change wheel: a guide to designing interventions. 1st ed. Sutton: Silverback Publishing; 2014.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique Taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95.
- Cosgrove D, MacMahon J, Bourbeau J, Bradley JM, O’Neill B. Facilitating education in pulmonary rehabilitation using the living well with COPD programme for pulmonary rehabilitation: a process evaluation. BMC Pulm Med. 2013;13:50.
- Williams S, Baxter N, Buxton M, Harrison A, Holmes S, Hughes E, McCarthy J, Morgan M, Price A, Restrick et al. IMPRESS Guide to Pulmonary Rehabilitation. British Thoracic Society Reports. 2011;3(2):ISSN 2040-2023.
- Rabinovich RA, Louvaris Z, Raste Y, Langer D, Van Remoortel H, Giavedoni S, Burtin C, Regueiro EM, Vogiatzis I, Hopkinson NS, et al. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J. 2013;42(5):1205–15.
- Schneider PL, Crouter S, Basset DR. Pedometer measures of free living physical activity: comparison of 13 models. Med Sci Sports Exerc. 2004;36(2):331–5.
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
- Singh SJ, Morgan MDL, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J. 1994;7:2016–20.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54.
- Briggs AH, Glick HA, Lozano-Ortega G, Spencer M, Calverley PMA, Jonese PW, Vestbo J; TORCH Investigators. Is treatment with ICS and LABA cost-effective for COPD? Multinational economic analysis of the TORCH study. Eur Respir J 2010;35(3):532–9.
- Perry M. Activity monitoring and low back pain. Chapter 1. Awarded PhD; University of Otago; 2010.
- Marcus BH, Forsyth LH. Motivating people to be physically active. 2nd ed. Champaign (IL): Human Kinetics; 2009.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trails. J Public Health Dent. 2011;71(1):1–21.
- Bellg A, Borrelli B, Resnick B, Hecht J, Minicucci D, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–51.
- Byron B, Rowe DA. Measuring free-living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studies. Contemp Clin Trials. 2016;47:172–84.
- King, N. Template analysis. In: Symon G, Cassell C, editors. Qualitative methods and analysis in organizational research. London: Sage; 1998.
- GOLD [Internet] [cited 2017 April 04]. Available from: http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf.
- Demeyer H, Burtin C, Hornikx M, Camillo CA, Van Remoortel H, Langer D, Janssens W, Troosters T. The minimal important difference in physical activity in patients with COPD. PLoS ONE 2016;11(4):1–11.
- Chaplin E, Hewitt S, Apps L, Bankart J, Pulikottil-Jacob R, Boyce S, Morgan M, Williams J, Singh S. Interactive web-based pulmonary rehabilitation programme: a randomised controlled feasibility trial. BMJ Open 2017;7:e013682.
- Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23(1):60–8.
- Varga J, Porszasz J, Boda K, Casaburi R, Somfay A. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med. 2007;101(11):2297–2304.
- Horton EJ, Mitchell KE, Johnson-Warrington V, Apps LD, Sewell L, Morgan M, Taylor RS, Singh SJ. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax. 2017;0:1–8.
- Main E. Airway clearance research in CF: the ‘perfect storm’ of strong preference and effortful participation in long-term, non-blinded studies. Thorax. 2013;68(8):701–2.
- O’Neill B, McKevitt A, Rafferty S, Bradley JM, Johnston D, Bradbury I, McMahon I. A comparison of twice- versus once-weekly supervision during pulmonary rehabilitation in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2008;88(2):167–72.
- van Remoortel H, Raste Y., Louvaris Z, Giavedoni S, Burtin C, Langer D. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One. 2012;7(6):e39198.
- Vooijs M, Alpay LA, Snoeck-Stroband JB, Beerthuizen T, Siemonsma P, Abbink JJ. Validity and usability of low-cost accelerometers for internet-based self-monitoring of physical activity in patients with chronic obstructive pulmonary disease. Interact J Med Res. 2014;3(4):e14.
- O’Neill B, McDonough SM, Wilson JJ, Bradbury I, Hayes K, Kirk A. Comparing accelerometer, pedometer and a questionnaire for measuring physical activity in bronchiectasis: a validity and feasibility study? Respir Res. 2017;18:16.
- Kinnunen TI, Tennant PWG, McParlin C, Poston L, Robson SC, Bell R. Agreement between pedometer and accelerometer in measuring physical activity in overweight and obese pregnant women. BMC Public Health. 2011;11:501. doi:10.1186/1471-2458-11-501.
- Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sports Exerc. 2009;41(7):1392–1402.
- Paxton RJ, Forster JE, Miller MJ, Gerron KL, Stevens-Lapsley JE, Christiansen CL. A feasibility study for improved physical activity after total knee arthroplasty. J Ageing Phys Act. 2017;26(1):1–21.
- Demeyer H, Louvaris Z, Frei A, Rabinovich RA, de Jong C, Gimeno-Santos E, Loeckx M, Buttery SC, Rubio N, Van der Molen T, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax. 2017;72(5):415–23.
- Moy ML, Collins RJ, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, Nguyen HQ, Cohen MD, Goodrich DE, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest. 2015;148(1):128–37.
- Vorrink SNW, Kort HSM, Troosters T, Zanen P, Lammers JWJ. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur Respir J. 2016;48(4):1019–102.
- Clarenbach CF, Sievi NA, Haile SR, Brack T, Brutsche MH, Fewy M, Irani S, Leuppi JD, Thurnheer R, Kohler M. Determinants of annual change in physical activity in COPD. Respirology. 2017;22(6):1133–39. doi:10.1111/resp.13035
- Booth ML, Bauman A, Owen N, Gore CJ. Physical activity preferences, preferred sources of assistance, and perceived barriers to increased activity among physically inactive Australians. Prev Med. 1997;26(1):131–7.
