Abstract
Combining objective tools with clinical decision (CD) may help clinicians identify the priority for pulmonary rehabilitation (PR) in patients with COPD. We aimed to assess the specificity, sensitivity and efficiency of a new tool, the Pulmonary Rehabilitation Decisional Score (PRDS), and its correlation with the BODE index (BI) and CD in assigning PR priority. We retrospectively compared the three methods (CD vs. PRDS vs. BI) in 124 patients. We assigned low priority (LP), high priority (HP) and very high priority (VHP) to PR based on a priori scores of PRDS (LP = 0–10; HP = 11–17; VHP ≥18) and BI (LP = 0–2; HP = 3–5; VHP ≥6) and compared these with CD. PR priority assigned by the different methods was similar among groups, but did not often refer to the same subjects. PRDS and BI showed very high concordance with CD in defining VHP (97.8% and 95.6% for PRDS and BI, respectively), but were less concordant with CD in assigning LP and HP. Both PRDS and BI differently evaluated 38/124 cases compared to CD (PRDS underprescribed 18 and overprescribed 20; BI underprescribed 19 and overprescribed 19). However, a direct comparison between PRDS and BI showed that the discordance decreased to 8 underprescriptions and 10 overprescriptions (efficiency ∼85%). An objective instrument such as the PRDS can enhance CD with additional information on new aspects such as disability and fragility. PRDS and BI are nonetheless equally efficient at detecting discrepancies versus CD alone, especially when the priority for PR is defined as low or very high.
Keywords:
| Abbreviations | ||
| ADLs: | = | activities of daily living |
| BI: | = | BODE index |
| BMI: | = | body mass index |
| COPD: | = | chronic obstructive pulmonary disease |
| CD: | = | clinical decision by specialists; |
| CAT: | = | COPD Assessment Tool |
| FEV1: | = | forced expiratory volume |
| GPs: | = | general practitioners |
| HP: | = | high priority to rehabilitation |
| LP: | = | low priority to rehabilitation |
| MRC: | = | Medical Research Council |
| PR: | = | pulmonary rehabilitation |
| PRDS: | = | Pulmonary Rehabilitation Decisional Score |
| VHP: | = | very high priority to rehabilitation |
| 6MWT: | = | 6-minute walking test |
Introduction
A recent retrospective study (Citation1) evaluated with an ad-hoc 17-item instrument – the Pulmonary Rehabilitation Decisional Score (PRDS) – the assignment of priority for pulmonary rehabilitation (PR) made by the specialist during an outpatient visit. The idea of the Authors was to develop a multi-face tool able to provide a more structured indication for assigning PR priority in chronic obstructive pulmonary disease (COPD) patients (Citation2–Citation5). This is a particularly important question in the current period of limited resources, in which health providers need instruments to define the “priority setting” and “budget impact” of PR to cover the costs and optimize resources invested in this field (Citation6–Citation8). A PRDS score ≤10 was associated to low priority (LP) for rehabilitation, defined as access to PR after 60 days, PRDS scores 11–17 indicated a high priority (HP) for rehabilitation, defined as an access to PR within 30–60 days, and a PRDS score ≥18 indicated a very high priority (VHP), defined as admission to PR within 30 days (Citation1).
After the development of this tool, the second step was to compare the PRDS to other scales or indexes used in the clinical practice for patient assessment. The BODE index (Citation9) (BI) is a 4-item multidimensional scoring system and capacity index used to test patients who have been diagnosed with COPD and to predict long-term survival (Citation10–Citation15), hospital readmissions for acute exacerbations of COPD (AECOPD) (Citation11) with fewer prediction for COPD exacerbations treated at home (Citation16). Although BI was not designed specifically to assess the need for PR, it investigates parameters that are closely influenced by PR (Citation17). Thus, the BI score can be used, similarly to PRDS, as an index for assigning PR priority.
The present study is a post-hoc analysis on data from the previous study (Citation1), aimed to: 1) correlate the levels of PR priority obtained with PRDS, BI and routine clinical decision (CD); 2) compare and correlate PRDS and BI to each other; and 3) test the sensitivity, specificity and effectiveness of each tool in assigning PR priority.
Methods
This was a retrospective study, approved by our local Scientific Board (CTS 24/11/2015) and by the Ethics Committee of Istituti Clinici Scientifici Maugeri IRCCS (EC#2017). It consisted of a post-hoc analysis of data collected in a two-month period in a previous study (Citation1) on the appropriateness of decision on priority to PR access as measured by the PRDS.
Measurements
The patient population has been described elsewhere (Citation1). Data were retrieved from specialist reports compiled at the outpatient visit using (when necessary) medical reports of respiratory functional status and exercise tolerance sent to the GP. Clinical data, forced expiratory volume (FEV1), clinical tools such as Medical Research Council (MRC) dyspnea scale and disease impact measured by the COPD Assessment Tool (CAT), exercise tolerance measured by 6-min walk test (6MWT), disability, ADLs participation, adherence to treatment; anxiety and depression status; and frailty condition with level of care need were collected as described elsewhere (Citation1). All data were used to retrospectively calculate the PRDS score, which was then compared with the PR priority prescription made by the specialist in the report as follows: according to our current regional recommendations (i.e. Lombardy region), low priority (LP) for rehabilitation was defined as access to PR available not before 60 days, high priority (HP) for rehabilitation as access available within 30–60 days, and very high priority (VHP) for admission to rehabilitation as access available within 30 days. The PRSD score ranges from 0 to 34.
The BI (Citation9) score ranges from 0 to 10. For this study, following the same criteria used for assigning PRDS (Citation1), we identified a priori BODE scores for defining the three PR groups of priority (Citation1):
Group 1 or LP = this was the Low Priority group in which Rehabilitation was considered as NOT YET a priority, with access recommended after >60 days. It was assigned to PRDS scores ≤10 and BI scores ≤2 (<30% of maximum BI score).
Group 2 or HP = this was the High Priority group in which Rehabilitation was considered as a priority (YES) but with access not immediate, i.e. within 30-60 days (Outpatient clinic, day hospital). It was assigned to PRDS scores from 11 to 17 and BI from 3 to 5 (between 31% and 50% of maximum BI score)
Group 3 or VHP = this was the Very High Priority group where prompt Rehabilitation was recommended (YES) with admission within 30 days (i.e. Inpatient). It was assigned for PRDS score ≥18 and BI≥ 6 (>51% of maximum BI score)
In order to better compare concordances and discrepancies between the two methods, in particular for borderline cases with PRDS score close to 10 (9–12) or BI very near to 2 (2–3) – which can make the difference in assigning LP or HP rehabilitation priority – we introduced the definition of “close to decisional limit” (CDL).
Statistical analysis
We retrospectively analyzed 124 clinical records containing specialist clinical indication for rehabilitation as LP (n = 41), HP (n = 37) and VHP (n = 46) and compared these indications to the priority classification for access to rehabilitation as based on PRDS and BI.
All data were evaluated for Gaussian distribution (Shapiro–Wilk test) before applying statistical analysis using the Graph Pad Software (Prism 4) and the R programming language (Citation18). Depending on this result, Student’s t test or Wilcoxon test was used for comparison between groups. One-way ANOVA was applied for comparing three groups or more. If ANOVA was significant, the Holm–Bonferroni post hoc method was used to confirm differences between groups. A p value <0.05 was considered statistically significant. For comparisons between CD, PRDS and BI, in terms of LP, HP and VHP frequency, Pearson chi-squared test was used, with Monte Carlo correction in the case of low values. A p value <0.05 was considered as significant.
Results
shows a similar distribution between PRDS, BODE and CD in identifying rehabilitation priority. However, the three decisional instruments did not often describe the same subjects. For details, see appendix.
Figure 1. Panel A shows PRDS score distribution with respect to clinical evaluation at entry for each type of “setting” (LP, HP and VHP; for both LP vs. VHP and HP vs. VHP p < 0.0001); Panel B refers to BI distribution with respect to clinical evaluation at entry for each type of “setting” (LP, HP and VHP; for both LP vs. VHP and HP vs. VHP, p < 0.0001). Legend: The horizontal lines represent PRDS or BI decisional values. Box plots refer to median value and interquartile ranges. Circles indicate outliers.
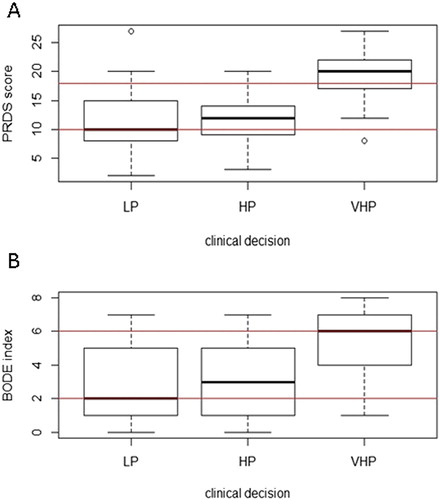
Table 1. Distribution of patient population according to PRDS, BI and Clinical decision (CD)
Both PRDS and BI showed a very high concordance with CD in the VHP group: 45/46 cases for PRDS (97.8%) and 44/46 cases for BI (95.6%). On the contrary, both tools showed differences with respect to CD in assigning patients into LP and HP classes. Concordances and discrepancies in attributing patients to the different groups of allocation by the three different tools are shown in and , respectively. To facilitate comparison between groups, we decided to consider HP and VHP as a single positive group of priority for rehabilitation <60 days (Rehabilitation YES) versus LP (Rehabilitation NOT YET). Concordances () were analyzed in terms of sensitivity, specificity and efficiency, first considering CD as the reference against which to compare PRDS and BI, and then considering PRDS vs. BI. Also for discrepancies (), interpreted as overprescription and underprescription of one tool versus the other, firstly we considered CD as the reference against which to compare PRDS and BI and then PRDS versus BI. With respect to CD, several underprescriptions and a few overprescriptions resulted as CDL. When we compared directly PRDS to BI the number of over and underprescriptions decreased, as a proof of the good correlation between the two methods (R = 0.7495, p-value <0.0001). In particular, out of 18 discrepancies 12 were CDL for PRDS (for details see appendix).
Table 2. Concordance between clinical decision, PRDS, BI in terms of Specificity (concordance in indicating LP, i.e. all real negative), Sensitivity (concordance in indicating HP or VHP, i.e. all real positive) and Efficiency (total concordance).
Table 3. Details for data which were differently evaluated by the tools: comparison of PRDS and BI with clinical decision and between PRDS and BI
Table 1 appendix: Efficiency, underprescription and overprescription of PRDS vs BI.
shows PRDS score (Panel A) and BI score (Panel B) with respect to CD (LP, HP and VHP). Median PRDS score for LP was 10, for HP 12 and for VHP 20. Differences were observed for LP versus VHP and HP versus VHP (both p < 0.0001). Median BI score was 2 in LP, 3 in HP and 6 in VHP setting. As for PRDS and with the same statistical significance, differences were observed for LP versus VHP and HP versus VHP (both p < 0.0001).
shows correlation plot between PRDS score and BI where patients are graded for PR priority by Clinical Decision and allocated in different sectors of the plot on the basis of PRDS and BODE PR priority.
Figure 2. Correlation plot between PRDS score and BI. Patients graded for PR priority by Clinical Decision, are allocated in different sectors of the plot on the basis of PRDS and BODE PR priority. Legend: cases are represented as signs in a scatter plot. Different signs and colors have been used for the three groups of rehabilitation priority according to CD: group 1 (white circle) for LP; group 2 (grey triangle) for HP and group 3 (black circle) for VHP. The oblique black line is the Regression line PRDS score (y) versus BI (x). Horizontal blue lines identify PRDS score limits (continue for LP-HP and dashed for HP-VHP). Vertical blue lines identify BI limits (continue for LP-HP and dashed for HP-VHP). The four blue lines split the plot into nine different sectors indicating PR priority by PRDS and BI: A. Discordance VHP PRDS-LP BI; B. Discordance HP PRDS-LP BI; C. Concordance LP; D. Discordance VHP PRDS-HP BI; E. Concordance HP; F. Discordance LP PRDS-HP BI; G. Concordance VHP; H. Discordance HP PRDS-VHP BI; I. Discordance LP PRDS-VHP BI.
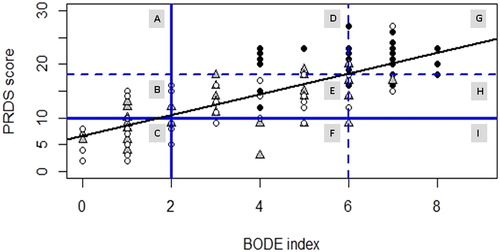
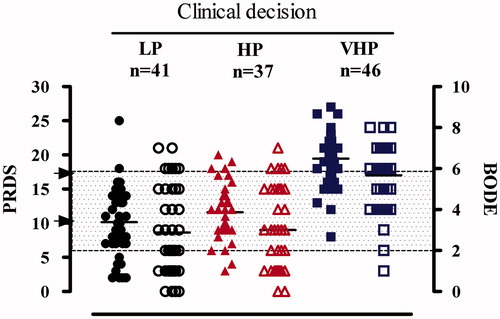
Figure 1 appendix shows clinical evaluation at entry for each type of “setting” (LP, HP and VHP) and the corresponding PRDS score and BI. Single dots represent cases (filled shapes for PRDS and empty form for BI) and black lines represent the median values within the group. Grey box defines areas for BI score: ≤2 white; 2–6 dots; ≥6 white; black arrows indicate cut off (for PRDS scores).
The regression line (m = 1.9437, q = 6.6094, R-squared = 0.5618, R = 0.7495, p < 2.2e-1) shows a good correlation between the two tools. The two horizontal and two vertical blue lines split the plot into nine different sectors (named A to I), identifying concordance or discordance in assigning rehabilitation priority by PRDS and BI. Details for interpretation are reported in the legend to . Underprescription by PRDS with respect to BI was observed in 22 cases (17.7% of the whole sample): PRDS estimated 8/22 patients as LP in contrast to BI’s estimate of HP/VHP while in the remaining 14 cases PRDS assigned HP in contrast to BI’s estimate of VHP. Overprescriptions of PRDS versus BI were found in 24 cases (19.3% of the whole sample): for 10/24 PRDS estimated HP/VHP while BI estimated them as LP, while the remaining 14 were graded by PRDS as VHP and instead by BI as HP ().
Discussion
The need for rehabilitation strategies to optimize function and reduce disability in COPD patients is a clear priority and a challenge for researchers (Citation19). Despite overwhelming evidence of benefits obtained from rehabilitation, a widespread implementation of PR is lacking and the ‘landscape’ of multidisciplinary programs remains very scattered with a consequent risk of restricted access available only for a limited number of patients (Citation20–24). Indeed, PR for COPD patients could be offered in hospital gyms, community centers or at home and could be provided irrespective of the availability of a structured education program (Citation25).
However, PR resources, when available, are underutilized and only severe COPD patients commonly receive rehabilitation (Citation26), and more than 1/3 of COPD patients drop out of programs (Citation26). The main causes are related to patients’ motivation and transport. The patients who drop out are those with worse functional tests, more exacerbations, steroid use and smoking habit (Citation27): for this reason, a more accurate patient selection grading the priority for access to rehabilitation/programs could be useful to the healthcare system. To adequately identify patients’ level of priority for PR programs, there is a logical need for multidimensional diagnostic, clinical, and functional tools based on criteria of respiratory function, comorbidities, disability, and frailty (Citation7). The efficacy of PR is closely related to a good subject selection, choosing the patient with the highest potential benefits (Citation5) and respecting a priority access list. However, in the real world, the choice of the most appropriate candidates for PR and of the appropriate setting in which to conduct PR often depends on funding schemes, the patient’s willingness to undergo/continue a program, the length of the waiting lists, socioeconomic reasons, and so on (Citation28–31). Hence, the selection of candidates for PR should not be based only on respiratory function but rather on a global assessment of the extrapulmonary consequences of COPD, proven to be reversible with rehabilitation (Citation5). The literature suggests that the ideal candidate for rehabilitation is a symptomatic subject with impaired functional status and low participation in ADLs, high utilization of healthcare resources, and suffering from the systemic consequences of COPD (Citation5).
The proposed PRDS score, based on findings from the literature and our own clinical experience, considers the patient from a holistic point of view in terms of lung function, disability, clinical severity, and psychological, behavioral and frailty criteria. This has produced a 17 item-score allowing to grade more appropriately the priority for PR (Citation1), and consequently ensuring a better allocation of health resources.
From our previous data, an adequate PR prescription was clearly more frequent when the clinical decision suggested a very high priority for rehabilitation. On the contrary, some discrepancies arose when the specialist had to make a decision in cases of either a low or high priority. Underprescription for PR access occurred in 19% of patients and was more evident in the group proposed for the LP list, while overprescription occurred in 25% of the sample and was more frequent in the HP group (Citation1).
This paper has demonstrated that both PRDS and BI evaluation, with similar efficiency (around 70%), show discrepancies with respect to clinical decision, which suggests many more overprescriptions for rehabilitation. However, if PRDS and BI are compared directly one to the other, they show a higher efficiency (about 85%) indicating that the respective discrepancies versus clinical decision mostly occur in common cases and result in PRDS-BODE concordances. This is true especially considering LP and VHP indications.
PRDS underprescribed 17.7% of BI priority indications, mainly suggesting a lower priority access (HP) with respect to VHP access by BI. On the contrary PRDS indicated that 19.3% of patients would have been granted access to rehabilitation with more urgency with respect to BI allocation (PRDS overprescriptions) (). The explanation for these differences between PRDS and BI was related in most cases to situations where scores were very close to the decision-making levels established to differentiate LP, HP and VHP. These were situations for which the evaluation of the clinician became decisive, guiding the final decision for rehabilitation priority.
Very few cases of discordances not close to the decision-making levels between PRDS and BI occurred, and are presented in Appendix. The possible reasons for the different allocation between PRDS and BI to PR can be understood in the light of the different range attributed to some functional items by the two scales. There are common items between PRDS and BI (respiratory function parameters, disability and BMI) that could justify the agreement between the two methods. However, it is important to underline that these parameters have different scoring criteria and thresholds, so they "weigh" differently in the calculation of the total score (i.e. the BMI item in the BI only differentiates the possible critical situation of underweight, while PRDS also considers the other extreme, i.e. overweight).
This study is the first to show that the PRDS scale may be very useful in the rehabilitation field, because it evaluates other parameters in addition to the respiratory, clinical and disability parameters (considered in the BI), that are fundamental to help the clinician in deciding the rehabilitation priority. Considering such PRDS parameters, the clinician could decide to more urgently rehabilitate a patient who is unstable due to depression, lifestyle factors, or number of exacerbations, even though less critical from a respiratory, disability and clinical function point of view (i.e. with a lower rating on BI).
A very important conclusion from our study is that when PRDS and BI, easily obtained from historical parameters and clinical information gathered during the outpatient visit, suggest the same PR priority, the respiratory specialist can, using these tools, confirm the appropriateness of this choice. The time and resources thus saved can be dedicated by clinicians to evaluate with more attention the cases of discordant priority between the two tools, so as to identify patients with particular needs and requiring a more “tailored” setting.
Possible limitations of our study are its retrospective design, the ad-hoc development of the decisional tree, and the (sometimes) arbitrary choice of cut-points used, without a prospective validation cohort. A prospective study is needed to evaluate patient follow-up and to verify if the choice of rehabilitation priority, guided by PRDS as a complement to BI, leads to positive and lasting results for the patient.
Conclusions
The new PRDS score might be a complement to the BI in helping the clinician to assign rehabilitation priority. While the PRDS includes some parameters common to the BI, it presents different methods of interpretation (cut-offs) and contains additional parameters such as disability and fragility that can guide the clinician in defining the rehabilitation priority. The two instruments, nevertheless, have good correlation and are equally effective at detecting discrepancies versus clinical decision alone, especially when the priority for PR is defined as low or very high. Future studies are needed to prospectively validate the internal consistency of the PRDS items in relation to PR benefits and outcome, and to compare the efficacy of PR priority decisions based on the 3 methods combined (PRDS, BI and clinical decision) vs. based on clinical decision alone. In future studies, we will also compare if the PRDS will help us reduce costs in hospital stays or lower priority patient income.
Acknowledgements
The authors are grateful to prof. Bartolome Celli for his important suggestions to improve the manuscript and thank Rosemary Allpress for the English revision of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Vitacca M, Comini L, Barbisoni M, Francolini G, Paneroni M, Ramponi JP. A pulmonary rehabilitation decisional score to define priority access for COPD patients. Rehabil Res Pract. 2017;2017:5710676. doi:10.1155//2017/5710676.
- Langer D, Hendriks E, Burtin C, Probst V, van der Schans C, Paterson W, Verhoef-de Wijk M, Straver R, Klaassen M, Troosters T, et al. A clinical practice guideline for physiotherapists treating patients with chronic obstructive pulmonary disease based on a systematic review of available evidence. Clin Rehabil. 2009;23(5):445–62. doi:10.1177/0269215509103507.
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4s–42s.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST.
- Spruit MA, Augustin IM, Vanfleteren LE, Janssen DJ, Gaffron S, Pennings HJ, Smeenk F, Pieters W, van den Bergh JJ, Michels AJ, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J. 2015;46(6):1625–35. doi:10.1183/13993003.00350-2015.
- Spruit MA, Pitta F, Garvey C, ZuWallack RL, Roberts CM, Collins EG, Goldstein R, McNamara R, Surpas P, Atsuyoshi K, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J. 2014;43(5):1326–37.
- Ambrosino N, Clini EM. Response to pulmonary rehabilitation: toward personalised programmes? Eur Respir J. 2015;46(6):1538–40. doi:10.1183/13993003.01125-2015
- Dal Negro RW, Celli BR. The BODECOST Index (BCI): a composite index for assessing the impact of COPD in real life. Multidiscip Respir Med. 2016;11:10. doi 10.1186/s40248-016-0045-4
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–12. doi:10.1056/NEJMoa021322.
- Celli BR, Cote CG, Lareau SC, Meek PM. Predictors of survival in COPD: more than just the FEV1. Respir Med. 2008;102 (Suppl 1):S27–S35.
- Ko FW, Tam W, Tung AH, Ngai J, Ng SS, Lai K, Au KF, Hui DS. A longitudinal study of serial BODE indices in predicting mortality and readmissions for COPD. Respir Med. 2011;105(2):266–73. doi:10.1016/j.rmed.2010.06.022.
- Moberg M, Vestbo J, Martinez G, Williams JE, Ladelund S, Lange P, Ringbaek T. Validation of the i-BODE index as a predictor of hospitalization and mortality in patients with COPD participating in pulmonary rehabilitation. COPD. 2014;11:381–7. doi:10.3109/15412555.2013.836171.
- Pedone C, Scarlata S, Forastiere F, Bellia V, Antonelli Incalzi R. BODE index or geriatric multidimensional assessment for the prediction of very-long-term mortality in elderly patients with chronic obstructive pulmonary disease? A prospective cohort study. Age Ageing. 2014;43(4):553–8. doi:10.1093/ageing/aft197
- Chen CZ, Ou CY, Yu CH, Yang SC, Chang HY, Hsiue TR. Comparison of global initiative for chronic obstructive pulmonary disease 2013 classification and body mass index, airflow obstruction, dyspnea, and exacerbations index in predicting mortality and exacerbations in elderly adults with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2015;63(2):244–50. doi:10.1111/jgs.13258.
- de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Divo M, Zulueta JJ, Berto J, Zagaceta J, Sanchez-Salcedo P, Cabrera C, et al. Prognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTE. Thorax. 2014;69(9):799–804. doi:10.1136/thoraxjnl-2014-205770.
- Motegi T, Jones RC, Ishii T, Hattori K, Kusunoki Y, Furutate R, Yamada K, Gemma A, Kida K. A comparison of three multidimensional indices of COPD severity as predictors of future exacerbations. Int J Chron Obstruct Pulmon Dis. 2013;8:259–71. doi:10.2147/COPD.S42769.
- Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26(4):630–6. doi:10.1183/09031936.05.00045505
- R Core Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available from: https://www.R-project.org/.
- Committee NIHMRC, O'Mara A, Rowland JH, Greenwell TN, Wiggs CL, Fleg J, Joseph L, McGowan J, Panagis JS, Washabaugh C, et al. National Institutes of Health Research Plan on Rehabilitation: NIH Medical Rehabilitation Coordinating Committee. Phys Ther. 2017;97 (4):104–407. doi:10.1093/ptj/pzx026
- Janssens W, Corhay JL, Bogaerts P, Derom E, Frusch N, Dang DN, Kibanda J, Ruttens D, Thyrion L, Troosters T, et al. How resources determine pulmonary rehabilitation programs: a survey among Belgian chest physicians. Chron Respir Dis. 2018;12:1479972318767732. doi:10.1177/1479972318767732.
- Arne M, Emtner M, Lisspers K, Wadell K, Stallberg B. Availability of pulmonary rehabilitation in primary care for patients with COPD: a cross-sectional study in Sweden. Eur Clin Respir J. 2016;3:31601. doi:10.3402/ecrj.v3.31601.
- Bowen JM, Campbell K, Sutherland S, Bartlett A, Brooks D, Qureshi R, Goldstein R, Gershon AS, Prevost S, Samis L, et al. Pulmonary rehabilitation in Ontario: a cross-sectional survey. Ont Health Technol Assess Ser. 2015;15(8):1–67.
- Camp PG, Hernandez P, Bourbeau J, Kirkham A, Debigare R, Stickland MK, Goodridge D, Marciniuk DD, Road JD, Bhutani M, et al. Pulmonary rehabilitation in Canada: a report from the Canadian Thoracic Society COPD Clinical Assembly. Can Respir J. 2015;22(3):147–52.
- Desveaux L, Janaudis-Ferreira T, Goldstein R, Brooks D. An international comparison of pulmonary rehabilitation: a systematic review. Copd. 2015;12(2):144–53. doi:10.3109/15412555.2014.922066.
- Alison JA, McKeough ZJ, Johnston K, McNamara RJ, Spencer LM, Jenkins SC, Hill CJ, McDonald VM, Frith P, Cafarella P, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology. 2017;22(4):800–19. doi:10.1111/resp.13025.
- Sundh J, Lindgren H, Hasselgren M, Montgomery S, Janson C, Ställberg B, Lisspers K. Pulmonary rehabilitation in COPD – available resources and utilization in Swedish primary and secondary care. Int J Chron Obstruct Pulmon Dis. 2017;12:1695–704. doi:10.2147/COPD.S135111.
- Almadana Pacheco V, Pavon Masa M, Gomez-Bastero Fernandez AP, Muniz Rodriguez AM, Tallon Moreno R, Montemayor Rubio T. Patient profile of drop-outs from a pulmonary rehabilitation program. Arch Bronconeumol. 2017;53(5):257–62. doi:10.1016/j.arbres.2016.06.010
- Nici L, ZuWallack RL. Pulmonary rehabilitation: future directions. Clin Chest Med. 2014;35(2):439–44. doi:10.1016/j.ccm.2014.02.015.
- Italian Health Ministry Report. Metodologia per la definizione dei criteri/parametri di appropriatezza ed efficienza dei ricoveri di riabilitazione ospedaliera. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2159_ulterioriallegati_ulterioreallegato_0_alleg.pdf Last access 10 August 2018.
- Vitacca M, Aliprandi G, Testa A, Facchi E, Errera D, Panizza F, Tassi G, Marinoni T, Scarcella C, Bettoncelli G. Il Governo Clinico della BPCO a Brescia: focus sulla Riabilitazione. Rassegna di Patologia dell'Apparato Respiratorio. 2012;27:34–40.
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, Spruit MA, Masefield S, Casaburi R, Clini EM et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–86. doi:10.1164/rccm.201510-1966ST.
Appendix
Table 1 (appendix) shows details on efficiency, underprescription and overprescription of PRDS vs. BI. To better investigate the discrepancies between PRDS and BI in cases which are not CDL, we considered and report here some of the parameters used to compute the PRDS score and BI.
