Abstract
Domiciliary noninvasive mechanical ventilation (NIMV) is used for treating patients with hypercapnic chronic obstructive pulmonary disease (COPD). We aimed to evaluate the association between adherence to the treatment and subsequent hospitalizations and costs.
Data from 54 (27 adherent; 27 non-adherent) patients with COPD who were undergoing NIMV treatment at home for 6 months. We assessed adherence based on digitally recorded data and checked hospital records for clinical and laboratory data, rehospitalization rates, and costs during the following 6 months.
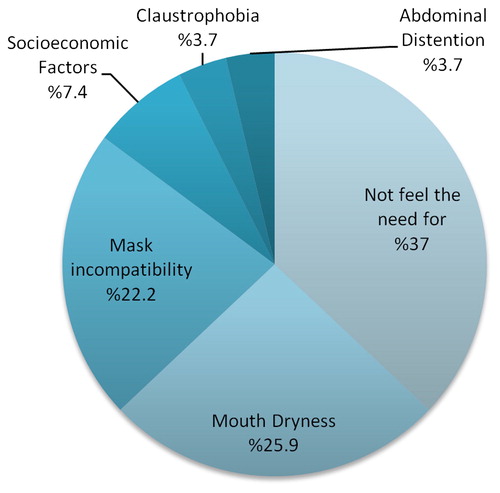
Nocturnal NIMV usage, mean daily usage of the device, and time to first hospitalization were higher in the treatment-adherent group (p < .001, p < .001, and p=.006, respectively). The percentage of active smokers, device leaks above 30 L/min, length of hospital stay, rehospitalization rates, and costs were significantly higher in the treatment-non-adherent group (p = 05, p = 006, p = 004, p = 006, and p = 01, respectively). The most frequent reasons for not using NIMV in the treatment-non-adherent group were a decreased need, dry mouth, mask incompatibility, and gastrointestinal complaints.
Adherence to NIMV treatment decreases the subsequent hospitalizations rates and noncompliance leads to complications. Findings of this study may help physicians in convincing patients diagnosed with COPD of the need for correct NIMV use to prevent hospitalizations and reduce the costs of COPD treatment.
Introduction
Noninvasive mechanical ventilation (NIMV) reduces the need for intubation and in-hospital mortality in patients with chronic obstructive pulmonary disease (COPD) exacerbations and acute hypercapnic respiratory failure. NIMV treatment is considered to be a method whose benefits do not leave any room for discussion in the presence of appropriate indications during COPD exacerbation. However, there is insufficient evidence on the need and long-term benefits of NIMV use in patients during the stable disease periods (Citation1). The 2008 National UK COPD audit showed that one-third of patients discharged from the hospital after exacerbations were re-admitted within 90 days. Another study showed that 20% of hospitalized patients with COPD were re-admitted within 28 days (Citation2). Therefore, the contribution of NIMV to the reduction of morbidity and mortality during the stable period needs to be investigated.
In a study analyzing cost-effectiveness of NIMV treatment in patients with severe hypercapnic respiratory insufficiency due to COPD, the annual cost of hospitalization for the patients with long-term domiciliary NIMV was 43,024 Euros/year, whereas that for patients without NIMV was 153,306 Euros (Citation3). Similar studies have demonstrated that the long-term use of domiciliary NIMV for patients with advanced-stage COPD reduced re-admissions and hospitalization costs; however, there is insufficient data on the treatment compliance, re-admission rate, and hospitalization costs for patients.
Treatment noncompliance of patients with indications for long-term NIMV use in Turkey is a major problem in the management of COPD. Increasing NIMV compliance in patients is crucial in reducing recurrent hospital admissions and secondary healthcare costs. The aim of this study was to evaluate the level of treatment compliance in patients diagnosed with severe COPD and who have been recommended long-term domiciliary NIMV therapy and those with health reports for home device use for determining re-admission rates and evaluating the cost-effectiveness of treatment compliance versus noncompliance.
Materials and methods
Study design, population, and approval
We designed a retrospective cohort study for evaluating data from patients diagnosed with severe COPD who were being followed up and treated at the Health Sciences University, Yedikule Chest Diseases and Chest Surgery Training and Research Hospital.
Our study was conducted in line with the Declaration of Helsinki tenets, and our institutional ethics committee approved it (Protocol No. 2016/21). Patients enrolled in this study were given informed consent forms in accordance with the International Conference on Harmonization-Good Clinical Practice (ICH-GCP) guidelines before participating in the study, and they signed them.
Definitions
We considered patients adherent to the treatment if they used NIMV for at least 4 h a day on at least 70% of treatment days, whereas those not meeting these criteria were considered to be non-adherent.
Patient selection
NIMV was indicated in patients with acute hypercapnic respiratory failure after acute COPD exacerbations and all patients were prescribed long-term oxygen treatment.
We included the following patients diagnosed with COPD prescribed domiciliary NIMV for 6 initial months:
Those with PaCO2 levels in arterial blood gas >55 mmHg or
Those with PaCO2 levels in arterial blood gas between50 and 55 mmHg with
Nocturnal desaturation (%Sat <88 for at least 5 min) or
Two hospitalizations in 1 year
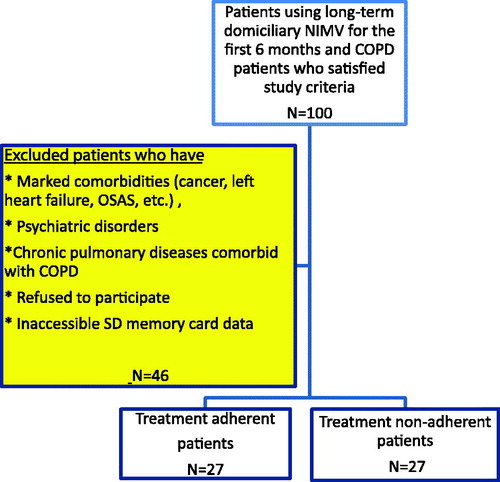
We excluded patients if
they presented with severe comorbidities (cancer, left heart failure, unstable angina pectoris, etc.),
they had psychiatric disorders (which might interfere with NIMV use),
they had chronic pulmonary diseases comorbid with COPD (such as fibrothorax, scoliosis, bronchiectasis, cystic fibrosis, and pulmonary fibrosis),
they had a history of obstructive sleeping apnea syndrome,
they refused to participate in the study, or
data in their digital Secure Digital (SD) memory card data could not be accessed
The study was conducted between January and July 2016 after screening for patients using domiciliary NIMV machines. All NIMV devices were used in the bilevel positive airway pressure-spontaneous (BiPAP-S) mode without a humidifier. All patients had proper fitting oronasal masks.
In all, 100 patients diagnosed with COPD satisfied the study criteria. After excluding patients who met the exclusion criteria, we enrolled 54 patients in the study; we classified 27 of them as adherent and 27 as non-adherent to the treatment (). We also recorded hospital admissions of the patients within the 6 months after their NIMV prescriptions ().
Data collection
We examined health board report lists and collected information concerning NIMV usage from the SD memory cards in the patients’ devices. Accessed data included the device mode, inspiratory positive airway pressure (IPAP)-expiratory positive airway pressure (EPAP) values, mean leakage, number of hours used per day, time zone used, and number of days used.
In addition, we collected data on patient histories about cigarette smoking, body mass index (BMI), biomass exposure, BECK scores, and reasons for not using the NIMV device (in the case of treatment-non-adherent patients). We also obtained the spirometric values, laboratory variables, and hospitalization information for each patient using the hospital database. Blood gas samples were obtained 2 h later after NIMV usage. Experienced doctors obtained arterial blood gas samples from radial arteries of patients with under 2 L/min nasal oxygen therapy. Records for hospital re-admissions included ward-intensive care hospitalizations in our hospital and in outpatient centers.
Although we did not include total treatment costs in the cost analysis because of large variations in prescribed treatments, we used the sum of the ward and intensive care hospitalization costs as representative of total costs.
Outcomes
Primary outcome
Rehospitalization rates during follow-up in treatment-adherent and treatment-non-adherent patients
Secondary outcomes
Cost of hospitalizations in treatment-adherent and treatment-non-adherent patients
Times to first hospitalization in both groups
Lengths of hospitalization in both groups
Comparison of PaCO2 levels before and after using domiciliary NIMV treatment in both groups
Statistical analysis
We transferred collected data to the Statistical Package for the Social Sciences (SPSS) version 23 software. We obtained central tendency measures, like the mean and standard deviation for numerical variables, and calculated frequency distributions (number and percentage) for categorical variables in study data. We evaluated the presence of differences between the two groups using an independent Sample t-test and a Mann–Whitney U-test under appropriate conditions. On the other hand, we used a chi-squared test for assessing associations between categorical variables; our results are presented in tables.
We calculated the sample size required for analysis using patients’ rehospitalization rates. According to the literature, rehospitalization rates in patients diagnosed with COPD using long-term domiciliary NIMV are 40% in treatment-adherent patients and 75% in treatment-non-adherent patients (Citation4). The number of patients required for analysis was calculated as 27 in both groups. The strength of our study was 80%, and results were evaluated at a 95% confidence interval, with a p < .05 considered as significant.
Results
Demographic characteristics of patients enrolled in the study
The mean age of patients enrolled in the study was 64.1 years (12 women and 42 men). We found no statistically significant association between treatment-adherent and treatment-non-adherent groups in terms of age, sex, smoking status, BODE index, biomass exposure, or BMI (all p values were >.05). Our results showed that the rehospitalization rate of patients with obesity was 33.3%, whereas that of patients who were not obese was 63.6% (p = .03).
shows the results of a comparison of clinical and demographic characteristics of the treatment-adherent and treatment-non-adherent patients.
Table 1. Differences in clinical and demographic data between treatment-adherent and treatment-non-adherent patients.
Blood gases, NIMV variables, and COPD medications in treatment-adherent and treatment-non-adherent patients
We found no statistically significant differences between treatment-adherent and treatment-non-adherent groups in terms of IPAP-EPAP values, blood gas pH (power of hydrogen), partial pressure of carbon dioxide (pCO2), bicarbonate (HCO3), mean base deficit before and after NIMV treatment, pCO2 values before and after treatment, or COPD medications (p > .05). In addition, we found no statistical differences between the mean of the total length of NIMV usage in treatment-adherent (40.4 ± 29.9 months) and treatment-non-adherent patients (31.4 ± 20.9 months) (p > .05). Treatment-non-adherent patients had higher mean partial pressure of oxygen (pO2) values prior to NIMV treatment than treatment-adherent patients; the mean length of use of treatment-adherent patients was higher than that in treatment-non-adherent patients (p = 03 and p < .001*, respectively). Treatment-adherent patients used the device more often during the night (between 00:00 and 08:00) and the mean leakage of the device (<30 L/min) was also higher (p < .001* and p = 006, respectively). Patients in the treatment-non-adherent group reported that the most frequent reasons for not using NIMV included the impression that they did not need the treatment, dry mouth, mask incompatibility, and association to gastrointestinal complaints, in order of frequency. shows the data concerning NIMV use in the different groups.
Table 2. Comparison of NIMV usage and COPD medications between treatment-adherent and treatment-non-adherent patients.
Rehospitalization rates and cost
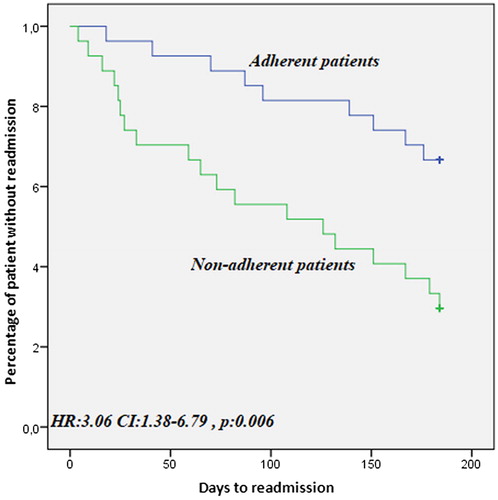
We found a statistically significant difference between treatment-adherent and treatment-non-adherent patients in terms of the total number of hospitalizations after the treatment (p = 006). We calculated the post-hoc power of the present study to be 86%. The number of hospitalizations in the wards after treatment, hospital stay lengths, and average cost of hospitalizations were significantly higher in treatment-non-adherent patients than in treatment-adherent patients (p = 004, p = 004, and p = 01, respectively). In addition, the time to the first hospitalization during follow-up was significantly higher in treatment-adherent patients (HR: 3.06; CI: 1.38–6.79; p = 006; ).
Figure 3. Kaplan–Meier curve of event-free survival treatment-adherent and treatment-non-adherent patients.
When we analyzed variables impacting rehospitalization of patients, we found that BMI, prior ward hospitalizations, and BECK scores were correlated to rehospitalization of the patients (p=.05, p=.03, and p=.03, respectively). Our multivariate analysis for investigating correlations between rehospitalization rates and BMI, prior ward hospitalizations, BECK scores, and adherence revealed that treatment-non-adherent patients with more prior ward hospitalizations had a higher risk for rehospitalization ().
Table 3. Logistic regression analysis estimating the independent association between adherence and rehospitalization.
We found no statistically significant differences in the number of admissions to the intensive care unit (p > .05) between the groups. shows the results of comparisons of group data on hospitalization and hospitalization costs.
Table 4. Comparison of hospitalization rates and costs between treatment-adherent and treatment-non-adherent patients.
Discussion
The use of NIMV in hospitals for treating acute respiratory failure associated with COPD has shown a significant increase over the last 15–20 years. Similarly, the incidence of long-term domiciliary NIMV use in patients with chronic respiratory failure is also on the rise. Domiciliary NIMV treatment for patients with COPD can reduce respiratory muscle work, restore chemosensitivity of the central nervous system (suppressed by the chronichypercapnia), and eliminate alveolar hypoventilation.
However, literature findings on long-term domiciliary NIMV use in patients with COPD are contradictory, and conflicting data are an indication that better studies are needed (Citation5–7). In Turkey, there is a lack of sufficient data on this subject.
The aim of our study was to determine associations between device use and number of rehospitalizations and hospitalization costs in patients using long-term domiciliary NIMV. Our results suggest that the use of long-term domiciliary NIMV treatment in patients with appropriate indications is effective for reducing patient rehospitalizations and associated costs.
In a study conducted by Altınöz et al., patients with BMI >30 and those using long-term domiciliary NIMV lived longer (Citation8). On multivariate regression analysis of a multicentric prospective cohort study conducted by Borel et al., the rehospitalization or mortality rate in patients with BMI <21 (HR: 1.54) was higher than those in patients with BMI >30 (HR: 0.66) (Citation9). In our study, we found no statistically significant differences in mean BMIs between the treatment-adherent and treatment-non-adherent groups. This suggests that COPD and high BMI phenotype, which demonstrates benefit from NIMV treatment, should be better defined although there are studies showing that patients with obesity benefit more from long-term domiciliary NIMV treatment. In addition, Borel et al. demonstrated that the 1-year rehospitalization rate for patients with obesity was 13% compared with the 31% for patients not diagnosed with obesity. Conversely, results of our study showed that the rehospitalization rate in patients with obesity was 33.3%, whereas that in patients who were not diagnosed with obesity was 63.6% (p = 03). We found the rates of rehospitalization in our study to be higher in both groups than those reported in the study by Borel et al., which can be attributed to the fact that the hospitalization rate in patients with COPD is higher in Turkey, based particularly on socioeconomic factors.
Patients in our study were grouped as treatment-adherent and treatment-non-adherent based on at least 4 h of NIMV-use per day, with those patients recording a rate of >70% compliance considered as treatment-adherent patients. To evaluate time intervals of the remaining 30%, we examined the mean daily NIMV usage hours and found that treatment-adherent patients presented a mean daily usage of 8.3 h per day and treatment-non-adherent patients presented a mean daily usage of 2.1 h per day. This difference was statistically significant (p < .001).
The time period during which treatment-adherent patients used the NIMV treatment most frequently was between 23:59 and 08:00, as recommended (Citation10), whereas treatment-non-adherent patients used the NIMV treatment most frequently from 16:00 to 23:59 (p < .001). Also, the device leakage rate that was supposed to be minimized during treatment was found to be lower in the treatment-adherent patients (p=.006), suggesting a need for increasing frequency of controls and to explain, in more detail, the treatment process for ensuring cooperation of patients.
The most common reasons for not using the NIMV machine in the treatment-non-adherent group was the “impression of no treatment requirement” in 37%, followed by “dry mouth” in 25.9% (). In the study by Criner et al., 23% of NIMV complications were mask-related irritation and 13% were due to rhinitis or aerophagia (Citation11).
One of the secondary endpoints of our study was the comparison of improvement in pCO2 values before and after treatment between the treatment-adherent and treatment-non-adherent groups. The pCO2 value in the treatment-adherent group was 16.5 mmHg, and it was 12.5 mmHg in the treatment-non-adherent group. This non-statistically significant difference was probably enough to impact the rehospitalization rate in patients.
According to our relative risk calculations, the risk of hospitalization within the following 6 months in the treatment-adherent patients was 2.11 times higher than that in the treatment-non-adherent patients (CI: 1.1739–3.7967, p = .01) ().
In the randomized controlled multicentric study conducted by Köhnlein et al., the long-term use of NIMV by patients with COPD reduced the number of rehospitalizations during a 1-year follow-up (Citation12). However, the study had technical problems, and patients were followed up every 3 months. The number of hospitalizations at the end of 3, 6, and 12 months decreased in the NIMV treatment-adherent group, whereas the same increased at the end of the 9th month in treatment-non-adherent group (Citation12). In our 6-month follow-up study, the number of hospitalizations was significantly lower in the treatment-adherent NIMV patients than in the treatment-non-adherent patients although the fact that follow-up was shorter than that reported in other studies may have influenced long-term outcomes.
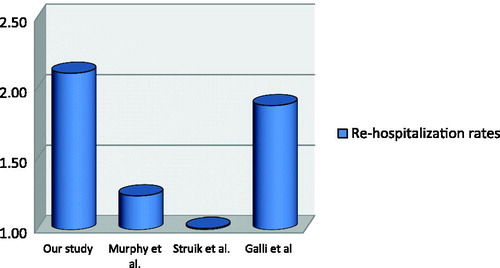
Results of the randomized controlled multicentric RESCUE study conducted by Struik et al. in 2014 identified no significant differences between patients using long-term NIMV and those receiving standard treatment with regard to rehospitalization rates (Citation7). In our study, the mean rehospitalization rate in the treatment-adherent group was 0.4 compared with that in the treatment-non-adherent group (1.2; p = .006). Similarly, the results of a multicentric, randomized controlled trial conducted by Murphy et al. in 2017 revealed a decreased rehospitalization rate within 1 year of the start of treatment (89% in the group receiving oxygen therapy alone; 72% in the group receiving NIMV + oxygen therapy) (Citation13). In our study, we found the rehospitalization rate in the treatment-non-adherent patients was 70% and that in the treatment-adherent patients was 33.3%.
In a study by Galli et al., a time-to-event analysis of the time until death or rehospitalization revealed that event-free survival of patients using long-term domiciliary NIMV was longer than that of patients not using it (Citation4). In our study, the effect of NIMV use on the time to rehospitalization was longer in the treatment-adherent group (HR: 3.06; CI: 1.38–6.79; p=.006). Therefore, we concluded that the length of hospital stay was longer in treatment-non-adherent patients (p=.004) (). In their study, Galli et al. found no significant difference in the length of stay between the two groups. The reason for this may be differences in the analyses; we included all the lengths of stay within 6 months and they only took the length of the first hospitalization into account. However, we consider this data to be clinically important because the time spent in hospital raises the risk for hospital-acquired infections in patients with COPD.
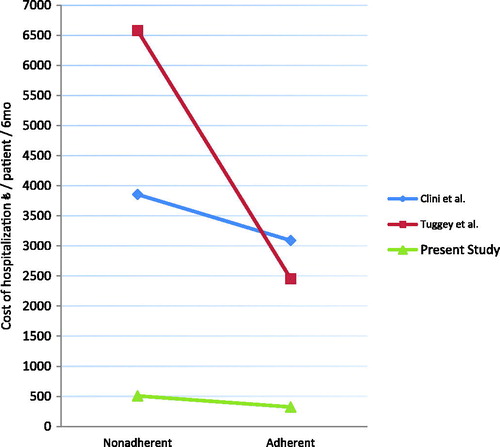
In our study, we showed that the effective use of long-term domiciliary NIMV treatment reduced the total cost of hospitalizations by 184,95 € per patient over 6 months. According to a retrospective cost analysis conducted by Tuggey et al. in 2003, the cost per patient was reduced by 4,126 € per patient over 6 months in patients using long-term domiciliary NIMV (Citation3). The retrospective cost analysis performed by Clini et al. in 2009 showed that the group undergoing long-term domiciliary NIMV treatment demonstrated a reduction in costs (765 Euros/6 months/patient) compared with the group receiving standard treatment (Citation14) (). Cost components in these studies include medications used, hospitalizations, and oxygen use.
Limitations
We are aware of the limitations of our study. The fact that our study was a retrospective cohort study led to the relatively short follow-up of patients because SD memory cards used in NIMV machines were able to record a maximum of 1 year of data. But, studies have indicated that longer follow-up may produce different results, implying a weakness of our study. Moreover, differences in the brand and technical specifications of NIMV devices used by different patients because of social security institution rules may have affected the accuracy of our results.
In addition, we were not able to establish correlations with mortality in patients who died before study visits because data in their SD cards were not available, leading to a selection error. In addition, other selection errors are conceivable because some patients chose not to participate in the study and others had no SD cards in their machines. We conducted our study with patients from a single center; thus, our study population may not reflect data of the whole country.
Conclusions
Ensuring compliance with long-term domiciliary NIMV treatment in patients having appropriate indications can result in a significant reduction in rehospitalizations and treatment costs. After registering the device, it is necessary to explain, in detail, the importance of its use to the patients and to carefully follow the device guidelines. To the best of our knowledge, ours is the first study of its kind in Turkey. We will present our results to social security institution in a report. We believe there is a need for prospective studies evaluating the association between long-term domiciliary NIMV treatment and rehospitalization (and cost-effectiveness) in patients diagnosed with COPD.
Disclosure of interest
The authors declare having no financial, consulting, or personal relationship conflicts of interest.
References
- Chu CM, Chan VL, Lin AW, Wong IW, Leung WS, Lai CK. Readmission rates and life-threatening events in COPD survivors treated with non invasive ventilation for acute hypercapnic respiratory failure. Thorax. 2004;59(12):1020–5. doi:10.1136/thx.2004.024307. PMID:15563699.
- Steer J, Norman EM, Afolabi OA, Gibson GJ, Bourke SC. Dyspnoea severity and pneumonia as predictors of in-hospital mortality and early readmission in acute exacerbations of COPD. Thorax. 2012;67(2):117–21. doi: 10.1136/thoraxjnl-2011-200332. PMID:21896712
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax. 2003;58(10):867–71. PMID:14514940
- Galli JA, Krahnke JS, James Mamary A, Shenoy K, Zhao H, Criner GJ. Home non-invasive ventilation use following acute hypercapnic respiratory failure in COPD. Respir Med. 2014;108(5):722–8. doi: 10.1016/j.rmed.2014.03.006. PMID: 24702885
- Lloyd-Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Farre R, Fauroux B, Robert D, Schoenhofer B, Simonds AK, Wedzicha JA. Patterns of home mechanical ventilation use in Europe: results from the Eurovent survey. Eur Respir J. 2005;25(6):1025–103. doi:10.1183/09031936.05.00066704. PMID:15929957
- Robert D, Argaud L. Clinical Review: Long term noninvasive ventilation. Crit Care. 2007;11(2):210. doi:10.1186/cc5714. PMID:17419882
- Struik FM, Sprooten RT, Kerstjens HA, Bladder G, Zijnen M, Asin J, Cobben NA, Vonk JM, Wijkstra PJ. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826–34. doi:10.1136/thoraxjnl-2014-205126. PMID:24781217.
- Altinoz H, Adiguzel N, Salturk C, Gungor G, Mocin O, Berk Takir H, Kargin F, Balci M, Dikensoy O, Karakurt Z. Obesity might be a good prognosis factor for COPD patients using domiciliary noninvasive mechanical ventilation. Int J Chron Obstruct Pulmon Dis. 2016;11:1895–901. doi:10.2147/COPD.S108813. PMID:27578969.
- Borel JC, Pepin JL, Pison C, Vesin A, Gonzalez-Bermejo J, Court-Fortune I, Timsit JF. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology. 2014;19(6):857–65. doi:10.1111/resp.12327. PMID:24912564
- McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, O'Donoghue FJ, Barnes DJ, Grunstein RR. Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009; 64(7):561–6. doi:10.1136/thx.2008.108274. PMID:19213769.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest. 1999;116(3):667–75. PMID:10492269.
- Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, Karg O, Laier-Groeneveld G, Nava S, Schönhofer B, Schucher B, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014; 2(9):698–705. doi: 10.1016/S2213-2600(14)70153-5. PMID: 25066329.
- Murphy PB, Rehal S, Arbane G, Bourke S, Calverley PMA, Crook AM, Dowson L, Duffy N, Gibson GJ, Hughes PD, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177–86. doi:10.1001/jama.2017.4451. PMID:28528348.
- Clini EM, Magni G, Crisafulli E, Viaggi S, Ambrosino N. Home non-invasive mechanical ventilation and long-term oxygen therapy in stable hypercapnic chronic obstructive pulmonary disease patients: comparison of costs. Respiration. 2009;77(1):44–50. doi:10.1159/000127410. PMID:18417954.