Abstract
The COMCOLD score was developed to quantify the impact of comorbidities on health status in patients with chronic obstructive pulmonary disease (COPD). The objective of this study is to evaluate the association between health status in outpatients with COPD according to COMCOLD score and the GOLD 2017 groups according to symptoms (B and D vs. A and C) and exacerbations (C and D vs. A and B). 439 patients were included. The average score was 2.4 ± 3. 48% of cases had a COMCOLD score >0. The most symptomatic patients (B and D vs. A and C) had a higher score: 3 ± 3.3 vs. 1.3 ± 2.1 (p < 0.001), in contrast with the groups with a higher risk of exacerbation (C and D vs. A and B) in which there was no significant difference: 3 ± 3.5 vs. 2.2 ± 3.0 (p = 0.055). The most symptomatic patients (B and D) showed a greater prevalence of depression, peripheral artery disease and heart disease with an adjusted OR of 3.04 [CI95%: 1.36; 6.86], 2.49 [CI95%: 1.17; 5.29], and 4.41 [CI95%: 2.50; 7.75], respectively. Moreover, no relationship was found between the comorbidities defined by the COMCOLD score and the GOLD 2017 groups with the greatest risk of exacerbation (C and D). The greatest effect on health status was found in those patients with COPD belonging to the most symptomatic groups (B and D), with depression, peripheral artery disease, and heart disease being the main comorbidities involved.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading public health issue, not only due to its elevated prevalence and high associated health care costs, but also due to the high resulting morbidity and mortality and decrease in health-related quality of life (HRQoL) (Citation1). Numerous studies have shown that between 60 and 90% of patients with COPD suffer from an associated comorbidity, with an average of four chronic illnesses per patient (Citation2–5); stroke, cardiovascular disease, lung cancer, diabetes mellitus, osteoporosis, muscular involvement, and anxiety and/or depression are among the most prevalent comorbidities (Citation6–9).
In spite of the important role comorbidities play in the evolution of COPD, we have not had the appropriate evaluation tools available for decades. In recent years, several indexes have been developed to measure the impact of different diseases on patient prognosis. To date, the Charlson comorbidity index (Citation10) has been the most widely used. Developed and validated in patients hospitalized in the 1980s, the use of this index has several limitations in patients with COPD: first, the index is not specific to these patients and, as a result, it does not evaluate the specific comorbidity of this disease (Citation8); second, having been designed decades ago, it places great value on diseases that are now better controlled. Similarly, indexes such as the COTE (Citation8) or CODEX (Citation11) aim to estimate mortality in patients with COPD.
However, some of the most prevalent comorbidities in these patients do not worsen survival but may greatly decrease quality of life (Citation12–14). A comprehensive patient evaluation must take into account the impact different diseases have on both HRQoL and state of health. The COMCOLD (COMorbidities in Chronic Obstructive Lung Disease) score (Citation15) has been developed to quantify the impact of different comorbidities (depression, anxiety, peripheral artery disease, cerebrovascular disease and symptomatic heart disease) on the aforementioned aspects based on the fact that these diseases will affect exercise tolerance, the intensity of symptoms – cough, dyspnea and wheezing – and the development of exacerbations (Citation3, Citation12–18), decisive factors when determining the treatment to follow (Citation19).
While women suffering from COPD as well as patients with the overlap COPD-asthma phenotype appear to show worse health status according to the COMCOLD score (Citation20), the score for these individuals was not evaluated in the different GOLD 2017 groups (A, B, C, and D) (Citation19).
The objectives of this study are (1) to classify patients with COPD according to the new GOLD 2017 classification, grouped by symptoms (A and C vs. B and D) and risk of exacerbation (A and B vs. C and D) and to evaluate the possible association with health status according to the COMCOLD score. (2) To evaluate the prevalence of a panel of comorbidities described as common in patients with COPD (not included in the score) and their relationship with the GOLD 2017 classification.
Material and methods
This is a cross-sectional, observational study of patients with COPD monitored by the pulmonology department at Hospital Universitario Nuestra Señora de Candelaria (HUNSC) from January 1, 2012 to December 31, 2014. The study population represents the southern region and area surrounding the capital of the island of Tenerife, with a reference population of 452,000 inhabitants and 22 healthcare areas.
The inclusion criteria included the following: age greater than 40 years, current or former smoker with a pack-years index greater than or equal to 10 and a forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of less than 70% after administering 400 micrograms of salbutamol, as well as having been monitored by a pulmonologist for at least 6 months. Exclusion criteria included the presence of a chronic respiratory disease other than COPD and the presence of chronic airflow obstruction without exposure to tobacco smoke or with a pack-years index less than 10. Patient recruitment was done consecutively according to the order they attended appointments with no selection criteria other than those mentioned above for inclusion or exclusion.
Following approval from the Ethics Committee at the hospital, an electronic health record (EHR) for each patient was used to collect data on the following parameters upon inclusion: age, sex, body mass index (BMI) (weight in kg/height in m2), history of tobacco use (current smoker if they had smoked at least one cigarette in the last 6 months, former smoker if they had been a smoker and continuously abstained for at least 6 months) and intensity according to the pack-years index ([number of cigarettes per day × years smoking]/20) and dyspnea evaluated using the modified Medical Research Council scale (mMRC) (Citation21). With regard to comorbidities, information was collected concerning cardiovascular risk factors: arterial hypertension, diabetes mellitus, dyslipidemia, sleep apnea-hypopnea syndrome and obesity; the presence of cardiovascular disease such as arrhythmia, ischemic heart disease, congestive heart failure, stroke, peripheral artery disease and chronic kidney disease; presence of anxiety and depression in addition to a personal history of bronchial asthma. The Charlson comorbidity index (both baseline and age-adjusted) (Citation8) was calculated for each of the patients. This index considers the following 19 medical conditions: myocardial infarction, angina, cardiovascular disease, cerebrovascular disease, dementia, COPD, connective tissue disease, gastrointestinal disease, mild or severe liver disease, complicated or uncomplicated diabetes mellitus, stroke, kidney failure, cancer, leukemia, lymphoma, secondary metastasis, and AIDS. The score ranges from 1 to 6 for each item, and the total score provides an index of severity. Age is adjusted by calculating each decade after 40 years of age as one point in the original Charlson comorbidity index. A point is added for each decade after 40 years of age, up to a maximum of 4 points (1 point for ages 41–50, 2 points for ages 51–60, 3 points for ages 61–70, 4 points for 71 years of age or older). The FEV1, FVC and FEV1/FVC ratio forced spirometry results were also recorded, stratifying patients according to the degree of severity included in the GOLD 2009 document (Citation22) and categories in GOLD 2017 (19). According to GOLD 2009, COPD severity is classified into the following stages: mild (stage 1) FEV1 ≥ 80%; moderate (stage 2) FEV1 ≥ 50% and <80%; severe (stage 3) FEV1 ≥ 30% and <50%; and very severe (stage 4) FEV1 < 30%. The GOLD 2017 document adds a letter (A, B, C, and D) to stages 1–4, providing information about patient symptoms and risk of exacerbation. Thus, groups A and B are those with <2 exacerbations per year without hospitalization while groups C and D represent patients with ≥2 exacerbations per year and/or ≥1 hospitalization. Additionally, groups A and C correspond to patients with dyspnea between 0 and 1, evaluated using the mMRC scale, and/or who score below 10 on the COPD Assessment Test (CAT) (Citation23). In contrast, groups B and D include patients with mMRC ≥2 and/or CAT scores ≥10. In the present study, the level of our patients’ symptoms was assessed using the mMRC.
Finally, the COMCOLD score (Citation15) was calculated for each patient using the weighted score for specific comorbidities: depression (6 points), anxiety (4 points), peripheral artery disease (3 points), cerebrovascular disease [stroke or transient ischemic attack] (3 points), and symptomatic heart disease [coronary disease and/or heart failure] (3 points). Minimum score = 0 and maximum score = 19. The total score provides an index of severity.
Statistical analysis
An initial descriptive analysis was done using the average ± standard deviation (SD) for quantitative variables and frequency (percentage) for qualitative variables. The Wilcoxon test was used when comparing the COMCOLD score in the two groups because it was not normally distributed. The prevalence of each comorbidity was analyzed using the Clopper-Pearson estimation method implemented in the R binGroup package (Citation24). Logistic regression models were created for each of the comorbidities using GOLD 2017 symptoms together with GOLD 2017 exacerbations as risk predictors. Additionally, the same analysis was done after adjusting for the FEV1/FVC ratio and pack-years index. Any associated probability below 5% was considered significant. The interaction between symptoms and exacerbations was not evaluated in the models. Finally, a negative binomial regression model was created and adjusted to predict the COMCOLD score using GOLD 2017 symptoms, GOLD 2017 exacerbations, FEV1/FVC ratio, and pack-years index.
Results
446 patients were recruited. Seven of these patients (1.5%) were excluded due to incorrect data collection. In the end, 439 patients were included. describes the characteristics of the patients with COPD. On average, patients were 70 years old, primarily male, with a % predicted FEV1 of 55 ± 20 and more than one third of the sample were current smokers (35%). Looking at the GOLD 2017 categories, 142 (32%) were classified as GOLD A, 207 (48%) as GOLD B, 10 (2%) as GOLD C and 80 (18%) as GOLD D. 34% of patients had an mMRC <2 and 80% belonged to the groups with low risk of exacerbations. Group B showed a higher score on the Charlson comorbidity index (; Figure S1).
Figure 1. Charlson Comorbidity Index score according to GOLD 2017 categories. The figure illustrates Box and Whiskers plots: the big square represents the median and small square represents mean ± standard deviation.
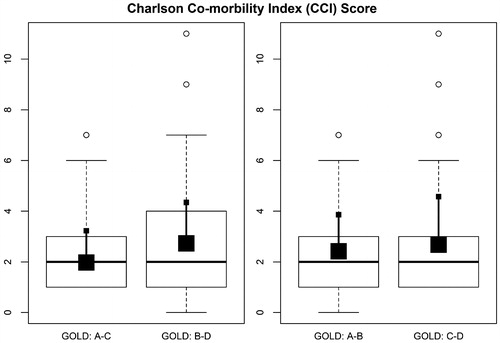
Table 1. Characteristics of the population with chronic obstructive pulmonary disease.
With regard to comorbidities, our patients show a high rate of cardiovascular comorbidity (). With regard to each of the comorbidities included in the COMCOLD score, the most symptomatic patients (B and D) showed a higher prevalence of depression, peripheral artery disease and heart disease, with a crude OR of 3.20 [CI95%: 1.45; 7.07], 2.31 [CI95%: 1.11; 4.84], and 3.35 [CI95%: 1.95; 5.75], respectively (). The risk of comorbidity was maintained after performing the logistic regression analysis adjusted for FEV1/FVC ratio and pack-years index (). Furthermore, no significant relationship was found between the comorbidities defined by the COMCOLD index and the groups with the greatest risk of exacerbation (C and D) after adjusting for FEV1/FVC ratio and pack-years index. With respect to the comorbidities not included in the score, the most symptomatic patients showed a higher presence of arrhythmia and chronic kidney disease with an adjusted OR of 2.74 [CI95%: 1.53; 4.89] and 2.96 [CI95%: 1.44; 6.07], respectively (). No relationship was detected with the groups with greatest risk of exacerbation.
Table 2. Prevalence and crude/adjusted odds ratios for comorbidities in relation to GOLD 2017.
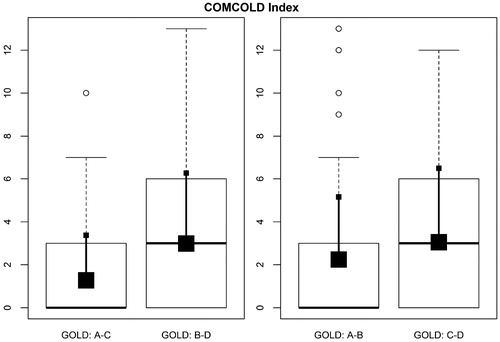
The range of COMCOLD scores obtained in our study population was 0–13 and the average score was 2.4 ± 3. 48% of the patients had a COMCOLD score >0 (range: 3–13). Of the 211 patients, 24% had a score of 3, 2% of 4, 14% of 6, 0.7% of 7, 2.3% of 9, 4% of 10, 0.5% of 12, and 0.5% of 13. According to the GOLD 2017 classification, 31% of patients classified as group A, 56% of those in group B, 40% in group C, and 57% in group D had a score of >0 (). The most symptomatic patients with COPD (B and D vs. A and C) had a higher score: 3 ± 3.3 vs. 1.3 ± 2.1 (p = 0.0001) (; Figure S2), in contrast with the groups with a higher risk of exacerbation (C and D vs. A and B) in which there was no significant difference: 3 ± 3.5 vs. 2.2 ± 3 (p = 0.055) (). In the multivariate analysis, belonging to the most symptomatic groups significantly increased the total COMCOLD score, multiplying on average by 2.4 (p < 0.001), adjusted for the remaining variables. The increase in COMCOLD score in patients with the greatest risk of exacerbation was not significant (p = 0.37).
Discussion
The following can be concluded from our study: (1) Nearly 50% of patients with COPD have an altered health status according to COMCOLD score. (2) Those patients with COPD in the GOLD 2017 groups with higher grades of dyspnea (B and D) show a greater effect on health status and a higher number of comorbidities. (3) Depression, peripheral artery disease, and heart disease are the comorbidities most closely related to the most symptomatic groups (B and D).
Health-related quality of life (HRQoL) shows the impact of a disease and its treatment on the subject’s lifestyle (Citation25). Furthermore, functional status reflects the ability to complete everyday physical, emotional and social tasks (Citation26). These two terms – HRQoL and functional status – are often used indiscriminately, despite the fact that they represent incomparable concepts that at times are imperfectly related (Citation25, Citation27). Finally, in its most general interpretation, health status refers to the set of effects and consequences that an individual’s deteriorating health status has on their ability to carry out and enjoy daily activities.
In recent years, interest in the impact diseases have on the health status of patients with COPD has taken center stage, particularly due to the discrepancy between subjective data indicated by patients and objectively measurable data. Factors such as the presence of respiratory distress, nutritional status, disease evolution status, socioeconomic level, and the ability to exercise seem to be related to HRQoL in patients with COPD beyond the mere chronic limitation of airflow (Citation28).
With respect to ability to exercise, the grade of dyspnea is a decisive factor in its evaluation. Dyspnea, the main symptom of COPD and reason most patients seek medical attention, encompasses both qualitative and quantitative aspects, resulting in a considerable heterogeneity with which this disease can present (Citation29). Activity is more restricted in those patients with greater dyspnea and the disease has a greater impact on their daily life (Citation30–34). The HEED study (Citation35) showed that dyspnea is present even in the mildest stages of the disease and, along with comorbidities, is one of the factors closely linked to health status measured both through the St. George’s Respiratory Questionnaire (SGRQ) (Citation36) and the CAT questionnaire (Citation23). Consistent with these results, Lee et al. (Citation16) described that in patients with COPD and mild-moderate chronic airflow obstruction, factors such as age, active tobacco use, dyspnea and the number of comorbidities (primarily congestive heart failure, dyslipidemia, and depression) were significantly associated with a higher score on the SGRQ. With respect to comorbidities, Frei et al. (Citation15) validated the COMCOLD score, determining how the presence of depression, anxiety, cerebrovascular disease, peripheral artery disease, heart failure, and ischemic heart disease impacted health status in patients with COPD, showing a good correlation with each of the components on the Chronic Respiratory Questionnaire (CRQ) [Pearson correlation coefficient: CRQ dyspnea: –0.25 (–0.34, –0.16); CRQ fatigue: –0.38 (–0.46, –0.30); CRQ emotional: –0.49 (–0.56, –0.41); CRQ mastery: –0.35 (–0.43, –0.26)].
In our study, those patients with a greater degree of dyspnea (mMRC >1) showed a greater probability of having an altered health status, showing that certain comorbidities (in this case, cardiovascular disease and psychiatric disorders) influence symptomatology in our patients. These results coincide with several publications. Burgel et al. (Citation3) described dyspnea as the main factor associated with the SGRQ score, with depression and ischemic heart disease being the main comorbidities involved in worse health status. Along the same lines, Bacells et al. (Citation14) reported that anxiety/depression worsened average HRQoL according to the SGRQ. Ng et al. (Citation37) demonstrated that depression (but not anxiety) was related to a decrease in HRQoL after adjusting the analysis for both the degree of dyspnea and for hospitalizations, results which are similar to those described by Burgel et al. (Citation3). With regard to cardiovascular disease, Jones et al. (Citation12) stated that patients with COPD and cardiovascular disease showed a modest increase in SGRQ score compared to subjects without said comorbidities. This difference was statistically significant but did not reach minimal clinically important difference. Consistent with this study, Burguer et al. (Citation3) found that ischemic heart disease impacted HRQoL, although its effect was modest. Similarly, Patel et al. (Citation38) noted that stable patients with COPD and ischemic heart disease had a worse health status, worse ability to exercise and greater degree of dyspnea. In light of these results, having determined that those patients with a greater degree of dyspnea show a higher COMCOLD score seems reasonable, implying the relevant role these comorbidities play in the development of symptomatology as well as their impact on health status. Depression, peripheral artery disease and heart disease were the main comorbidities associated with groups with a greater degree of dyspnea, results similar to those obtained by Kahnert et al. (Citation18) where mental illness and ischemic heart disease were associated with the most symptomatic patients with COPD (GOLD 2017 group B + D vs. A + C) with an OR of 1.58 (CI95%: 1.253–2.007; p ≤ 0.001) and 1.90 (CI95%: 1.452–2.493; p ≤ 0.001), respectively. However, unlike the above mentioned study, we found no increase in cardiovascular comorbidity or psychiatric disorders in the groups with greatest risk of exacerbation (C + D) when compared to the groups with lower risk (A + B).
Furthermore, the average COMCOLD score obtained in this study was lower than that described in the literature. Pérez-Manchón et al. (Citation20) obtained an average score of 5.29 points (CI95%: 4.3–6.2), which could be related to the high prevalence of depression/anxiety found in their sample (36% and 30%, respectively). A study published by González-Gutierrez et al. (Citation39) describes a psychiatric comorbidity prevalence of 36% in stable patients with COPD (pure anxiety disorder: 19%, only depression: 9.8%, and mixed anxiety and depression: 7.3%) with an infradiagnosis rate of 76%. Our figures are lower than those described, which may be related to the low proportion of women included in the sample (21%) with respect to that described in the study by Pérez-Manchón et al. (32.8%) (Citation20). Female patients appear to be more prone to psychological impairment, which correlates well with some specific symptomatic aspects of the disease, such as dyspnea (Citation40). This difference may also be due to a low infradiagnosis rate for psychiatric comorbidities as a result of not using specific questionnaires for their detection. An article published by Miravitlles et al. (Citation41) states that 74.6% of stable patients with COPD treated in outpatient clinics in Spain suffer from depression according to Beck's Depression Inventory (BDI).
These results lead us to consider the following: documents on the management of COPD classify disease severity and treatment according to patient symptoms, the degree of functional limitation and the number of exacerbations, based on results from clinical trials whose perspective is far from reality in which a large number of patients with COPD and relevant comorbidities are excluded (Citation42). Not taking these comorbidities into account can lead to error since the degree of dyspnea, effort limitation and even degree of functional affectation may be related to factors beyond obstruction (Citation9, Citation38, Citation43–45). In our sample, group B had the highest COMCOLD score and Charlson comorbidity index. Han and coworkers (Citation46) suggest that cardiovascular comorbidities are more frequent in groups B and D and can explain breathlessness more than COPD, particularly in patients with borderline lung function (FEV1/FVC <0.7 but > lower limit of normal [LLN]). Multiple studies showed GOLD classification led to more false positives and LLNs to more false negative diagnoses, especially in the elderly (Citation46–50). These patients with borderline lung function could “erroneously” be diagnosed with COPD and consequently be incorrectly treated since their symptoms are primarily related to the presence of cardiovascular disease (Citation46). In this sense, GOLD 2017 makes a timid though groundbreaking recommendation with regard to the possibility of de-escalation of bronchodilator therapy in those group B patients who do not show improved dyspnea after starting treatment with dual bronchodilator therapy, suggesting a return to monotherapy (Citation19).
The strength of our study lies in that it is the first to evaluate the relationship between COMCOLD score and GOLD 2017 group (according to symptoms/exacerbations), focusing on the importance of comorbidities in symptomatology and health status in patients with COPD and, consequently, their classification in the GOLD scheme. With regard to limitations, the characteristics of our study (cross-sectional) do not allow for causal relationships between comorbidities and/or COMCOLD scores and GOLD 2017 groups. Additionally, there may be an information bias since information was recorded from patient EHRs. However, it is important to keep in mind that the current standardization of diagnostic criteria minimizes the difference in criteria among the studies consulted. Furthermore, given that the prevalence of depression and anxiety was lower than that described in the literature, it is very likely that there is a high infradiagnosis rate for mental illness in our patients, which may have influenced the low overall COMCOLD score. Moreover, we must also highlight that the value of the COMCOLD index as an indicator of the health impact of comorbidities in patients with COPD has not been established in other populations beyond Swiss and Dutch patients with COPD. Finally, in our study, the level of patients’ symptoms was assessed using the mMRC and not CAT. The instrument used to determine the level of symptoms could influence the distribution of patients (Citation46).
To conclude, the greatest effect on health status according to COMCOLD score was found in those patients with COPD belonging to the most symptomatic GOLD 2017 groups (B and D), with depression, peripheral artery disease and heart disease being the main comorbidities involved. Furthermore, given that dyspnea has a multifactorial component, we must question any recommendation that supports an increase in treatment without first evaluating factors such as comorbidities which can impact COPD symptomatology.
Declaration of interest
All authors declare that there is no conflict of interest or funding in this manuscript.
Supplemental Material
Download PDF (132 KB)References
- Miravitlles M, Mario C, Guerrero T, Gisbert S. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest. 2003;123:784–791.
- García-Olmos L, Alberquilla A, Ayala V, García-Sagredo P, Morales L, Carmona M, de Tena-Dávila MJ, Pascual M, Muñoz A, Salavdor CH, et al. Comorbidity in patients with chronic obstructive pulmonary disease in family practice: a cross sectional study. BMC Fam Pract. 2013;14:11.
- Burgel PR, Escamilla R, Perez T, Carré P, Caillaud D, Chanez P, Pinet C, Jebrak G, Brinchault G, Court-Fortune I, et al. Impact of comorbidities on COPD-specific health-related quality of life. Respir Med. 2013;107:233–241.
- Van Manen JG, Bindels PJ, IJzermans CJ, van der Zee JS, Bottema BJ, Schadé E. Prevalence of comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J Clin Epidemiol. 2001;54:287–293.
- Crisafulli E, Costi S, Luppi F, Cirelli G, Cilione C, Coletti O, Fabbri M, Clini EM. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax. 2008;63:487–492.
- Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med. 2000;160:2653–2658.
- Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1:73–83.
- Divo M, Cote C, de Torres JP, Casanova C, Marín JM, Pinto-Plata V, Zulueta J, Cabrera C, Zagaceta J, Hunninghake1 G, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–156.
- Miller J, Edwards LD, Agustí A, Bakke P, Calverley PM, Celli B, Coxson HO, Krim C, Lomas DA, Miller BE, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107:1376–1384.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Almagro P, Soriano JB, Cabrera FJ, Boixeda R, Alonso-Ortiz MB, Barreiro B, Díaz-Manglano J, Mario C, Heredia JL. Short and medium-term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest. 2014;145:972–980.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, Pérez T, Soler-Cataluña JJ, van Der Molen T, Adamek L, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med. 2011;105:57–66.
- de Torres JP, Casanova C, Hernández C, Abreu J, Montejo de Garcini A, Aguirre-Jaime A, Celli B. Gender associated differences in determinants of quality of life in patients with COPD: a case series study. Health Qual Life Outcomes. 2006;4:72.
- Balcells E, Gea J, Ferrer J, Serra I, Orozco-Levi M, de Batlle J, Rodriguez E, Benet M, Donaire-González D, Ante JM, et al. Factors affecting the relationship between psychological status and quality of life in COPD patients. Health Qual Life Outcomes. 2010;8:108.
- Frei A, Muggensturm P, Putcha N, Siebeling L, Zoller M, Boyd CM, ter Riet G, Puhan MA. Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J Clin Epidemiol. 2014;67:904–911.
- Lee H, Jhun BW, Cho J, Yoo KH, Lee JH, Kim DK, Lee JD, Jung KS, Lee JY, Park HY. Different impacts of respiratory symptoms and comorbidities on COPD-specific health-related quality of life by COPD severity. Int J Chron Obstruct Pulmon Dis. 2017;12:3301–3310.
- Putcha N, Puhan MA, Hansel NN, Drummond MB, Boyd CM. Impact of co-morbidities on self-rated health in self-reported COPD: an analysis of NHANES 2001-2008. COPD. 2013;10:324–332.
- Kahnert K, Alter P, Young D, Lucke T, Heinrich J, Huber RM, Behr J, Wacker M, Biertz F, Watz H, et al. The revised GOLD 2017 COPD categorization in relation to comorbidities. Respir Med. 2018;134:79–85
- Global strategy for the diagnosis, management and prevention of COPD. Global initiative for chronic obstructive lung disease (GOLD); 2017. Available from: http://www.goldcopd.org.
- Pérez-Manchón D, Álvarez-García GM. Comprehensive assessment of chronic obstructive pulmonary disease in primary care. Quality of life and mortality associated comorbidity. Aten Primaria. 2017;49:255–256.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest.1988;93:580–586.
- Global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD); 2009. Available from: http://www.goldcopd.org.
- Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654.
- Zhang B, Bilder C, Biggerstaff B, Schaarschmidt F, Hitt B, binGroup. 2018: Evaluation and Experimental Design for Binomial Group Testing. R Package Version 2.1–1.
- Spilker B, Revicki DA. Taxonomy of quality of life. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia: Lippincort-Raven; 1996. p. 25–31
- Curtis JR, Martin DP, Martin TR. Patient-assessed health outcomes in chronic lung disease: what are they, how do they help us, and where do we go from here? Am J Respir Crit Care Med.1997;156:1032–1039.
- Patrick DL, Kinne S, Engelberg RA, Pearlman RA. Functional status and perceived quality of life in adults with and without chronic conditions. J Clin Epidemiol. 2000;53:779–785.
- de La Fuente Cid R, de La Iglesia Martínez F, Ramos Polledo V, Pellicer Vázquez C, Nicolás Miguel R, Diz-Lois Martínez F. Factor analysis of the health related quality of life of patients with stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2001;37:411–416.
- Vestbo J, Agusti A, Wouters EF, Bakke P, Calverley PM, Celli B, Coxson H, Crim C, Edwards D, Locantore N, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189:1022–1030.
- Ketekaars CAJ, Schlösser MAG, Mostert R, Huyer Abu-Saar H, Halfens RJG, Wouters EF. Determinants of health-related quality of life in patients with chronic obstructive pulmonary disease. Thorax. 1996;51:39–43.
- Miravitlles M, Molina J, Naberan K, Cots JM, Ros F, Llor C; EVOCA study. Factors determining the quality of life of patients with COPD in primary care. Ther Adv Respir Dis. 2007;1:85–92.
- Shavro SA, Ezhilarasu P, Augustine J, Bechtel JJ, Christopher DJ. Correlation of health-related quality of life with other disease severity indices in Indian chronic obstructive pulmonary disease patients. Int J Chron Obstruct Pulmon Dis. 2012;7:291–296.
- Reardon JZ, Lareau SC, ZuWallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Am J Med. 2006;119(10 suppl 1):32–37.
- Monteagudo M, Rodriguez Blanco T, Llagostera M, Valoro C, Bayona X, Ferrer M, Miravitlles M. Factors associated with changes in quality of life of COPD patients: a prospective study in primary care. Respir Med. 2013;107:1589–1597.
- Jones PW, Brusselle G, Dal Negro RW. Patient-centred assessment of COPD in primary care: experience from cross sectional study of health-related quality of life in Europe. Prim Care Respir J. 2012;21:329–336.
- Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved COPD specific version of the St. George Respiratory Questionnaire. Chest. 2007;132:456–463.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60e7.
- Patel ARC, Donaldson GC, Mackay AJ, Wedzicha JA, Hurst JR. The impact of ischemic heart disease on symptoms, health status, and exacerbations inpatients with COPD. Chest. 2012;141:851–857
- González-Gutiérrez MV, Guerrero Velázquez J, Morales García C, Casas Maldonado F, Gómez Jiménez FJ, González Vargas F. Predictive model for anxiety and depression in Spanish patients with stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2016;52:151–157.
- Di Marco F, Verga M, Reggente M, Maria Casanova F, Santus P, Blasi F, Allegra L, Centanni S. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respir Med. 2006;100:1767–774.
- Miravitlles M, Molina J, Quintano JA, Campuzano A, Perez J, Roncero C. Factors associated with depression and severe depression in patients with COPD. Respir Med. 2014;108:1615–1625.
- Wedzicha JA, Zhong N, Ichinose M, Humphries M, Fogel R, Thach C, Patalano F, Banerji D. Indacaterol/glycopyrronium versus salmeterol/fluticasone in Asian patients with COPD at a high risk of exacerbations: results from the FLAME study. Int J Chron Obstruct Pulmon Dis. 2017;12:339–349.
- García-Rio F, Soriano JB, Miravitlles M, Muñoz L, Duran-Tauleria E, Sánchez G, Sobradillo V, Ancochea J. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS One. 2014;9:e105220.
- Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, Kinney GL, McDonald MN, Brigham EP, Wise RA, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151:68–77.
- von Leupoldt A, Taube K, Lehmann K, Fritzsche A, Magnussen H. The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest. 2011;140:730–736.
- Han J, Dai L, Zhong N, Young D. Breathlessness or health status in chronic obstructive pulmonary disease: the impact of different definitions. COPD. 2015;12:115–125.
- Güder G, Brenner S, Angermann CE, Ertl G, Held M, Sachs AP, Lammers JW, Zanen P, Hoes AW, Störk S, et al. "GOLD or lower limit of normal definition? a comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study”. Respir Res. 2012;13:13.
- Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, Jensen RL, Falaschetti E, Schouten JP, Hankinson JL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051.
- Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: use percentage of FEV1/FVC ratio below the fifth percentile, not <70%. Chest. 2007;131:349–355.
- Schermer TR, Smeele IJ, Thoonen BP, Lucas AE, Grootens JG, van Boxem TJ, Heijdra YF, van Weel C. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur Respir J. 2008;32:945–952.