Abstract
The aim of our study was to evaluate the prevalence and predictors of obstructive sleep apnea (OSA) in patients with chronic obstructive pulmonary disease (COPD) undergoing inpatient pulmonary rehabilitation programs (PRPs). A retrospective data review of consecutive stable patients with a known diagnosis of COPD, admitted for PRP between January 2007 and December 2013. Full overnight polysomnography (PSG) and Epworth Sleepiness Scale (ESS) were assessed in all patients. Out of 422 evaluated patients, 190 (45%) showed an Apnea Hypopnea Index (AHI) ≥ 15 events/hour and underwent OSA treatment. Patients with OSA were significantly younger and had a less severe airway obstruction as compared to patients without OSA. There were no significant differences in cardiac comorbidities nor in arterial blood gases. As expected, patients with OSA showed significantly more severe diurnal symptoms, as assessed by the ESS and higher body mass index (BMI). However, only 69 out of 190 patients with OSA (36.3%) showed an ESS >10, whereas 25% of them had BMI ≤25 and 41% of them had a BMI <30. In all, 68% of patients with OSA were discharged with continuous positive airway pressure (CPAP), 15% with Bilevel ventilation, and 17% without any ventilatory treatment. In conclusion, in the population studied, the combination of OSA and COPD was frequent. BMI and ESS values commonly considered cutoff values for the prediction of OSA in the general population may not be accurate in a subgroup of patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with several comorbidities contributing to different phenotypes, with related mortality rates and reduced physical activity (Citation1–3). There is evidence that the combination of obstructive sleep apnea (OSA) and COPD, also known as the “overlap syndrome,” is not common in the general hospital population (1.0–3.6%), whereas it is highly prevalent in patients with either OSA (7.6–55.7%) or COPD (2.9–65.9%) (Citation4). Patients with overlap syndrome have been shown to have greater nocturnal oxygen desaturation (NOD) (i.e., reduced mean pulse oximetric oxygen saturation (SpO2), increased sleep time spent with SpO2 <90% (T90)), and worse sleep quality than patients with only OSA. Overlap syndrome is associated with more frequent cardiovascular morbidity, poorer health-related quality of life (HRQL), more frequent COPD acute exacerbations (AECOPD), and increased medical costs (Citation4).
Pulmonary rehabilitation programs (PRPs) play a corner role in the comprehensive management of patients with COPD. After a PRP, patients, either in a stable state or following an AECOPD report benefits in symptoms, HRQL, and exercise tolerance (Citation5). This is also true when complex comorbidities and different phenotypes are present (Citation6, Citation7).
We wondered whether the reported prevalence of OSA in patients with COPD could be observed also in patients who are eligible for pulmonary rehabilitation (Citation4). Therefore, the aim of this real-life retrospective study was to evaluate the prevalence and predictors of OSA in patients with COPD who underwent an inpatient PRP. Our secondary aim was to identify the appropriate management of these patients either with continuous positive airway pressure (CPAP) or noninvasive ventilation (NIV).
Methods
The protocol was approved by the Ethical Committee (Number 844 CEC on July 25th, 2012) of the Istituti Clinici Scientifici Maugeri, IRCCS, Pavia, Italy. All patients admitted to the Scientific Institute of Pavia gave informed consent to the use of their data for scientific purposes.
Study participants
We conducted a retrospective review of consecutive stable patients with a known diagnosis of COPD, admitted for the first time to a PRP between January 2007 and December 2013 at the Pulmonary Rehabilitation Unit of the Istituti Clinici Scientifici Maugeri, IRCCS Pavia, Italy, a referral center for pulmonary rehabilitation, diagnosis and management of OSA, and chronic care (Citation8), including prescription of home NIV and CPAP (Citation9, Citation10). All the admitted patients with a diagnosis of COPD, regardless of functional severity, underwent the PRP. Eligibility criteria for and components of PRP were according to accepted guidelines (Citation5).
The diagnosis and severity of COPD were confirmed by spirometry according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Citation1). At admission, all patients were in a stable condition, as assessed by the absence of any worsening of symptoms, and stability in arterial blood gases (no acidosis, defined as pH <7.35). All patients received their regular treatment with inhaled bronchodilators with or without inhaled steroids according to current guidelines for their disease stage (Citation1). Patients with a history of more than two AECOPD per year requiring antibiotics or those who were referred to the PRP after an acute exacerbation requiring mechanical ventilation were defined as “frequent exacerbators.”
OSA was defined as the presence of 15 or more obstructive respiratory events per hour of sleep (Citation11).
Exclusion criteria were as follows: concomitant restrictive diseases, major comorbidities (e.g., psychiatric conditions, cancer, end-stage renal failure) or familiar conditions preventing long-term home ventilatory programs. Patients screened elsewhere or already treated with long-term mechanical ventilation were also excluded.
Measurements
At admission, demographic, anthropometric data, and reported cardiac comorbidities (including ischemic and valvular heart disease, heart failure, systemic hypertension, and arrhythmias) were recorded and all patients underwent evaluation of the following:
Epworth Sleepiness Scale (ESS) score (Citation12)
Lung function, performed according to the American Thoracic Society (ATS) guidelines (1Citation4), by means of a body plethysmograph (MasterScope Body Jeager – Wuerzburg Germany) or a water sealed spirometer (EOS Biomedin, Padova, Italy), using the predicted values according to the ERS/ATS Task Force (Citation13).
Arterial blood gases, performed by means of an automated analyzer (Radiometer 800Flex, Copenhagen, Denmark) on samples from the radial artery with the patients in the sitting position for at least 1 hour. Patients under long-term oxygen therapy (LTOT) were assessed under their usual inspiratory oxygen fraction.
Standard sleep evaluation was performed, with a full overnight polysomnography (PSG) (Nox T3 Sleep Monitor, Nox Medical, Reykjavik, Iceland) analyzed and scored according to the American Academy of Sleep Medicine (AASM) 2007 Guidelines (Citation14). SpO2, heart rate, nasal flow, thoracic-abdominal movements, and body position were monitored in all cases.
After diagnosis, CPAP was proposed to all patients with OSA, irrespective of the presence of hypercapnia. CPAP titration was performed either by full-night manual titration according to the AASM Guidelines (Citation14), or by automatic titration with auto-set systems (Citation15). The interface, whether nasal or oronasal mask, was prescribed as appropriate according to patient requirements.
If CPAP treatment was unable to correct hypoventilation or not well tolerated, NIV in Bilevel mode was prescribed (Citation5). Hypoventilation was defined either as the persistence of diurnal arterial carbon dioxide tension (PaCO2) ≥ 45 mmHg or periodic oxygen desaturations higher than 4% associated with a transcutaneous carbon dioxide tension (PtcCO2) increase in ≥5 mmHg from baseline for more than 5 minutes continuously, at least once during the night (Citation14, Citation16).
The setting of NIV was adjusted to achieve an effective correction of the obstructive events and the normalization of the gas exchanges (Citation14).
Patients were discharged with CPAP or NIV if the patient showed good adherence to the therapy, defined as an average use of at least 4 h/night, as indicated by the memory card of the device.
Statistical analysis
Descriptive statistics are reported as mean ± standard deviation (SD) for continuous variables and as numbers (percentage frequencies) for discrete variables.
Between-group differences were calculated by means of an independent t-test and the Mann–Whitney test, where appropriate.
A multiple logistic regression analysis was performed to assess which of the tested variables could be predictive of OSA. Independent variables with a p value <.02 in the univariate analysis were included in the multivariable model. A linear regression analysis was then performed to assess the relationship between Apnea Hypopnea Index (AHI) and some dependent variables such as body mass index (BMI) and ESS.
Differences in the frequency of variables between independent groups were tested by the Fisher’s Exact test or χ2 test as appropriate. A probability value of p < .05 was considered significant.
Statistical procedures were performed using the Statistix 9 Analytical Software (URL https://www.statistix.com).
Results
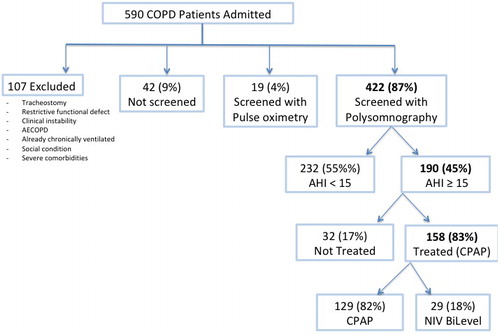
shows the flow chart of the study. Between January 2007 and December 2013, 590 patients with COPD were referred for the first time to a PRP. Out of these patients, 422 were included and underwent a full PSG. In all, 190 (45%) of these patients showed an AHI ≥15 events/hour and underwent OSA treatment.
Figure 1. Flow chart of the study. COPD: chronic obstructive pulmonary disease; AECOPD: acute exacerbations of chronic obstructive pulmonary disease; AHI: apnea-hypopnea index; CPAP: continuous positive airway pressure, NIV: noninvasive ventilation.
shows the demographic, anthropometric, physiological, and clinical characteristics of the patients. Patients with OSA were significantly younger and had a less severe airway obstruction as compared to patients with COPD without OSA. There were no significant differences in cardiac comorbidities nor in arterial blood gases. “Frequent exacerbators” were significantly less represented in the OSA group as compared to the non-OSA group.
Table 1. Demographic, anthropometric, physiological, clinical characteristics of patients.
As expected, patients with OSA had a significant higher BMI, even if 41% of them showed a BMI <30 and 25% even ≤25, whereas 117 (75%) non-OSA patients showed a BMI ≤25. Furthermore, patients with OSA showed significantly more severe diurnal symptoms, as assessed by the ESS. However, only 69 out of 190 patients with OSA (36.3%) showed an ESS >10, which is commonly considered as the OSA threshold (Citation17).
In the OSA group, mean nocturnal SpO2 was lower, whereas the oxygen desaturation index (ODI) and T90 were significantly higher than in the non-OSA group. However, 21 out of 147 (14%) patients with OSA had an ODI <15 events/h, whereas 12% of patients without OSA showed an ODI> 15 events/h.
Multivariate logistic regression analysis showed that BMI and ESS predicted the probability of concomitant OSA (OR: 1.12, p = .05 and OR: 1.06, p = .003, respectively). Linear regression analysis showed a very low although significant correlation between AHI and BMI and ESS (R2=0.2, p = .001 and R2 =0.1, p = .001, respectively).
In all, 158 out of 190 patients (83%) underwent CPAP treatment, which was prescribed at discharge to 129 of them (82%). Following the initial attempt with CPAP, 29 out of 158 patients (18%) were shifted to and discharged with NIV. In all, 32 out of 190 (17%) patients with OSA were discharged without any treatment due to refusal or intolerance.
In all, 21 out of the 129 (17%) patients discharged with CPAP and all of the patients treated with NIV were hypercapnic at baseline (p = .0001). shows the other baseline characteristics of patients discharged with CPAP or NIV. Patients discharged with NIV showed higher mean PaCO2 levels and lower mean forced expiratory volume in 1 second (FEV1). Furthermore, they showed lower AHI and ODI, whereas there was no difference in the other PSG parameters.
Table 2. Baseline characteristics of patients discharged with CPAP or NIV.
Discussion
In our study, the prevalence of the combination of OSA and COPD in patients hospitalized for a PRP was 45%. The prevalence of OSA in the literature is extremely variable due to several factors, including the AHI threshold used as diagnostic criterion, the characteristics of the population, the severity of airway obstruction, prevalent phenotype, the presence of exacerbation at assessment, and concomitant comorbidities. Chaouat et al. reported an OSA prevalence of 11%, using an AHI threshold of ≥20 events/h in a subgroup of patients with severe airway obstruction (Citation18). De Miguel et al. found a 28% prevalence, with an AHI diagnostic threshold of ≥10 events/h in unselected patients with COPD and moderate airway obstruction (Citation19). On the other side, Turcani et al. (Citation20) reported a prevalence similar to ours (51%), but their population was made up of patients hospitalized for AECOPD, which are per se among the contributing factors of such a high prevalence, as previously demonstrated by Marin et al. (Citation21) There is evidence that the prevalence of OSA increases with advancing age (2Citation4), our results may be affected by the aging effect.
Such a broad variability in prevalence among patients with COPD with different severity classes may be related to the effect of lung mechanics on the collapsibility of the upper airways. The lower prevalence of OSA in more severe patients might be partially explained by the increase in end-expiratory lung volumes, preventing upper airways collapse. As a matter of fact, increased lung volumes and hyperinflation have been shown to increase the traction on the upper airway reducing its collapsibility and thus the probability to have OSA (Citation22–25). As a result, patients diagnosed with COPD, with a more severe level of airway obstruction and consequent increased end-expiratory lung volumes, might be protected against OSA. OSA patients were slightly but significantly younger, which may have at least partially contributed to the milder airway obstruction. Also Sanders et al. found that a decrease in OSA prevalence paralleled the decrease in FEV1 (Citation26).
As expected, patients with OSA showed a higher mean BMI, which was an independent predictor of concomitant OSA. However, a high proportion of patients with OSA (41.3%) showed a BMI <30 with 15% even <25. As a consequence, the absolute BMI value alone cannot be used to rule out the presence of OSA in patients with COPD, especially if a threshold value of 35 is used. Furthermore, the correlation between BMI and AHI was extremely low, with a significant dispersion of value distribution. This fact is of crucial importance considering that a BMI value of 35 has been previously considered as an exclusion criterion of OSA in patients with COPD in studies aimed at assessing the effectiveness of domiciliary NIV (Citation27).
Our patients with OSA showed a significantly higher ESS score as compared to patients without and this variable was an independent predictor of OSA in patients with COPD. Nevertheless, the vast majority of patients with an AHI ≥15 events/h (78%) showed an ESS <10/24, which is the commonly considered threshold value above which the probability of OSA is considered significantly higher (Citation17). Consequently, also this variable with the usual cutoff value should not be considered a reliable indicator of OSA, at least in our patients. This finding is consistent with previous data by Faria et al. (Citation28), who found that an ESS cutoff value of 10, was poor at both predicting and ruling out OSA. Also Turcani et al. (Citation20) found a significant correlation between increased AHI, increased BMI and ESS. However, they did not assess whether specific ESS threshold values were able to rule out OSA.
In our study, awake arterial blood gases did not differ between groups. Therefore, this measurement cannot be used to predict OSA in these patients. No variable associated with OSA such as SpO2, ODI, and T90 was correlated with the risk of concomitant OSA in our patients. These findings, in patients with overlap syndrome compared to only patients with COPD, are consistent with those of Lacedonia et al. (Citation29) and Marin et al. (Citation21). Finding a significantly higher ODI in patients with concomitant OSA may lead to the conclusion that night pulsossimetry might be enough to screen for obstructive events. Actually, our data showed that this was not the case. In fact, 21 of the 190 (11%) patients with OSA showed a non-diagnostic value of ODI (<15 events/h), underestimating the real prevalence of OSA. This issue is scarce and contradictory in the literature. Turcani et al. (Citation20) found out that ODI and AHI were superimposable in identifying OSA. In contrast, results reported by Scott et al. (Citation30) were more in line with ours. In fact, they showed that a value of ODI>15 events/hour was neither sensitive nor specific enough to accurately screen for OSA in patients with COPD. The concomitant use of oxygen therapy in a subgroup of COPD patients may explain the non-diagnostic value of ODI in our study.
Sixty-eight percent of all patients with OSA were discharged with CPAP, whereas only 15% were treated with NIV and 17% refused or were intolerant to any ventilatory treatment. In fact, patients treated with NIV were significantly more hypercapnic than patients treated with CPAP. Hypercapnia may have played a role in influencing patients’ tolerance to CPAP. Furthermore, patients treated with NIV suffered from more severe airway obstruction and less severe obstructive and desaturative nocturnal events.
Our therapeutic procedure entails initiating CPAP even in hypercapnic patients with OSA, and shifting to NIV only in case of failure such as persistent diurnal hypercapnia, signs of nocturnal hypoventilation or poor tolerance (3Citation4). Only 18% of patients who underwent the CPAP trial required NIV, although at least a third of all the patients with OSA were hypercapnic, confirming previous reports questioning whether hypercapnia necessarily requires NIV in these patients (Citation31).
We evaluated a selected group of patients with COPD admitted to a PRP without a phenotype characterization. Therefore, our results cannot be generalized to all patients diagnosed with COPD.
Conclusion
Our data show that at least in the population studied, the combination of OSA and COPD, the so-called “overlap syndrome,” is frequent. BMI and ESS were independent predictive factors of OSA. However, a fairly noteworthy percentage of patients with OSA showed a BMI <30 and an ESS <10, indicating that values commonly considered as cutoffs of OSA prevalence in the general population may not be accurate enough in patients with COPD. Finally, our results may question studies of long-term NIV in COPD, excluding patients with OSAS only on the basis of reported symptoms or BMI.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of Chronic Obstructive Lung Disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP.
- Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, Rutten EP, Op 't Roodt J, Wouters EF, Franssen FM. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–35. doi:10.1164/rccm.201209-1665OC.
- Mantoani LC, Dell'Era S, MacNee W, Rabinovich RA. Physical activity in patients with COPD: the impact of comorbidities. Expert Rev Respir Med. 2017;11(90):685–98. doi:10.1080/17476348.2017.1354699.
- Shawon MS, Perret JL, Senaratna CV, Lodge C, Hamilton GS, Dharmage SC. Current evidence on prevalence and clinical outcome of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev. 2017;32:58–68. doi:10.1016/j.smrv.2016.02.007.
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi:10.1164/rccm.201309-1634ST.
- Griffo R, Spanevello A, Temporelli PL, Faggiano P, Carone M, Magni G, Ambrosino N, Tavazzi L. Frequent coexistence of chronic heart failure and chronic obstructive pulmonary disease in respiratory and cardiac outpatients: Evidence from SUSPIRIUM, a multicentre Italian survey. Eur J Prev Cardiol. 2017;24:567–76. doi:10.1177/2047487316687425.
- Ambrosino N, Venturelli E, de Blasio F, Paggiaro P, Pasqua F, Vitacca M, Vagheggini G, Clini EM. A prospective multicentric study of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease and different clinical phenotypes. Respiration. 2015;89:141–147. doi:10.1159/000371471.
- Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12:CD005305. doi:10.1002/14651858.CD005305.pub4.
- Fanfulla F, Grassi M, Taurino AE, D'Artavilla Lupo N, Trentin R. The relationship of daytime hypoxemia and nocturnal hypoxia in obstructive sleep apnea syndrome. Sleep. 2008;31:249–255.
- Vitacca M, Nava S, Confalonieri M, Bianchi L, Porta R, Clini E, Ambrosino N. The appropriate setting of noninvasive pressure support ventilation in stable COPD patients. Chest. 2000;118:1286–1293.
- Wang Y, Hu K, Liu K, Li Z, Yang J, Dong Y, Nie M, Chen J, Ruan Y, Kang J. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16:1123–1130.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545.
- Pellegrino R, Viegi G, Brusasco V, et al. Series ‘‘ATS/ERS Task Force: standardisation of lung function testing”. Interpretative strategies for lung function test. Eur Respir J. 2005;26(5):948–968.
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- Iber C, Ancoli-Istrael S, Chesson AL, Quan SF. The AASM Manual for the scoring of sleep associated events: rules, terminology and technical specifications, 1st ed. Wetchester (IL): American Academy of Sleep Medicine; 2007.
- Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, Parthasarathy S, Quan SF, Rowley JA; Positive Airway Pressure Titration Task Force; American Academy of Sleep Medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–171.
- Kitajima T, Marumo T, Shima H, Shirata M, Kawashima S, Inoue D, Katayama Y, Itotani R, Sakuramoto M, Fukui M. Clinical impact of episodic nocturnal hypercapnia and its treatment with noninvasive positive pressure ventilation in patients with stable advanced COPD. Int J COPD. 2018;13:843–853. doi:10.2147/COPD.S153200.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235.
- Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. doi:10.1164/ajrccm.151.1.7812577.
- de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath. 2002;6(1):3–10. doi:10.1007/s11325-002-0003-6.
- Turcani P, Skrickova J, Pavlik T, Janousova E, Orban M. The prevalence of obstructive sleep apnea in patients hospitalized for COPD exacerbation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(3):422–428. doi:10.5507/bp.2014.002.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi:10.1164/rccm.200912-1869OC.
- Appelberg J, Nordahl G, Janson C. Lung volume and its correlation to nocturnal apnoea and desaturation. Respir Med. 2000;94:233–239. doi:10.1053/rmed.1999.0730.
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148.
- Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–2131. doi:10.1152/jappl.1988.65.5.2124.
- Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30(2):179–186.
- Biselli P, Grossman PR, Kirkness JP, Patil SP, Smith PL, Schwartz AR, Schneider H. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J Appl Physiol. (1985). 2015;119(3):266–271. doi:10.1152/japplphysiol.00455.2014.
- Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, O'Connor GT, Punjabi NM, Shahar E; Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. doi:10.1164/rccm.2203046.
- Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. doi:10.1016/S2213-2600(14)70153-5.
- Faria AC, da Costa CH, Rufino R. Sleep Apnea Clinical Score, Berlin Questionnaire, or Epworth Sleepiness Scale: which is the best obstructive sleep apnea predictor in patients with COPD? Int J Gen Med. 2015;8:275–281. doi:10.2147/IJGM.S86479.
- Lacedonia D, Carpagnano GE, Aliani M, Sabato R, Foschino Barbaro MP, Spanevello A, Carone M, Fanfulla F. Daytime PaO2 in OSAS, COPD and the combination of the two (overlap syndrome). Respir Med. 2013;107(2):310–316. doi:10.1016/j.rmed.2012.10.012.
- Scott AS, Baltzan MA, Wolkove N. Examination of pulse oximetry tracings to detect obstructive sleep apnea in patients with advanced chronic obstructive pulmonary disease. Can Respir J. 2014;21(3):171–175.
- Toraldo DM, De Nuccio F, Nicolardi G. Fixed-pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): a 24-month follow-up study. Sleep Breath. 2010;14(2):115–123. doi:10.1007/s11325-009-0291-1.
- Ozsancak A, D’Ambrosio C, Hill NS. Nocturnal noninvasive ventilation. Chest. 2008;133:1275–1286. doi:10.1378/chest.07-1527.
