Abstract
Pulmonary rehabilitation has been established as the standard of care for patients with symptomatic chronic obstructive pulmonary disease (COPD). Benefits include improvements in exercise tolerance, dyspnoea and quality of life; magnitude of benefit is generally greater than for any other COPD therapy. A wide range of professional organizations and standards documents have recommended pulmonary rehabilitation; benefits accrue across the spectrum of disease severity.
However, pulmonary rehabilitation is provided to only a tiny fraction of those chronic obstructive pulmonary disease (COPD) patients who would benefit. International estimates posit that only 1–2% of COPD patients receive pulmonary rehabilitation. In contrast, other COPD therapies, bronchodilators and oxygen therapy in particular, are much more widely available. The costs of pulmonary rehabilitation should not be a major barrier, as costs are comparable to other therapies.
In seeking strategies to increase pulmonary rehabilitation availability, it can be argued that a demonstration of a life prolongation benefit would be of great help. Therapies that improve survival have a high priority for patients, for their health care providers and for payers. A well-designed survival study has never been performed. Although efforts are underway to organize such a trial, even in a best-case scenario it will be a number of years before the results are available.
Keywords:
Pulmonary rehabilitation is no longer a young therapy. It can be said to have been conceptualized by Alvan Barach in the 1950s (Citation1) and reduced to practice by Thomas Petty in the late 1960s (Citation2). This paper will review the success of pulmonary rehabilitation: in establishing a firm scientific basis and undeniable evidence of effectiveness. It will also detail its failure: its poor availability for patients who would benefit. This is clearly true for patients with COPD, and likely even more so for patients with other chronic pulmonary conditions. This paper will finish by suggesting a way forward that might, in time, result in better availability.
In the years following organization of the first multi-disciplinary team to deliver pulmonary rehabilitation by Tom Petty, programmes proliferated in the United States and elsewhere. However, questions persisted regarding the benefits to be expected: were they strictly psychological (Citation3)? In the 1990s, exercise scientists established the physiological basis of the exercise programmes for COPD that are the core of pulmonary rehabilitation (Citation4). They introduced concepts such as limb muscle dysfunction and dynamic hyperinflation that elucidate why exercise tolerance is so poor. Limb muscle dysfunction explained why exercise levels that were achievable in pulmonary rehabilitation were capable of improving muscle function in the COPD patient although they would not be a training stimulus in healthy subjects (Citation5). The concept of dynamic hyperinflation explains why any intervention that either decreases breathing rate or speeds expiratory airflow will result in improved exercise tolerance (Citation6, Citation7). A key demonstration was that improving limb muscle function through exercise training reduces ventilatory requirements at a given level of exercise (Citation8). This allows a slower breathing rate and, thereby, less dynamic hyperinflation (Citation6). The result is less dyspnoea. Thus, a therapy that does not improve lung function can improve exercise tolerance even in the patient whose exercise tolerance is limited by the lungs. Furthermore, it has been demonstrated that a substantial fraction of COPD patients (especially those with mild-to-moderate disease) are limited by leg fatigue and not primarily by dyspnoea (Citation9, Citation10); for these patients improving leg muscle function has a direct effect on exercise tolerance. In fact, pulmonary rehabilitation reduces exertional dyspnoea and increases exercise tolerance to a greater extent than any other COPD therapy (Citation11). This is important because reductions in exercise tolerance lead to inability to perform activities of daily living; this inability is often the chief complaint of the COPD patient. These physiological concepts have allowed design of adjuncts to exercise programmes that have made pulmonary rehabilitation even more effective (Citation12). For example, published studies have demonstrated that inhaling supplemental oxygen (Citation13), employing noninvasive ventilation (Citation14) and instituting good bronchodilator therapy (Citation15) can enhance the benefits of exercise programmes.
Pulmonary rehabilitation has been promoted as standard of care by every relevant professional organization in authoritative documents (e.g. (Citation16–18)). The 2018 Global Initiative for Chronic Obstructive Lung Disease (GOLD) document (Citation19) concludes
The benefits to COPD patients from pulmonary rehabilitation are considerable and rehabilitation has been shown to be the most effective therapeutic strategy to improve shortness of breath, health status and exercise tolerance. Pulmonary rehabilitation is appropriate for most patients with COPD; improved functional exercise capacity and health related quality of life have been demonstrated across all grades of COPD severity.
Against this background, it is difficult to discern why pulmonary rehabilitation is not generally available and universally recommended for symptomatic COPD patients. But that availability is poor is undeniable. A 2013 review of the international literature found seven relevant studies published between 1995 and 2013, capturing 791 programmes across seven countries (Citation20). This review concludes ‘the annual national capacity for pulmonary rehabilitation…consistently translated to ≤1.2% of the estimated COPD population’. In the United States, a Medicare database examination was conducted by Nishi et al. in 2016 (Citation21). Medicare claims data were reviewed based on a 5% sampling of beneficiaries. The review spans the period before and after pulmonary rehabilitation becoming a Medicare benefit (in 2010). It was discerned that pulmonary rehabilitation participation rate increased from 2.6% in 2003 to 3.7% in 2012. It may further be argued that this low rate is likely an overestimate of participation: many COPD patients lack Medicare and those without Medicare are even less likely receive pulmonary rehabilitation.
This lack of availability has not gone unnoticed. A 2015 American Thoracic Society/European Respiratory Society (ATS/ERS) Policy Statement (Citation22) dealt with strategies to enhance the implementation of pulmonary rehabilitation. It concludes that ‘the ATS and ERS commit to undertake actions that will improve access to and delivery of PR services for suitable patients. They call on their members and other health professional societies, payers, patients, and patient advocacy groups to join in this commitment’. But this call seems to have gone largely unanswered.
In seeking to discern ways in which availability of pulmonary rehabilitation might be increased, it is useful to make comparisons with other COPD therapeutic modalities. The two that come to mind are inhaled bronchodilators and long-term oxygen therapy. In terms of availability, the contrasts with pulmonary rehabilitation are profound. Bronchodilators are widely…almost universally…used in COPD patients in first-world nations. Similarly, long-term oxygen therapy is widely available to patients who demonstrate severe resting hypoxemia.
Can this difference in availability be ascribed to rehabilitation’s more modest clinical benefits? Much the opposite, actually. presents the semi-quantitative analysis, comparing the benefits of pulmonary rehabilitation to those of a long-acting bronchodilator (either a long-acting beta-agonist (LABA) or an anti-cholinergic long-acting muscarinic antagonist (LAMA)). Very few head-to-head studies have been performed (exposing the same subject to the two interventions), so studies summarizing the responses in different subjects, but with the same outcome measure were collected. Panel A compares the responses to constant work rate cycle ergometer exercise tests, a measure of endurance exercise tolerance. Responses were taken from the ERS Statement on the use of exercise testing in the evaluation of interventional efficiency (Citation23), with average responses being calculated by weighing the individual study’s responses according to the number of subjects studied in the given study. The responses to bronchodilator represent 10 LABA and 9 LAMA studies with a total of 1847 subject’s responses; rehabilitation represents 11 studies with 559 subjects. The horizontal line represents the postulated minimum clinically important difference (MCID) of 105 seconds (Citation23, Citation24). It is apparent that bronchodilator administration results, on average, in an increase in exercise tolerance that approximates the MCID while rehabilitation yields a response roughly three times larger.
Figure 1. Semi-quantitative comparison of the benefits of pulmonary rehabilitation with inhaled long-acting bronchodilator administration (beta agonist or anticholinergic). (a) exercise tolerance improvement assessed by increase in exercise time in constant work rate cycle ergometer testing. (c) health-related quality of life improvement assessed by decrease in SGRQ total score. (b) dyspnoea reduction assessed by increase in Transitional Dyspnoea Index. The horizontal line in each panel represents the postulated MCID. See text for details of analysis.
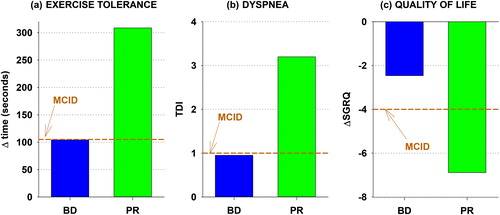
presents, in similar format, the health-related quality of life benefits, as assessed by the St. George’s Respiratory Questionnaire (SGRQ), for the two interventions. The bronchodilator responses are gleaned from a 2014 Cochrane Library network meta-analysis (Citation25) and the rehabilitation responses from a 2015 Cochrane Library review (Citation26). The bronchodilator response represents six LABA and seven LAMA studies including approximately 13,000 subject responses; the rehabilitation response represents 19 studies including 1146 subjects. Decreases in SGRQ indicate benefit: on average, a long-acting bronchodilators fail to confer a clinically important quality of life benefit (a decrease in SGRQ score of 4); rehabilitation yields a benefit well exceeding the MCID.
presents the dyspnoea benefits, assessed by Mahler’s Transitional Dyspnoea Index. Increases indicate reduced dyspnoea; 1 unit is the MCID. The responses are from a review composed by Mahler et al. (Citation27) comprised of four LABA and three LAMA studies with 4163 subjects and seven rehabilitation studies with 476 subjects. The average responses show that bronchodilators achieve the MCID (1 unit), but rehabilitation exceeds it threefold.
So, clearly, the lack of uptake of pulmonary rehabilitation cannot be related to its lack of efficacy; it is a very efficacious therapy. Can it be cost? While costs are difficult to compare head-to-head, in the United States the ‘list price’ of a full rehabilitation programme, a year of a single long-acting inhaled bronchodilator and a year of supplemental oxygen are all in the range of a few thousand dollars. In fact, a British analysis rated pulmonary rehabilitation to be more cost-effective (lower cost per quality-adjusted life-year) than bronchodilator therapy (Citation28). This is not to say that the cost to the consumer is necessarily similar. In the United States, third-party payer coverage for both bronchodilator and oxygen therapy is generally good; for rehabilitation it is poor. But third-party payer coverage cannot be taken as a reflection of the value of a therapy.
Given its effectiveness and reasonable costs, it is worth trying to understand why the availability of rehabilitation is so poor. Specifically, what do bronchodilators and oxygen therapy have that rehabilitation lacks? For bronchodilators, the answer is rather straightforward: bronchodilators have marketing: a sales force to promote their products to prescribers, the ability to advertise to consumers and a team of professionals to lobby regulators and legislators. Moreover, an extensive programme of clinical research studies is targeted at defining the product’s benefits. These tools are brought to bear to promote availability, consumer demand, prescriber acceptance and third-party coverage.
For oxygen therapy, the reason for its high availability is more subtle. Oxygen suppliers and device manufacturers do not have a sales force, advertising presence or lobbying arm comparable to that of the pharmaceutical industry. Their footprint in clinical research exploring the benefits of long-term oxygen therapy is very small. What oxygen therapy has, is the results of two studies, performed almost 40 years ago, that included just over 300 subjects between them. These studies, the NOTT (Citation29) and MRC (Citation30) trials, demonstrated that, in COPD patients with severe resting hypoxemia, long-term oxygen therapy prolongs life.
Therapies that improve survival have a special place in the pantheon of medical treatments. It is difficult to deny patients a treatment, almost irrespective of cost, if it prolongs life. One need look no further that the US television advertisements touting ‘a chance to live longer’ in support of cancer chemotherapy agents that prolong life, on average, only a few months. Therapies that prolong life have a high priority…for patients, their physicians and health care systems.
It seems unlikely that pulmonary rehabilitation will ever have a major league marketing operation at its disposal. But what of a survival benefit? It seems quite plausible that an intervention that improves fitness and often increases physical activity might enhance survival, but this possibility has never been adequately tested. Troosters, et al., in their 2005 State of the Art Review of pulmonary rehabilitation (Citation31), outlined the difficulty in performing a mortality trial:
So far no study has convincingly shown evidence of improved survival after pulmonary rehabilitation…Because patients who enroll in pulmonary rehabilitation are generally in a relatively stable state, their likelihood of dying in the short term is rather low. Hence the absolute reduction in mortality is likely to be relatively modest. Studies investigating patients with higher mortality risk (e.g., after discharge from the hospital for an acute exacerbation) may be more successful in finding effects on survival.
In other words, the sample size of an investigation trying to determine mortality benefits in stable COPD patients undergoing rehabilitation would be very large (many thousands) unless the survival advantage was huge. Whether rehabilitation after hospitalization yields mortality benefits has yet to be tested. A 2016 Cochrane review collected relevant data from eight studies (with a total of 347 rehabilitation and 323 control participants), none of which was powered for a mortality outcome (Citation32). The analysis revealed a trend for a mortality benefit that was nowhere near statistically significant.
It can be hypothesized that a convincing demonstration that pulmonary rehabilitation improves survival would yield a reformulation of health policy, resulting in improved access and uptake of this therapy. Such a study would necessarily be a multi-centre effort, randomizing well more than a thousand post-hospitalization patients to either a rehabilitation or a control group and featuring a follow-up period of a year or greater. Efforts are underway to organize such a trial but, even in a best-case scenario, it will be a number of years before the results are available.
In conclusion, for pulmonary rehabilitation, the next few years are likely to be crucial. Will it wither or will it prosper?
Declaration of interest
Dr. Casaburi reports personal fees from Boehringer-Ingelheim Pharmaceuticals, Astra Zeneca Pharmaceuticals, GlaxoSmithKline Pharmaceuticals and Medimmune, and has equity interest in Inogen, outside the submitted work.
Acknowledgment
R. Casaburi holds the Grancell/Burns Chair in the Rehabilitative Sciences.
References
- Barach AL, Bickerman HA, Beck G. Advances in the treatment of non-tuberculous pulmonary disease. Bull N Y Acad Med. 1952;28(6):353–84.
- Petty TL, Nett LM, Finigan MM, Brink GA, Corsello PR. A comprehensive care program for chronic airway obstruction. Methods and preliminary evaluation of symptomatic and functional improvement. Ann Intern Med. 1969;70(6):1109–20.
- Belman MJ. Exercise in chronic obstructive pulmonary disease. Clin Chest Med. 1986;7(4):585–97.
- Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159(4 Pt 2):S1–S40.
- Casaburi R. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001;33(7 Suppl):S662–S70.
- Porszasz J, Emtner M, Goto S, Somfay A, Whipp BJ, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest. 2005;128(4):2025–34.
- Casaburi R, Porszasz J. Reduction of hyperinflation by pharmacologic and other interventions. Proc Am Thorac Soc. 2006;3(2):185–9.
- Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143(1):9–18.
- Saey D, Debigare R, LeBlanc P, Mador MJ, Cote CH, Jobin J, Maltais F. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(4):425–30.
- Casaburi R, Maltais F, Porszasz J, Albers F, Deng Q, Iqbal A, Paden HA, O'Donnell DE; 205.440 Investigators. Effects of tiotropium on hyperinflation and treadmill exercise tolerance in mild to moderate chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(9):1351–61.
- Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(13):1329–35.
- Wijkstra PJ, Wempe JB. New tools in pulmonary rehabilitation. Eur Respir J. 2011;38(6):1468–74.
- Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med. 2003;168(9):1034–42.
- Ambrosino N, Palmiero G, Strambi SK. New approaches in pulmonary rehabilitation. Clin Chest Med. 2007;28(3):629–38, vii.
- Casaburi R, Kukafka D, Cooper CB, Witek TJ Jr., Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127(3):809–17.
- Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, Greening NJ, Heslop K, Hull JH, Man WD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax. 2013;68 Suppl 2:ii1–30.
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(5 Suppl):4S–42S.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018 [accessed 2018 Jul 10]. http://goldcopd.org.
- Desveaux L, Janaudis-Ferreira T, Goldstein R, Brooks D. An international comparison of pulmonary rehabilitation: a systematic review. COPD. 2015;12(2):144–53.
- Nishi SP, Zhang W, Kuo YF, Sharma G. Pulmonary rehabilitation utilization in older adults with chronic obstructive pulmonary disease, 2003 to 2012. J Cardiopulm Rehabil Prev. 2016;36(5):375–82.
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, Spruit MA, Masefield S, Casaburi R, Clini EM, et al. An official American thoracic society/European respiratory society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–86.
- Puente-Maestu L, Palange P, Casaburi R, Laveneziana P, Maltais F, Neder JA, O'Donnell DE, Onorati P, Porszasz J, Rabinovich R. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47(2):429–60.
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–5.
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Mahler DA, Witek TJ, Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103.
- Zoumot Z, Jordan S, Hopkinson NS. Emphysema: time to say farewell to therapeutic nihilism. Thorax. 2014;69(11):973–5.
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391–8.
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681–6.
- Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(1):19–38.
- Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;(12):CD005305.
