Abstract
Chronic obstructive pulmonary disease (COPD) is a risk factor of post-operative complications after lung cancer resection. The influence of the “frequent exacerbator (FE)” phenotype (at least three exacerbations per year) is unknown.
Postoperative outcomes of frequent exacerbators (POFE) was a prospective observational study of patients with COPD undergoing lung resection for cancer. The inclusion criteria were: age >40 years, FEV1/FVC <70%, non-urgent surgery for lung cancer, filled out self-questionnaires. The primary outcome was assessment of postoperative pulmonary complications (purulent tracheobronchitis, atelectasis, pneumonia, acute respiratory failure, need of mechanical ventilation). Secondary outcomes encompassed the prevalence of the FE phenotype and its impact on postoperative complications.
A total of 682 patients were screened from June 2014 to October 2015. 93 patients with COPD were included, 21 (23%) were FE. Postoperative tracheobronchitis, atelectasis pneumonia or respiratory failure (isolated or associated) occurred in 47%, 48%, 26%, and 38% of patients, respectively. Non-invasive and invasive mechanical ventilation were necessary in 4 (4%) and 22 (23%) patients. Purulent tracheobronchitis, pneumonia and hypercapnia (this last requiring noninvasive mechanical ventilation) were more frequent in FE (p = 0.043, 0.042, 0.015); however the number of patients wth at least one respiratory complication was not different (76% vs. 52%, p = 0.056). In all patients, multivariate logistic regression identified two independent factors of postoperative respiratory complications: male sex (OR 10.6 [95% CI 1.97–57.6], p = 0.006) and the FE phenotype (OR 6.33 [1.04–38.39], p = 0.045).
Occurrence of postoperative complications in patients with COPD is high. FE phenotype is an independent risk factor.
Introduction
Chronic obstructive pulmonary disease (COPD) is as a systemic inflammatory disorder associated with an increased risk of primary lung cancer (Citation1). This risk is two- to fivefold greater compared with non-COPD smokers (Citation2). Thus, when considering patients with non-small cell lung cancer (NSCLC) for curative resection, associated COPD is a frequent concern, because of increased post-operative risk (Citation3,Citation4). The 30-day mortality varies according to the extent of pulmonary resection, with figures, in patients with COPD, ranging 6–24% after pneumonectomy, and 2–5% after lobectomy (Citation5,Citation6). Respiratory complications are important determinants of mortality and are mainly represented by pneumonia and respiratory failure (Citation7). Thus, progresses in surgical and intensive care as well as an expanded understanding of the pathophysiology of COPD after lung resection may help improve outcomes.
It is well known that exacerbations play a negative impact on COPD evolution, (Citation8) and the frequent exacerbator (FE) phenotype has been recently described (Citation9). This phenotype defines patients with several annual exacerbations associated with a deterioration of respiratory symptoms, requiring modification of the self-managed treatment, prescription of antibiotics, and corticosteroids and/or hospitalization (Citation9).
The new Global Initiative for Obstructive Lung Disease (GOLD) classification is based not only on data of lung function tests, but also on the history of exacerbations and symptom scores (dyspnoea, global impact of the disease) (Citation10). Kim and colleagues explored the impact of the new GOLD classification in post-operative complications of thoracic and non-thoracic surgery (Citation11). In that retrospective cohort study of 405 patients with COPD, GOLD C-D was associated with a significantly higher occurrence of complications; however only 14 patients had thoracic surgery (Citation11). No other study evaluated the impact of the new GOLD staging in postoperative complications after lung resection of NSCLC. Furthermore, to date, no study has assessed the frequence of FE phenotype among patients with COPD requiring lung resection, nor the impact of FE phenotype on the occurrence of respiratory complications after lung resection. Thus, we aimed at evaluating prospectively the incidence of postoperative respiratory complications (purulent trachobronchitis, atelectasis, pneumonia, acute respiratory failure) in patients with COPD with respect to the new GOLD stage and, in particular, in FE requiring pulmonary resection for cancer.
Methods
Postoperative outcomes of frequent exacerbators (POFE) was a single center, prospective, observational study involving patients undergoing lung resection for NSCLC at Paris Center University Hospital. IRB approval was obtained by the Ethics Committee of the French Society of Thoracic and Cardiovascular Surgery. The trial was registered at www.clinicaltrial.gov NCT 02268708. Patients were included after signed informed consent.
Age >40 years, eligibility for non-urgent anatomical lung resection, and COPD (FEV1/FVC <70%) were the inclusion criteria; minor age, deprivation of liberty, pregnancy, and inability to fulfill the questionnaries were exclusion criteria.
Respiratory questionnaries (see below in the section “Collected data”) were administered at surgery or anesthesia consultation. Patient's demographic and clinical characteristics were collected before surgery, intra- and postoperative data were collected after surgery. Follow-up information was obtained by phone interview 30 days after the intervention.
The primary endpoint was the rate of pulmonary complications (purulent tracheobronchitis, atelectasis, pneumonia, acute respiratory failure, need of noninvasive or invasive mechanical ventilation) after lung resection in patients with COPD. Secondary outcomes were assessement of the prevalence of the FE phenotype, and of respiratory postoperative pulmonary complication rates in this group, as well as ICU admission rate, postoperative non-respiratory complications, duration of hospitalization, as well as in-hospital, 30-day and 90-day postoperative mortality, Incidence of complications according to GOLD classification was another secondary endpoint.
Patients were included prospectively from June 2014 to October 2015 in the Thoracic surgery department of Paris Center University Hospital, Paris France. Postoperatvely, patients were admitted in the ICU in case of intra-operative or postoperative complications or for surveillance following local staff decisions, which were based on the risk of postoperative complications.
Collected data
Four validated questionnaires were administered during the consultation: the Medical Research Council Dyspnoea Scale (MRC), the COPD Assesment Test (CAT), the International Physical Activity Questionnaire (IPAQ), and the Hospital Anxiety and Depression Scale (HAD) (Citation12–14). At consultation, information on smoking history, existence of daily cough and sputum, number of exacerbations, frequency of respiratory physiotherapy and knowledge of bronchodilator treatment was systematically collected. FE phenotype was defined as at least three exacerbations during the year prior to the surgical intervention (Citation15).
Demographic data and respiratory functionnal tests were also collected at inclusion. We determined the ECOG Performance Status, American Score of Anesthesiology (ASA score), Charlson score (comorbidities) (Citation16), and Thoracoscore (inhospital mortality risk after thoracic surgery) (Citation17). The BODE (Body mass index, airflow obstruction, functional dyspnea, exercise capacity) and the GOLD groups were also determined (Citation13,Citation18).
For anesthesiological and surgical data the following data were collected: extent of resection, need of transfusion, intraoperative hypoxemia requiring CPAP (continuous positive air pressure) or inspiratory oxygen fraction (FiO2) of 100% for at least 15 minutes, and use of catecholamines.
Concerning postoperative data, we prospectively collected SAPS 2 score, use of invasive or noninvasive ventilation (with respective indications), occurrence of extra-thoracic infections, sepsis, cardiovascular complications (atrial fibrillation, myocardial infarction, acute pulmonary edema, pulmonary embolism) and renal failure.
Cancer stage was determined according to the 2009 UICC classification (pTNM) (Citation19).
Definitions
Purulent tracheobronchitis was defined by the presence of purulent bronchial secretions associated with fever >38.5 °C and leucocytosis >12 × 109/L, in the absence of pulmonary infiltrate (Citation20). Atelectasis was defined as a retractile condensation of a pulmonary territory visible on chest x-ray. Postoperative pneumonia was defined by the appearance of new pulmonary infiltrates associated with at least two of the following criteria: (i) fever >38.5 °C or hypothermia <35 °C; (ii) leucopenia <4 × 109/L or leucocytosis >12 × 109/L; (iii) purulent bronchial secretions. Bacteriological confirmation was obtained by the presence of microorganisms in the bronchial samples (ECBC > 107 CFU/ml, bronchial aspiration >105 CFU/ml, BAL > 104 CFU/ml, protected brush >103 CFU/ml).
Post-operative acute respiratory failure was defined by the presence of one of the following criteria: tachypnea with a respiratory rate >30/min, PaO2/FiO2 < 200, abdominal breathing, activation of accessory inspiratory muscles, and expiratory contraction of the abdominal muscles.
Statistics
Proportions were expressed as percentages, continuous variables by mean and standard deviation if normally distributed or by median and interquartile range otherwise. Percentages were compared by using the Chi-square or Fisher test as appropriate. Quantitative data were compared using t-test or Mann–Whittney test.
Thus, to assess variables indepentently associated with the occurrence of postoperative respiratory complications, we firstly performed an univariate analysis entering available clinical and pathological factors. Subsequently we entered in a multivariate logistic regression model factors associated at univariate analysis (with a p < 0.1) with postoperative complication. A stepwise selection procedure was employed. Significance was accepted at p < 0.05.
Sample size calculation
In order to show a difference of 25% in the occurrence of postoperative respiratory complications between FE and non-FE, with an α risk of 5% and a power of 90%, we evaluated that 72 patients had to be included in the study.
Statistical tests were performed with SPSS 21.0 statistical software (IBM Corporation, Armonk, NY, USA) and SEM software (Statistics in Epidemiology and Medicine, Mirefleur, France).
Results
General demographics
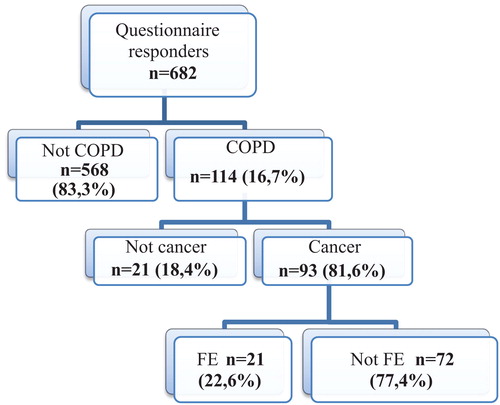
Between June 1, 2014 and October 31, 2015, 682 patients with suspected or proven operable lung cancer admitted in the thoracic surgery departement were screened. 114 (16.7%) had COPD. Of these patients, 93 had final diagnosis of NSCLC and were included in subsequent analysis. FE represented 22.6% of them (21 patients) ().
Median age was 68 years, and 68% of patients were men. Comparison of FE and non-FE is shown in . There was no difference in terms of comorbidities (cardiac, renal, or metabolic desease). FE had higher BMI (27 kg/m2 [25–29] vs. 24 kg/m2 [22–27], p = 0.02) and fibrinogen levels (5.1 g/l [4.6–6.6] vs. 3.8 g/l [3.2–4.6], p < 0.001) than non-FE.
Table 1. Demographic data.
Respiratory data
The median predicted FEV1 was 72% in the whole population of patients with COPD and the majority (61%) of patients had GOLD stage II. BODE and CAT scores were higher in FE (), whereas IPAQ was not significantly different. 17/93 patients (18%) reported antibiotic administration in the 21 days before surgery, three were FE and 14 non-FE (p = 0.59). eight patients (4 FE 19% and 4 non-FE 6%, p = 0.052) had a proven pre-operative bacterial colonization.
Table 2. Respiratory characteristics.
Intraoperative complications
Intraoperative complications are shown in . They occurred more frequently in the FE than in non-FE population (67 vs. 39%, p = 0.02) and were represented mainly by hypoxemia (p = 0.011).
Table 3. Interventional data.
Primary endpoint: postoperative respiratory complications
One-hundred forty eight postoperative respiratory complications were diagnosed. 55 patients (59%) had at least one postoperative respiratory complication (). About 1, 2, 3, and 4 postoperative respiratory complications occurred in 14, 10, 4, and 17 patients, respectively. Postoperative tracheobronchitis, atelectasis pneumonia or respiratory failure (isolated or associated) occurred in 47%, 48%, 26%, and 38% of patients, respectively.
Table 4. Respiratory complications.
Sixty patients (64%) were admitted in the intensive care unit. Curative noninvasive ventilation (NIV) took place in 23% (n = 4) of patients. Re-intubation and invasive mechanical ventilation were necessary in 4/93 patients. No in-hospital or 30-day death occurred. 90-day mortality was 4.3% (4/93).
Postoperative complications according to the FE phenotype and to GOLD stage
Global number of respiratory complications was higher in FE as compared to non-FE patients (p = 0.00058). Purulent tracheobronchitis occurred in 14/21 FE and 30/72 non-FE patients, respectively (p = 0.043), whereas pneumonia occurred in 9/21 FE and 15/72 non-FE patients, respectively (p = 0.042), and hypercapnia requiring NIV in 6/21 FE and 6/72 non-FE, respectively (p = 0.015). No differences were observed in the rate of atelectasis, respiratory failure, or need of invasive mechanical ventilation (). The number of patients wth at least one respiratory complication was not different between FE and non-FE patients (76% vs. 52%, p = 0.056) ().
At least one respiratory complication occurred in 11/23, 38/61, and 6/9 patients with COPD stage (GOLD 2011) I, II, and III, respectively (p = 0.8). When analyzing occurrence of at least one respiratory complication with respect to the 2013 GOLD classification, figures were 19/34, 7/16, 9/16, and 20/27 in stage A, B, C, and D, respectively (p = 0.78).
Nonrespiratory postoperative complications
Nonrespiratory postoperative complications are shown in . No difference in their occurrence was observed between FE and non FE COPD patients.
Table 5. Outcome after ICU admission complications.
Risk factors for postoperative respiratory complications
Risk factors associated with the occurrence of at least one respiratory complication at univariate analysis are shown in . To assess independent risk factors, we entered in the stepwise logistic regression model the following factors associated with postoperative complications with a p value <0.1 at univariate analysis (): sex; BMI; FVC; DLCO/AV, Thoracoscore, prolonged Fi O2 during surgery, FE Fenotype. Two independent factors of postoperative respiratory complications were identified: male sex (OR 10.6 (95% CI: 1.97, 57.6), p = 0.006) and the FE phenotype (OR 6.33 (95% CI: 1.04, 38.39), p = 0.045) ().
Table 6. Comparaison between patients presenting or not post operative pulmonary complications.
Table 7. Final results of stepwise multivariate logistic regression for risk factors of post operative pulmonary complications.
Discussion
In the present study we show that respiratory complications are frequent after lung resection in patients with COPD. The FE phenotype is often encountered among COPD patients requiring resection and is associated to an increased risk of respiratory complications, including purulent tracheobronchitis, pneumonia, and hypercapnia requiring noninvasive ventilation. On the other hand, higher GOLD stage (both 2011 and 2013) is associated with a non-significant increase in postoperative respiratory complications.
In the whole population of lung cancer patients undergoing resection in the study period, 16.7% had COPD, and the majority of them had GOLD stage II or D. Of note, in our population, GOLD stage percentages are different as compared to COPD populations usually described in the literature, probably because of the thoracic surgery context.
The FE phenotype accounted for 22.6% of this COPD population. The cutoff for the FE phenotype was at least three exacerbations per year, as suggested by Seemungal et al. (Citation15) This cutoff permits to single out highly severe patients with an increased risk of exacerbation. Some existing studies provide data on the incidence of FE phenotype among patients with COPD in a (non-thoracic) surgical context. In a post hoc analysis of the POET-COPD cohort (Citation21), FE accounted for 11.6% of cases among GOLD 1 and 2 patients, and 15.6% for GOLD 3-4. The ECLIPSE cohort (Citation22) also found an increasing rate of FE according to GOLD stage: 22% in GOLD 2 group, 33% in GOLD 3, and 47% in GOLD 4.
Less than half of the patients with COPD of our study (39%) were aware of a previous diagnosis of COPD. However, FE seemed to have better knowledge of their disease.
Furthermore, BODE and CAT scores were higher in the FE population, underlining probably a greater impact of COPD in everyday life of these patients. FEs had increased BMI and fibrinogen level, suggesting that overweight, and systemic inflammation are part of this specific phenotype (Citation23).
In our population, the great majority of patients (97%) had smoking history and only half of the population (49% and 52% of the FE) had really stopped smoking before the operation, despite incitation and anti-tobacco consultation. Of note, it is known that postoperative complications increase if smoking is not stopped (Citation24).
In our study, respiratory postoperative complications were frequent, occurring in 59% of patients and were even more frequent in the FE group (76% vs. 54%). In the literature, cardio-respiratory complications in thoracic surgery occurs in approximately 10% of patients in Europe, (Citation25) and in France Agostini et al. (Citation26) observed a 15% rate. These studies did not target patients with COPD or FE phenotype. Moreover, in these studies, purulent tracheobronchitis was not included among the respiratory complications. Prospective studies observed a higher respiratory complication rate of lung resection in general populations, and identified COPD as a risk factor of postoperative pneumonia (Citation7). Obviously the physiologic impact of COPD in patients undergoing lung resection explains the higher rate of respiratory complications. Indeed, the small size of this population induces an interpretation bias because of the possible lack of power.
The FE had a higher rate of postoperative respiratory complications but no difference in cardiovascular, renal or infectious complications. Together with FE phenotype, other risk factors for respiratory complications included male sex, higher BMI, DLCO, thoracoscore, and occurrence of intraoperative complications. Only FE phenotype and gender were identified as independent risk factors of postoperative respiratory complication at multivariate analysis. Male sex, COPD and comorbidities have been identified as risk factors of postoperative complication in the literature (Citation21,Citation27). On the other hand, results are quite in disagreement with literature with respect to BMI. In the study by the Epithor Group (Citation28) on 19,856 unselected patients undergoing lobectomy, overweight, and obesity were associated to a reduced risk of operative mortality and respiratory complications in thoracic surgery. Similar results were observed in an unselected population of consecutive pneumonectomies by our team (Citation29). In the present work we find the opposite result, that is overweight is a risk factor of respiratory complications. However, our work targeted the specific population of lung cancer associated with COPD. One hypothesis is that the combination of cancer, COPD and overweight would represent a specific phenotype at increased risk of postoperative complications. In this study, we did not perform a more profund assessment of nutritional status (e.g., by albumin or prealbumin measurement) or possible sarcopenia. This could have unraveled discrepancies between biological and radiological markers and BMI in the assessment of postoperative risk related to nutritional status in this specific population. A recent study showed the yeld of measures of psoas in the assessment of sarcopenia in patients undergoing lung surgery (Citation29). May be assessment of muscle mass in patients with COPD undergoing lung resection may help selecting specific cluster of patients at increased risk of postoperative complications.
This study highlights the importance of clinical data in estimating the perioperative risk in thoracic surgery. Indeed, the patient’s phenotype seems to influence the incidence of complications beyond functional paraclinical data. The European and American recommendations on postoperative risk in thoracic surgery are based on physiological tests (Citation25). Our study suggests the contribution of the clinical data (by simply identifying the exacerbator phenotype) in estimating the risk of postoperative complications.
Limitations of the study
This was a single center study that took place in a major oncologic thoracic center in France. Thus the results of the risk factors may have been influenced by a center effect. However it should be noted that the proportion of patients with COPD (16%) is similar to the literature (Citation26) despite FE definitions. Declarative data, including the frequency of exacerbations and the severity of the disease, may have induced an interpretation biais. However, it is difficult to overcome the subjective side of the severity of COPD or impact in the quality of life. It should be underlined that, according to the literature, the number of COPD exacerbations is more reliable, by self-filled questionnaires than by hospital data (Citation30).
Finally, few data exist in the literature on the proportion of FE in thoracic surgery, and no study to date described the FE profile of this specific population.
Further multi centers studies would be needed to better determine the influence of the FE phenotype in the morbidity and mortality after oncological pulmonary resection of non-small cell cancer.
Disclosure statement
Dr. Roche reports grants and personal fees from Boehringer Ingelheim, Pfizer, Novartis, personal fees from Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, Sanofi, Sandoz, 3M, Zambon, outside the submitted work.
Additional information
Notes on contributors
Suela Demiri
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Christine Lorut
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Antoine Rabbat
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Daniel Luu van Lang
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Aurelie Lefebvre
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Jean-François Regnard
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Charles-Marc Samama
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Daniel Dusser
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Nicolas Roche
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
Marco Alifano
SD contributed in article writing, study conception, patient inclusion, statistics, and submission approval. CL contributed in article writing, study conception, patient inclusion, and submission approval. AR, DLVL, AL, JFR, MS and DD are contributed in patient inclusion, submission approval, and manuscript revision. NR contributed in article writing, study conception, and submission approval. MA contributed in article writing, study conception, statistics, and submission approval.
References
- Raviv S, Hawkins KA, DeCamp MM, Kalhan R. Lung cancer in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(9):1138–46. doi:10.1164/rccm.201008-1274CI.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245–57. doi:10.1183/09031936.00133805.
- Licker MJ, Widikker I, Robert J, Frey J-G, Spiliopoulos A, Ellenberger C. Schweizer A, Tschopp JM. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81(5):1830–7. doi:10.1016/j.athoracsur.2005.11.048.
- Thomas PA, Berbis J, Baste J-M, Le Pimpec-Barthes F, Tronc F, Falcoz P-E., Dahan M7, Loundou A. Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg. 2015;149(1):73–82. doi:10.1016/j.jtcvs.2014.09.063.
- Strand T-E, Rostad H, Damhuis RAM, Norstein J. Risk factors for 30-day mortality after resection of lung cancer and prediction of their magnitude. Thorax. 2007;62(11):991–7. doi:10.1136/thx.2007.079145.
- Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer. 2002;37(1):95–101. doi:10.1016/S0169-5002(02)00014-4
- Schussler O, Alifano M, Dermine H, Strano S, Casetta A, Sepulveda S. Chafik A, Coignard S, Rabbat A, Regnard JF. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med. 2006;173(10):1161–9. doi:10.1164/rccm.200510-1556OC.
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–52. doi:10.1136/thorax.57.10.847
- Friedlander AL, Lynch D, Dyar LA, Bowler RP. Phenotypes of chronic obstructive pulmonary disease. COPD. 2007;4(4):355–84. doi:10.1080/15412550701629663.
- Yusen RD. Evolution of the GOLD documents for the diagnosis. management. and prevention of chronic obstructive pulmonary disease. Controversies and questions. Am J Respir Crit Care Med. 2013;188(1):4–5. doi:10.1164/rccm.201305-0846ED.
- Kim H-J, Lee J. Park YS, Lee C-H, Lee S-M, Yim J-J. Impact of GOLD groups of chronic pulmonary obstructive disease on surgical complications. Int J Chron Obstruct Pulmon Dis. 2016;11:281–7. doi:10.2147/COPD.S95046.
- Perez T, Burgel PR, Paillasseur J-L, Caillaud D, Deslée G, Chanez P, Roche N. Modified Medical Research Council Scale vs Baseline Dyspnea Index to evaluate dyspnea in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1663–72. doi:10.2147/COPD.S82408.
- Karloh M, Fleig Mayer A, Maurici R, Pizzichini MMM, Jones PW. Pizzichini E. The COPD assessment test: what do we know so far?: A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stages. Chest. 2016;149(2):413–25. doi:10.1378/chest.15-175214.
- Herrmann C. International experiences with the Hospital Anxiety and Depression Scale—a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi:10.1016/S0022-3999(96)00216-4
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–22. doi:10.1164/ajrccm.157.5.9709032.
- Birim O, Maat APWM, Kappetein AP, van Meerbeeck JP, Damhuis R A. M, Bogers AJJC. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardio-Thorac Surg. 2003;23(1):30–4. doi:10.1016/j.ejcts.2005.06.046
- Falcoz PE, Conti M. Brouchet L, Chocron S, Puyraveau M, Mercier M. Etievent JP, Dahan M. The thoracic surgery scoring system (Thoracoscore): risk model for in-hospital death in 15.183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg. 2007;133(2):325–32. doi:10.1016/j.jtcvs.2006.09.020.
- Ong K-C, Earnest A. Lu S-J. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest. 2005;128(6):3810–6. doi:10.1378/chest.128.6.3810.
- Eberhardt WE1, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A 3rd, Goldstraw P, Rami-Porta R. The IASLC lung cancer staging project: Proposals for the revision of the M descriptors in the forthcoming Eighth Edition of the TNM classification of lung cancer. J Thorac Oncol. 2015;10(11):1515–22. doi:10.1097/JTO.0000000000000673.
- Jose Belda, Cavalcanti M, Ferrer M, Serra M, Puig de la Bellacasa J, Canalis E, Torres A. Bronchial colonization and postoperative respiratory infections in patients undergoing lung cancer surgery. Chest. 2005;128(3):1571–9. doi:10.1378/chest.128.3.1571
- Beeh KM, Glaab T, Stowasser S, Schmidt H, Fabbri LM, Rabe KF, Vogelmeier CF. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial. Respir Res. 2013;14:116. doi:10.1186/1465-9921-14-116.
- Hurst JR. Vestbo J. Anzueto A. Locantore N. Müllerova H. Tal-Singer R. Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–38. doi:10.1056/NEJMoa0909883.
- Alifano M, Mansuet-Lupo A, Lococo F, Roche N, Bobbio A, Canny E. Schussler O, Dermine H, Régnard JF, Burroni B. et al. Systemic inflammation. nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PloS One. 2014;9(9):e106914. doi:10.1371/journal.pone.0106914.
- Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL. Gouskova NA, Hansel NN, Hoffman EA, Kanner RE et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–21. doi:10.1056/NEJMoa1505971.
- Novoa N, Jiménez MF, Aranda JL, Rodriguez M, Ramos J, Gómez Hernández MT. Varela G. Effect of implementing the European guidelines for functional evaluation before lung resection on cardiorespiratory morbidity and 30-day mortality in lung cancer patients: a case-control study on a matched series of patients. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2014;45(4):e89–93; discussion e93. doi:10.1093/ejcts/ezt596.
- Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat MS, Rajesh PB. Steyn RS, Singh S, Naidu B. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65(9):815–8. doi:10.1136/thx.2009.123083.
- Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier J-P. Varela G. Licker M, Ferguson MK, Faivre-Finn C, Huber RM, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. 2009;34(1):17–41. doi:10.1183/09031936.00184308.
- Thomas PA, Berbis J, Falcoz P-E, Le Pimpec-Barthes F, Bernard A, Jougon J. Porte H, Alifano M, Dahan M. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2014;45(4):652–9; discussion 659. doi:10.1093/ejcts/ezt452.
- Hervochon R, Bobbio A, Guinet C, Mansuet-Lupo A, Rabbat A, Régnard J-F. Roche N, Damotte D, Iannelli A, Alifano M. Body mass index and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg. 2017;103(1):287–95. doi:10.1016/j.athoracsur.2016.06.077.
- Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi:10.1186/1741-7015-11-181.