Abstract
Individuals with Chronic Obstructive Pulmonary Disease (COPD) experience sleep disturbances due to the impact of respiratory symptoms on sleep quality. We explored whether sleep disturbances in COPD are linked to heterogeneity of airway constriction.
The impact of breathing problems on sleep quality was measured in consecutive COPD outpatients with the COPD and Asthma Sleep Impact Scale (CASIS) questionnaire. Impulse oscillometry technique (IOS) was employed to assess heterogeneity of airway constriction. Subjects with a previous or concomitant diagnosis of asthma or obstructive sleep apnea (OSA) were excluded.
Fifty COPD subjects (M/F 40/10; age: 71 ± 8 yrs, Body Mass Index (BMI): 26.2 ± 4.7 kg/m2, Forced Expiratory Volume in the first second (FEV1): 65 ± 25% predicted; mean ± SD) were enrolled. The mean CASIS score was 36 ± 3.3, and the R5–R20 value was 0.2 ± 0.15 kPa s L−1. The CASIS score was significantly higher in subjects with increased R5–R20 (>0.07 kPa s L−1) (39 ± 24; p = 0.02) compared to normal R5–R20 (21 ± 17). When subjects were categorized on the basis of lung function in severely versus non severely obstructed (FEV1 ≤ or >50% predicted) or air trappers versus non air trappers (Residual Volume, RV ≥ or <120% predicted) the CASIS score remained unchanged (for FEV1: 37 ± 23 versus 33 ± 25, respectively, p = 0.61; for RV: 30 ± 20 versus 40 ± 23, p = 0.16).
Sleep disturbances due to COPD symptoms are associated with heterogeneity of airway constriction, possibly reflecting peripheral airway dysfunction.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease and among the leading causes of morbidity and mortality worldwide. The cardinal functional sign of COPD is chronic airway obstruction, which is permanent and progressive (Citation1). Despite the fixed nature of airflow limitation, the most common respiratory symptoms of COPD are of variable intensity and frequency, occurring during all parts of the 24-h day (Citation2). Kessler and colleagues found that more than half of COPD patients have daily and/or weekly variations of one or more symptoms (Citation3); typically, symptoms seem to be worst in the morning, particularly in patients with severe COPD (Citation4).
Efforts have been traditionally made to the relieving of respiratory symptoms occurring during the diurnal hours, since they severely impact on occupational and recreational activities, and are associated with worse health status and higher risk of COPD exacerbations (Citation5); on the other hand, little attention has been paid to COPD symptoms that occur during the night. Indeed, nocturnal respiratory symptoms, such as wheezing or chest tightness, as well as disturbed sleep quality have been recorded in the vast majority of patients with COPD (Citation6). Patients with nighttime symptoms appear to have significantly worse health status and higher healthcare resource utilization (Citation7,Citation8). A recent study performed in a Spanish cohort of COPD patients confirmed that almost two-third of subjects suffer from nocturnal symptoms (Citation5).
The mechanisms responsible for the variability in respiratory symptoms are largely unexplored. The ASSESS study showed that the prevalence of nighttime symptoms was comparable across all degrees of bronchial obstruction (Citation2). It has been hypothesized that the occurrence of air trapping in supine position could play an important pathogenetic role (Citation9). Indeed, the supine position during nighttime could lead to expiratory flow limitation (EFL). In COPD, EFL occurs because of reduction in lung elastic recoil or uncoupling of airway-to-parenchyma interdependence. In this context, the peripheral airways are more prone to collapse during expiration thus favoring EFL and, as a consequence, dyspnea. Other mechanisms, such as the decreased mucociliary clearance or increased vagal tone during the nocturnal hours could explain the circadian variability of respiratory symptoms. On this basis, we speculated that, in COPD individuals, a relationship between nocturnal disturbances and features of heterogeneity of airway constriction exists. The current study aimed to test this hypothesis.
Materials and methods
Subjects and study design
Consecutive subjects with an ascertained diagnosis of COPD according to GOLD (Global Initiative for Chronic Obstructive Lung Disease) recommendations (Citation1) attending the Outpatient Clinics of the University of Palermo, Italy were recruited from September 2016 to March 2017. All individuals aged >40 years, with spirometric impairment from mild to very severe airway obstruction and symptomatic despite optimal pharmacological treatment, in accordance with the international recommendations (Citation1). Patients had to be in stable conditions and able to fill the questionnaires. If an exacerbation occurred, the study evaluation was postponed for 4 weeks. Subjects were excluded if the risk of OSA, or restless leg syndrome (RLS) narcolepsy and other common sleep disordered breathing (SDB) were documented. In particular, we excluded the risk of OSA based on the absence of suggestive symptoms (lack of snoring, referred apneas, nicturia, daytime sleepiness), screening questionnaires (ESS questionnaire), and negative nocturnal oxyhemoglobin saturation monitoring. RLS was excluded on the basis of negative medical history (absence of nocturnal irresistible urge to move the legs either by itself or in response to uncomfortable paraesthesia of the legs). Narcolepsy was excluded because of the absence of excessive daytime sleepiness, cataplexy, sleep disruption, sleep paralysis, and hypnagogic hallucinations. Subjects suffering from severe comorbid conditions, such as active cancer, severe cardiac, renal, liver or neurological diseases that could influence the outcomes of the study, were also excluded. The study was approved by the Ethic Committee of the “Cervello Hospital-University of Palermo,” Italy (Protocol number 144), and written consent was obtained prior to the beginning of the study.
Methods
Each subject underwent clinical and functional assessments in a single visit, which took place between 8:00 and 9:00 am. The clinical assessment was based on the recording of respiratory symptoms and the administration of specific questionnaires. The CASIS questionnaire is a self-administered, 7-item scale evaluating sleep impairment associated with COPD and asthma. The questionnaire has been validated by Pokrzywinski et al. (Citation10) showing an internal consistency of 0.9 for the COPD population and a test–retest reliability of 0.84. Five items relate to disturbance falling asleep or staying awake during the day, whereas the remaining two items specifically address sleep quality. The response options range from 0 = never to 4 = very often, and the last two items are reverse scored. At the end, all items are summed together in order to obtain a total raw score. This is linearly transformed to a 0–100 total scale score. Higher scores suggest greater sleep deterioration in the previous week. The final score represented the main outcome of the current study.
The lack of history of sleep apnea episodes and the absence of signs and symptoms of sleep disorders suggestive of OSA, such as loud snoring, witnessed sleep apnea, previous diagnosis of arterial hypertension, and diurnal sleepiness allow us to exclude moderate or severe, symptomatic sleep apnea. This was also supported by the administration of the ESS questionnaire, which is composed by eight items assessing subjective daytime sleepiness (Citation11,Citation12) and with a cut-off score equal to 10 to rule out the risk of OSA.
Functional assessment included measurements of lung volumes and nocturnal oxyhemoglobin saturation monitoring. Assessment of heterogeneity of airway constriction was performed by IOS technique. Lung function was evaluated by spirometry (Biomedin, Padua, Italy) and body plethysmography (SensorMedics, Yorba Linda, CA, USA) according to current guidelines (Citation13,Citation14) in order to estimate the level of lung hyperinflation and air trapping. Nocturnal oxyhemoglobin saturation monitoring was carried out in all recruited subjects the night before the study with the use of the Nonin 2500 Palmsat. The cut-off of Oxygen Desaturation Index (ODI) to be reasonably confident to exclude a moderate-severe OSA was established as less than 15 oxygen desaturations per hour (oxygen desaturation was defined as a fall in oxygen saturation of 4% for at least 8 seconds) (Citation15).
IOS measurements were performed to investigate the occurrence of airway abnormalities (Jaeger Masterscreen, CareFusion Technologies, San Diego, CA, USA). IOS is a modification of forced oscillation technique (FOT) that uses different sound waves to calculate: R5 (total resistance), R20 (central resistance), as well as X5 (reactance), AX (area of reactance), and RF (resonant frequency). This technique differs from FOT because of different waveforms and data processing. In this respect, values obtained with both procedures are similar but not equal; in particular, Hellinckx and colleagues demonstrated that resistance at different frequencies is smaller with FOT than with IOS, becoming higher with decreasing frequency (Citation16). IOS is used to derive values for heterogeneity of airway obstruction as expressed by the R5–R20, (Citation17) although some studies assume that R5–R20 values are also related to peripheral airway impairment (Citation18–23). Subjects supported their cheeks to avoid upper airway shunting, while impulses were applied for 30 seconds during tidal breathing, and measurements were obtained in triplicate to reduce intratest variability. Quality and accuracy was assured by operators and artifacts due to swallows, phonation, cough, and significant leaks were avoided.
Data analysis
Statistical analysis was performed using Statview 5.0 (SAS Institute Inc., Cary, NC, USA). Unpaired t-tests were used to compare the CASIS scores between the study groups. We constructed simple regression models to evaluate the relationship between the CASIS score and lung function variables. In all analyses, a p value <0.05 was used. All data are expressed as mean ± SD.
Results
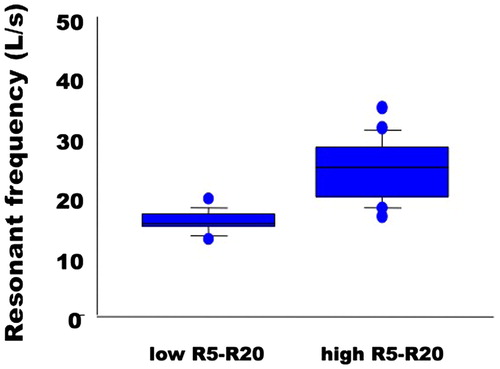
50 COPD subjects (M/F 40/10; Age: 71 ± 8 yrs, BMI: 26.2 ± 4.7 kg/m2) were enrolled. All of them were current or former smokers with a history of cigarette smoking of at least 10 pk-yrs. According to GOLD classification (Citation1) 10 subjects belonged to stage A, 19 to stage B, 3 to stage C, and 18 to stage D. At study entry, patients were receiving the following inhaled medications: LAMA, 10 subjects, LABA, 2 subjects, LABA/LAMA in fixed combination, 21 subjects; ICS/LABA, 2 subjects, ICS/LABA plus LAMA, 15 subjects. Of note, 8 subjects were under long-term oxygen treatment. Lung function characteristics are presented in , and data are expressed as % of predicted values. The mean CASIS score was 36 ± 3.3. In the entire study group, the R5–R20 value was 0.2 ± 0.15 kPa s L−1. The RF was significantly and positively correlated with R5–R20 values (r = 0.81, p < 0.0001).
Table 1. Functional characteristics of the study sample.
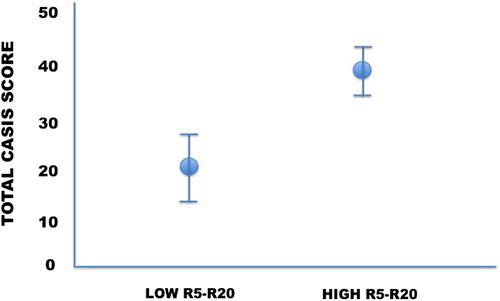
The simple linear regression between the CASIS score and the R5-R20 values was not statistically significant (p = 0.42) However, when we arbitrarily divided the study group according to R5–R20 value ≤0.07 kPa s L−1 (normal heterogeneity of airway constriction, 20% of the whole study group) and >0.07 kPa s L−1 (increased heterogeneity of airway constriction), the CASIS score was significantly higher (p = 0.02) in subjects with high R5–R20 (39 ± 24; p = 0.02) compared to those with low R5–R20 (21 ± 17) (). Interestingly, RF differed significantly between subjects with low and high R5–R20 values (). When subjects were categorized between severely versus non-severely obstructed (FEV1 ≤ or >50% predicted) or between those who had air trapping versus those who did not (RV ≥ or <120% predicted), the CASIS score remained unchanged (for FEV1: 37 ± 23 vs. 33 ± 25, respectively, p = 0.61); for RV: 30 ± 20 vs. 40 ± 23, p = 0.16). The regression analysis performed to explore the relationship between the CASIS score and X5 and AX values yielded trend for significance (p = 0.065 and p = 0.072, respectively); no relationship was found between CASIS score and RF (p = 0.67).
Finally, the CASIS score was not significantly associated with any other functional variable, such as FVC % predicted, FRC % predicted and TLC % predicted (p > 0.05 for all analyses). We further explored whether RV/TLC and FRC/TLC correlated with the CASIS, and we were unable to find significant relationships (for RV/TLC: p = 0.13, r = 0.24; for FRC/TLC: p = 0.09, r = 0.26).
Discussion
The current study aimed at assessing the relationship between nocturnal symptoms and heterogeneity of airway obstruction, exploring a potential mechanism responsible for the occurrence of circadian symptom variability in COPD. Our finding of significantly worse sleep disorders in subjects with higher R5–R20 suggests that heterogeneity of airway constriction is implicated in the circadian variability of respiratory symptoms.
The presence of nocturnal symptoms is a common finding in COPD patients (Citation2,Citation5). Several methods have been suggested to describe the occurrence of sleep disturbances (Citation10); The CASIS was designed to measure sleep impairment associated with chronic obstructive diseases, and provides a disease-specific instrument to measure nocturnal disturbances rather than more generic measures of sleep problems. In the ASSESS study, the CASIS score showed worse sleep quality in subjects experiencing more than one symptom (Citation24). In a recent study, higher CASIS scores were related to worse health-related quality of life (HRQoL) and higher scores in the Hamilton Anxiety and Depression Scale (HADS) (Citation5). Furthermore, Price et al demonstrated that in COPD subjects a poorer quality of sleep is associated with a worse health status, compared with patients without night symptoms (Citation25).
Despite the impact of COPD-related sleep disturbances and nocturnal symptoms on quality of life and health status, the underlying mechanisms are still unclear (Citation25). EFL occurring during the night could play a central role in the development of respiratory symptoms. At first, it is observed in COPD patients only in supine position, facilitating orthopnea, and later also in sitting or in orthostatic positions (Citation9). In COPD subjects, EFL causes air trapping and pulmonary hyperinflation. Several factors can be advocated to explain the occurrence of EFL during the night: increased airway resistance due to chronic inflammation and mucus; major airways collapse due to the destruction of the alveolar septa and consequent reduction of the elastic properties with premature airway closure during the expiratory phase; bronchial vagal hypertone typically occurring during the night (Citation26,Citation27). In supine position, because of changes of the gravitational forces and cephalad displacement of diaphragm, all lung volumes decrease. The reduction of expiratory lung volume when breathing at tidal volumes can favor EFL. The site where the system becomes flow-limited can be central or peripheral airways. Finally, a vagal hypertone during sleep could lead to EFL by increasing airflow resistance (Citation9,Citation28). Taken together, we favor the hypothesis that heterogeneity of airway obstruction occurring during the night is the major determinant of nocturnal symptoms and, therefore, circadian variability.
To explore our hypothesis, we employed measurements of IOS, which allow to assess heterogeneity of airway obstruction, and provide additional information to the traditional spirometric functional assessment (Citation17). Berger et al. demonstrated that abnormalities in distal airway function assessed by oscillometric frequency dependence of resistance and reactance are associated with increased in vivo lung inflammation (Citation29). In this perspective, several studies have demonstrated the use of the IOS parameter R5–R20 as a useful marker of heterogeneity of airway constriction at a single time-point, perhaps reflecting to some extent peripheral airway dysfunction. Kolsum et al. demonstrated a close association between R5–R20 and FEF25–75% predicted in patients with COPD (Citation30). In addition, review papers list IOS among the commonly accepted tools to investigate peripheral airway dysfunction (Citation26,Citation31–35). In COPD, higher heterogeneity of bronchial obstruction is associated with increased resonant frequency, which is related to resistive load; ( Citation36) our findings are in support of this relationship.
Pisi et al. showed that R5–R20 is increased in the vast majority of patients with COPD, and associated with increased airway obstruction and lung hyperinflation, as well as poorer health status (Citation37). The current findings confirm a close association between high CASIS scores and increased R5–R20, which was not demonstrated with other lung function variables. These observations suggest that sleep disorders in subjects with COPD could be more related to the status of heterogeneous airway dysfunction more than the level of airway obstruction. Indeed, changes in conventional spirometric outcomes are not useful to predict EFL, which is linked to chronic dyspnea (Citation38). EFL more than airway obstruction carries the risk of lung hyperinflation due to its negative consequences on work of breathing and inspiratory muscle function In this context, the measure of airway resistance and reactance can find subtle changes even in the setting of normal spirometry (Citation39,Citation40). An interesting observation is the lack of an association between sleep disorders and features of air trapping. Perhaps, increased heterogeneity of airway constriction relates to different components of sleep quality, whereas features of air trapping could account for disruption of sleep structure, as shown by Kwon and colleagues in subjects with COPD and concomitant OSAS using polysomnography (Citation18).
To our knowledge, this is the first study in which factors potentially implicated in the occurrence of circadian variability of COPD symptoms were explored. Subjects had different levels of severity and were under different treatments. This is potentially a confounding factor, thus advocating for confirming studies on larger cohorts. Overall, the results seem to demonstrate that patients with increased R5–R20 by IOS present with more nocturnal disturbances. In conclusion, increased heterogeneity in airway constriction occurring during nighttime gives a reasonable pathophysiological insight for sleep disturbances referred by COPD patients.
Disclosure
The author reports no conflicts of interest in this work.
Acknowledgments
Not applicable.
Additional information
Notes on contributors
Marco Basile
MB participated to the design of the study, recruited the patients, performed the study tests, collected and analyzed the data, and contributed to the writing of the manuscript.
Pierpaolo Baiamonte
PB, EM, SP performed the study tests, collected and analyzed the data and participated to the interpretation of the results and to the writing of the manuscript.
Emilia Mazzuca
PB, EM, SP performed the study tests, collected and analyzed the data and participated to the interpretation of the results and to the writing of the manuscript.
Stefania Principe
PB, EM, SP performed the study tests, collected and analyzed the data and participated to the interpretation of the results and to the writing of the manuscript.
Federica Pennavaria
FP recruited the patients, performed the study tests, and collected the data.
Alida Benfante
AB analyzed the data and participated to the interpretation of the results and to the writing of the manuscript.
Nicola Scichilone
NS conceived and design the study, recruited the patients, participated to the analysis of the data, and wrote the first version of the manuscript. He is the guarantor of the paper.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://goldcopd.org; 2017
- Miravitlles M, Worth H, Soler Cataluña JJ, Price D, De Benedetto F, Roche N, Godtfredsen NS, van der Molen T, Löfdahl CG, Padullés L. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014;15:122. doi: 10.1186/s12931-014-0122-1.
- Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, Ostinelli J. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264–272. doi:10.1183/09031936.00051110.
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25(8):2043–2048. doi: 10.1185/03007990903103006.
- Soler-Cataluña JJ, Sauleda J, Valdés L, Marín P, Agüero R, Pérez M, Miravitlles M. Prevalence and perception of 24-hour symptom patterns in patients with stable chronic obstructive pulmonary disease in Spain. Arch Bronconeumol. 2016;52(6):308–315. doi: 10.1016/j.arbres.2015.11.010.
- Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. doi: 10.1183/09059180.00004311.
- Roche N, Chavannes NH, Miravitlles M. COPD symptoms in the morning: impact, evaluation and management. Respir Res. 2013;14:112. doi:10.1186/1465-9921-14-112.
- Roche N, Small M, Broomfield S, Higgins V, Pollard R. Real world COPD: association of morning symptoms with clinical and patient reported outcomes. COPD. 2013;10(6):679–686. doi: 10.3109/15412555.2013.844784.
- Tantucci C. Expiratory flow limitation definition, mechanisms, methods, and significance. Pulm Med. 2013;2013:749860. doi: 10.1155/2013/749860.
- Pokrzywinski RF, Meads DM, McKenna SP, Glendenning GA, Revicki DA. Development and psychometric assessment of the COPD and Asthma Sleep Impact Scale (CASIS). Health Qual Life Outcomes. 2009;7:98. doi: 10.1186/1477-7525-7-98.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545.
- Masa JF, Corral J, Sanchez de Cos J, et al. Effectiveness of three sleep apnea management alternatives. Sleep. 2013;36(12):1799–1807
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005 Aug;26(2):319–38.
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–512
- Hang LW, Wang HL, Chen JH, Hsu JC, Lin HH, Chung WS7, Chen YF. Validation of overnight oximetry to diagnose patients with moderate to severe obstructive sleep apnea. BMC Pulm Med. 2015;15:24. doi: 10.1186/s12890-015-0017-z.
- Hellinckx J, Cauberghs M, De Boeck K, Demedts M. Evaluation of impulse oscillation system: comparison with forced oscillation technique and body plethysmography. Eur Respir J. 2001;18(3):564–570
- Williamson PA, Clearie K, Menzies D, Vaidyanathan S, Lipworth BJ. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung. 2011;189(2):121–129.
- Jarenbäck L, Ankerst J, Bjermer L, Tufvesson E. Flow-volume parameters in COPD related to extended measurements of lung volume, diffusion, and resistance. Pulm Med. 2013;2013:782052. doi: 10.1155/2013/782052.
- Crim C, Celli B, Edwards LD, Wouters E, Coxson HO, Tal-Singer R, Calverley PM; ECLIPSE investigators. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med. 2011;105(7):1069–1078. doi: 10.1016/j.rmed.2011.01.010.
- Anderson WJ, Lipworth BJ. Relationships between impulse oscillometry, spirometry and dyspnoea in COPD. J R Coll Physicians Edinb. 2012;42(2):111–115. doi: 10.4997/JRCPE.2012.204.
- Piorunek T, Kostrzewska M, Cofta S, Batura-Gabryel H, Andrzejczak P, Bogdański P, Wysocka E. Impulse oscillometry in the diagnosis of airway resistance in chronic obstructive pulmonary disease. Nippon Rinsho. 2015;838:47–52. doi: 10.1007/5584_2014_49.
- Tanaka H, Fujii M, Kitada J. [Further examination of COPD using spirometry, respiratory function test, and impulse oscillometry]. Nihon Rinsho. 2011;69(10):1786–1791
- Piorunek T, Kostrzewska M, Stelmach-Mardas M, Mardas M, Michalak S Goździk-Spychalska J, Batura-Gabryel H. Small airway obstruction in chronic obstructive pulmonary disease: potential parameters for early detection. Adv Exp Med Biol. 2017;980:75–82. doi: 10.1007/5584_2016_208.
- Miravitlles M, Iriberri M, Barrueco M, Lleonart M, Villarrubia E, Galera J. Usefulness of the LCOPD, CAFS and CASIS scales in understanding the impact of COPD on patients. Respiration. 2013;86(3):190–200
- Price D, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of night-time symptoms in COPD: a real-world study in five European countries. Int J Chron Obstruct Pulmon Dis. 2013;8:595–603.
- Eltayara L, Becklake MR, Volta CA, Milic-Emili J. Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. Am J Res and Crit Care. 1996;154(6):1726–1734.
- Boczkowski J, Murciano D, Pichot MH, Ferretti A, Pariente R, Milic-Emili J Expiratory flow limitation in stable asthmatic patients during resting breathing. Am J Res Crit Care. 1997;155(3):1036–1041.
- McNicholas WT. Impact of sleep in respiratory failure. Eur Respir J. 1997;10(4):920–933.
- Berger KI, Pradhan DR, Goldring RM, Oppenheimer BW, Rom WN, Segal LN. Distal airway dysfunction identifies pulmonary inflammation in asymptomatic smokers. ERJ Open Res. 2016;2(4). pii: 00066–2016.
- Kolsum U, Borrill Z, Roy K, Starkey C, Vestbo J, Houghton C, Singh D. Impulse oscillometry in COPD: identification of measurements related to airway obstruction, airway conductance and lung volumes. Respir Med. 2009;103:136–143.
- Carr TF, Altisheh R, Zitt M. Small airways disease and severe asthma. World Allergy Organ J. 2017;10(1):20.
- Usmani OS, Singh D, Spinola M, Bizzi A, Barnes PJ. Small airway dysfunction and bronchial asthma control: the state of the art. Respir Med. 2016;1:19–27.
- Cottini M, Lombardi C, Micheletto C. Small airway dysfunction and bronchial asthma control: the state of the art. Asthma Res Pract. 2015;13:13.
- Scichilone N, Contoli M, Paleari D, Pirina P, Rossi A, Sanguinetti CM, Santus P, Sofia M, Sverzellati N. Assessing and accessing the small airways; implications for asthma management. Pulm Pharmacol Ther. 2013;26(2):172–179.
- Contoli M, Bousquet J, Fabbri LM, Magnussen H, Rabe KF, Siafakas NM, Hamid Q, Kraft M. The small airways and distal lung compartment in asthma and COPD: a time for reappraisal. Allergy. 2010;65(2):141–151.
- Brashier B, Salvi S. Measuring lung function using sound waves: role of the forced oscillation technique and impulse oscillometry system. Breathe (Sheff). 2015;11(1):57–65.
- Pisi R, Aiello M, Zanini A, Tzani P, Paleari D, Marangio E, Spanevello A, Nicolini G, Chetta A. Small airway dysfunction and flow and volume bronchodilator responsiveness in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1191–1197.
- Boczkowski J, Murciano D, Pichot MH, Ferretti A, Pariente R, Milic-Emili J. Expiratory flow limitation in stable asthmatic patients during resting breathing. Am J Respir Crit Care Med. 1997;156(3 Pt 1):752–757.
- Frantz S, Nihlén U, Dencker M, Engström G, Löfdahl CG, Wollmer P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir Med. 2012;106(8):1116–1123. doi: 10.1016/j.rmed.2012.04.010.
- Oppenheimer BW, Goldring RM, Herberg ME, Hofer IS, Reyfman PA, Liautaud S, Rom WN, Reibman J, Berger KI. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132(4):1275–1282.