Abstract
The COPD assessment test (CAT) is a short questionnaire developed to help patients and clinicians to assess the impact of symptoms in routine clinical practice. We aimed to validate and to test the reproducibility of CAT in patients with bronchiectasis and correlate with the severity of dyspnea, aerobic and functional capacity, and physical activity in daily life. This is a cross-sectional study, patients with bronchiectasis underwent spirometry, cardiopulmonary exercise test (CPET), incremental shuttle walk test (ISWT), Saint George`s Respiratory Questionnaire (SGRQ), and received pedometer. CAT was applied twice (CAT-1 and CAT-2, 7 to 10 days apart). The severity of bronchiectasis was assessed by E-FACED and bronchiectasis severity index (BSI). A total of 100 patients were evaluated (48 ± 14 years, 59 women, FVC: 67 ± 22% pred, FEV1: 52 ± 25% pred). According to CAT, 14% patients presented low, 40% medium, 32% high, and 14% very high impact. The higher the CAT, the worse the severity of bronchiectasis, dyspnea, quality of life, performance on the CPET, and smaller the distance walked (DW) on the ISWT and number of steps (NS) per day. There was significant correlation between CAT and SGRQ, E-FACED, BSI, NS, ISWT, oxygen uptake, and workload at CPET. CAT-1 and CAT-2 presented similar values: 21 (13–26) and 19 (13–26), respectively. The CAT is a valid and reproducible instrument in patients with bronchiectasis presenting good correlation with clinical, functional, and quality of life measurements. This easy-to-use, easy-to-understand, quick, and useful tool may play an important role to assess the impact of bronchiectasis on both daily medical practice and clinical trial settings.
Introduction
Health-related quality of life (HRQL) is defined as the perception of the impact of health on an individual’s mood swings or satisfaction with life in areas they consider important (Citation1). Patients with bronchiectasis usually have an impaired in their HRQL due to a chronic pulmonary disease characterised by a productive cough, dyspnea, and reduced exercise capacity (Citation2–4).
Evaluation of HRQL is traditionally measured using questionnaires, and some have been validated in patients with bronchiectasis. The Saint George’s Respiratory Questionnaire (SGRQ) (Citation5) and Chronic Respiratory Disease Questionnaire (CRDQ) (Citation6) have been used for patients with bronchiectasis. Both evaluate relevant items of the disease, but they are lengthy questionnaires. The Leicester Cough Questionnaire (LCQ) is a quick questionnaire, but evaluates only the impact of cough (Citation7). There are two questionnaires designed specifically for patients with bronchiectasis. The Quality of Life Questionnaire for Bronchiectasis (QoL-B) (Citation8) that assess respiratory symptoms and physical, emotional, and functional condition, but it is also long questionnaire and often difficult understanding. The Bronchiectasis Health Questionnaire (BHQ) (Citation9) is a brief evaluation which includes aspects of daily life, sputum, and infection; however, the seven different possibilities of answer are difficult to interpret by the patient.
COPD assessment test (CAT) is a shorter, easy-to-complete, validated questionnaire based on the SGRQ (Citation10–13), recommended by GOLD (Citation14) to assess and quantify the impact of COPD symptoms in clinical practice, and sensible to changes in health status following exacerbations and after pulmonary rehabilitation (Citation15). Lee et al. (Citation16) performed the validity of the CAT for Korean bronchiectasis patients. However, the authors have used questionnaires for this validation (SGRQ and dyspnea questionnaire). The authors did not use a questionnaire for disease-specific symptoms, neither other clinical aspects, as exercise capacity.
As bronchiectasis is a disease with multidimensional clinical features similar to COPD (Citation17), the CAT could be an easily applied tool for evaluation of the impact of the disease on this population. However, before using it in bronchiectasis patients, the CAT has to be validated based on different clinical aspects. Thus, the objective of this study was to validate and test the reproducibility of the CAT questionnaire in patients with bronchiectasis and then correlate the scores with the severity of bronchiectasis, dyspnea, aerobic and functional capacity, and physical activity in daily life. The author’s hypothesis is that CAT is a valid test for bronchiectasis.
Methods
Subjects
From January 2013 to August 2015, patients were recruited from a tertiary referral university hospital. The inclusion criteria were as follows: diagnosis of bronchiectasis confirmed by high-resolution computed tomography, ≥18 years of age, and clinical stability (no change in medication dosage, quantity and/or colour of secretions, or symptoms of dyspnea in the last 4 weeks) (Citation17). Patients who had smoked more than 10 years, had severe cardiac disorders and/or other pulmonary diseases, were obese (body mass index [BMI] > 30 kg/m2), were unable to perform the functional and exercise evaluation tests, or had exacerbations between evaluations were excluded. The Institutional Ethical Committees has approved the study (#0921/11). All the procedures and any associated risks were described to the participants and informed consent was obtained from all patients.
Study design
This was a cross-sectional study composed of two days of evaluation. The first day, the volunteers performed spirometry and exercise evaluation tests (cardiopulmonary exercise test [CPET] and incremental shuttle walk test [ISWT]), answered the modified Medical Research Council (mMRC), SGRQ, and CAT-1, and received a pedometer. The severity of the disease was evaluated by E-FACED score. After 7–10 days, the second visit was conducted. Patients returned the pedometers and answered the CAT for the second time (CAT-2).
Assessments
Lung function
Spirometry was performed using the Ultima CPX system (MedGraphics Corporation®, St. Paul, MN). The technical acceptance criteria were performed according to previous guidelines (Citation18) and the variables were recorded in litres and percentages of the predicted value (Citation19).
COPD assessment test (CAT)
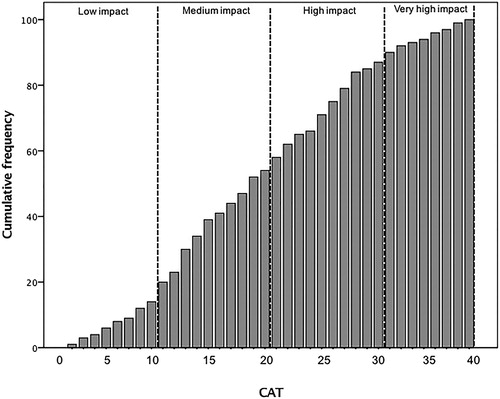
The CAT is a questionnaire developed for COPD patients (Citation10) and validated in Brazil (Citation12). It consists of eight items and the total scores range from 0 to 40, where 0 indicates no health-related quality of life impairment, and 40 indicates the greatest impact of the disease. The CAT is stratified in: 0–10 points as low, 11–20 points as medium, 21–30 points as high, and 31–40 points as very high to assess the impact of the disease in patient’s life (Citation13).
Saint George’s Respiratory Questionnaire (SGRQ)
This is a questionnaire developed to measure HRQL in COPD (Citation5, Citation20). The SGRQ has 50 items categorised into the following three domains: symptoms, activity, and impact. The total score ranges from 0 to 100, with 0 indicating no impairment of quality of life.
Modified Medical Research Council Scale (mMRC)
The mMRC scale evaluates the patients’ physical activities based on their dyspnea (Citation21). The total scores of MRC vary from 0 to 4 points. Higher scores indicate worse dyspnea.
E-FACED score
The E-FACED score was developed to evaluate the predictive capacity of exacerbations (Citation22) of bronchiectasis patients. This score was developed and validated in Latin America Countries (Citation22), based on FACED score (Citation23). The E-FACED consists of five variables: at least one severe exacerbation in previous year; FEV1, age, chronic colonisation by Pseudomonas aeruginosa, extension (number of lobes affected), and dyspnea (mMRC). The range of the E-FACED score is 0–9, and the higher the score, the greater the severity of disease. It is possible to define bronchiectasis as mild (score between 0 and 3), moderate (between 4 and 6), or severe (score between 7 and 9) (Citation22).
Bronchiectasis severity index (BSI)
The BSI is a combination of clinical, radiological, and microbiological features, is a strong predictor of morbidity and mortality and predicts one and four years/morbidity and mortality (Citation24) for patients with non-CF bronchiectasis. This assessment of severity tool was also found to give excellent predictions of hospital admissions, exacerbations, and quality of life. The score between 0 and 4 is considered mild bronchiectasis; 5–8 moderate bronchiectasis, and >9 is severe bronchiectasis (∼30% of mortality).
Cardiopulmonary exercise test (CPET)
The maximal incremental cycle ergometer test was carried out on an electromagnetically braked cycle ergometer (Corival®, LODE B.V. Medical Technology, Groningen, Netherlands) with gas exchange and ventilator variables analysed breath-by-breath (Medical Graphics Corporation-MGC, St. Paul, MN). The test was performed using a ramp protocol; the workload increasing was between 5 and 20 W per minute, according to patient’s capacity. The main outcomes for this test were pulmonary oxygen uptake (VO2, mL/minute) and peak workload (Watts).
Incremental shuttle walk test (ISWT)
The tests were conducted in a 10 m corridor. Two ISWTs were performed with at least 30 minutes of rest in between. The walking speed was dictated by an audio signal started at 0.5 m/s and progressively increased 0.17 m/s every minute according to a triple beep (Citation25). The distance walked (DW) was expressed in meters and in predicted values (Citation26). The test with the longest DW was used for correlation with the CAT.
Assessment of daily physical activity
The patients received the pedometer and were instructed to use them in the right pocket on the anterior surface of their clothes for five consecutive days during the weekdays. For analysis, the first and last days were discarded and daily number of steps (NS) was the mean over three consecutive days. Sedentary was considered when the NS walked was less than 5.000 steps/day, low level of physical activity when between 5.000 and 7.499 steps/day, moderate level of daily physical activity when between 7.500 and 9.999 steps/day, and good daily activity when the NS was higher than 10.000 per day (Citation27).
Statistical analysis
The sample size of 59 patients with bronchiectasis was based on a difference of 3.76 points (Citation10) and a variability of 7.3 (Citation12) between CAT-1 and CAT-2 measurements with the probability of type I error set as 5% and type II at 0.2. The final sample size was improved considering the correlations design for the study.
Data normality was analysed using the Shapiro–Wilk test. The CAT, mMRC, E-FACED, and the NS/day were expressed as median (interquartile range IQR), and other variables were expressed as means ± SD. To compare the CAT level of impact (low impact: 0–10, medium impact: 11–20, high impact: 21–30, very high impact: 31–40) and the predicted variables, ANOVA one way (post-hoc Bonferroni) or Kruskal–Wallis was performed. The Wilcoxon test was used to compare CAT-1 and CAT-2. Internal consistency was evaluated by Cronbach’s coefficient. The intraclass correlation coefficient (ICC) and its 95% of confidence interval (CI 95%) were used to evaluate the reliability of CAT. Additionally, Bland Altman analysis was performed between CAT-1 and CAT-2 values. The Spearman correlation between CAT-2, SGRQ, mMRC, E-FACED, DW at ISWT, NP, VO2, and workload at CPET was performed. The SPSS statistic package version 20 (SPSS Inc., Chicago, IL) was used. Statistical significance was considered as p < .05.
Results
A total of 150 patients were invited to complete the protocol evaluations, whose 42 were unavailable to perform all the evaluations and eight patients were excluded (one presented physical impairment, three had heart disease, and four were smokers). Then, 100 patients finished the protocol, 59 (59%) were women, and 17 were under home oxygen therapy. shows the patients’ characteristics.
Table 1. Patients characteristics.
Most of the patients presented with obstructive airflow limitation measured by spirometry and had a median of 2 on the mMRC scale. The NS showed 64% of the volunteers at sedentary or low physical activity in daily life and 36% of volunteers at active. Exercise capacity was reduced evaluated by VO2 and workload at the peak of CPET. According to E-FACED score, the majority of patients were classified as presenting mild/moderate severity and similar results were obtained when assessed by BSI ().
According to CAT scores, 14% of patients had low impact, 40% medium impact, 31% high impact, and 15% very high impact of the disease ().
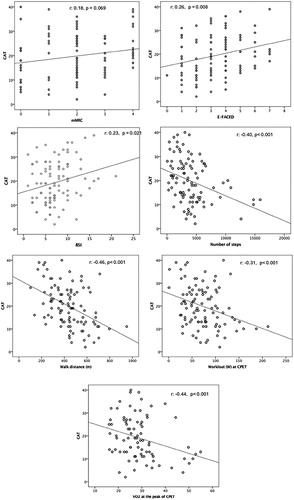
shows a reduction of exercise capacity and HRQL as CAT scores increase. The volunteers with low impact on the CAT had lower E-FACED scores, lower mMRC scores, better quality of life on the SGRQ, longer walked distance at the ISWT, better performance on the CPET, and more numbers of steps walked per day.
Table 2. Impact of CAT between the variables of different tests.
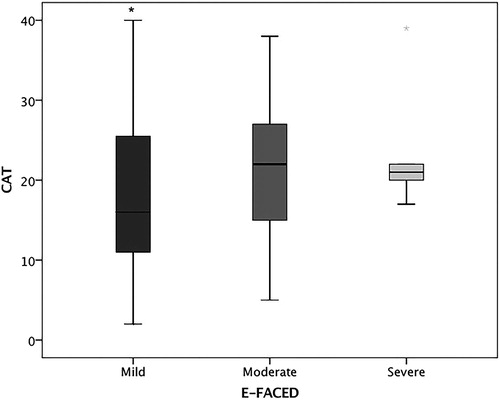
Five volunteers were categorised as E-FACED score severe, of those two had CAT medium impact, other two had CAT high impact, and one had CAT very high impact. The less severe patients at E-FACED had lower values on the CAT ().
Figure 2. CAT score according to bronchiectasis severity by E-FACED. *p<.05 vs. moderate and severe. Mild: E-FACED 0–3 points, moderate: E-FACED 4–6 points, and severe: E-FACED 7–9 points.
Of the total volunteers, 58% were infected by Pseudomonas aeruginosa. There was no difference at CAT between infected (17 [13–26]) and non-infected (20 [13–28]) volunteers (p = .19).
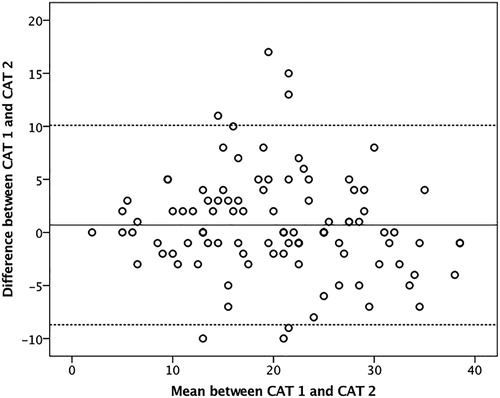
There was no difference between the median score of CAT-1 and CAT-2 (21 [13–26] vs. 19 [13–26], respectively), p = .20. The Cronbach’s Alpha was 0.91 between the total score of CAT-1 and CAT-2. The ICC was 0.84 (CI 95% 0.78–0.89), p < .001. All the ICCs for each domain were over than 0.78, p < .001. The Bland Altman analysis showed a mean bias and limits of agreement of 0.7 (CI 95% −8.7–10.1) expressed in .
Figure 3. Bland Altman plot. The solid line indicates the reference mean bias (0.7) and the dashed lines indicate the central mean bias and the upper and lower limits of agreement between the tests (CI 95% −8.7–10.1).
There were significant correlations between CAT and SGRQ total score (r = 0.74), SGRQ impact domain (r = 0.72), SGRQ activity domain (r = 0.61), SGRQ symptoms domain (r = 0.61), and p < .001. There were significant correlations between CAT and E-FACED score, BSI, NS, DW, workload, and VO2 at CEPT (). There was also significant correlation between CAT and FEV1 (%) (r = −0.28, p = .004) and FVC (%) (r = −0.33, p = .001).
Discussion
In this study, the CAT has proven to be a valid and reproducible questionnaire to evaluate patients with bronchiectasis. Concurrent validation of the CAT was made through good correlation with the SGRQ as has been observed in other studies in the COPD population (Citation10, Citation12).
Similar to our study, Lee et al. (Citation16) found good correlation (r = 0.72) between the SGRQ and the CAT in Korean patients with bronchiectasis, but the validation of the CAT was performed with SGRQ and dyspnea questionnaires. Additionally, there was a disproportion between the severities of CAT scores (75% of patients had CAT scores below 20 and less than 5% had scores between 30 and 40). In this study, we performed the CAT validation with several tools (CPET, ISWT, NS, SGRQ, mMRC) and had the majority of patients (71%) distributed among moderate (CAT: 11–20) and high impact (CAT: 21–30) of disease.
Significant correlations were also found between CAT and both BSI and E-FACED scores. These correlations were weak; however, it is important to emphasise that these tools represent different aspects of the disease. There was no significant correlation between the CAT and mMRC scale. This can be explained because mMRC is one-dimensional tool to assess dyspnea and may not explain all complexity of symptoms related to patients with bronchiectasis. It is plausible, therefore, to assume that these correlations are not linear. Nevertheless, our data suggest that CAT may be a better tool to be used in patients with bronchiectasis since better represent patient’s symptoms and also correlates to prognostic scores, keeping simplicity in its application, as described before (Citation22, Citation23).
Although FEV1 is used as a marker of severity of airway obstruction, it does not always reflect the clinical and functional condition of the patient. This fact justifies the correlation observed between lung function and the CAT. In this way, the use of scores with multidimensional aspects like the CAT, E-FACED, and BHQ is essential in the evaluation of patients with bronchiectasis.
The greater the impact of the disease on the patient’s life evaluated by the CAT, the worse the VO2 and the workload on the CPET, the shorter the DW during the ISWT, and the fewer the NS a day. Similar data was also observed by other authors (Citation4, Citation28). These correlations show that the CAT is associated with functional aspects, exercise capacity, and daily life activities.
The CAT was able to differentiate worse exercise capacity and quality of life according to its stratification in terms of the impact of the disease (mild, moderate, high, or very high impact). The results demonstrated that the higher the CAT score, the worse the dyspnea and quality of life assessed by SGRQ, the lower exercise capacity, and the fewer the NS in daily life. This result shows that the CAT, an easy-application questionnaire, properly discriminates the impact of the disease in several clinical aspects.
Excellent reproducibility of the CAT was observed within 7–10 days apart. The Bland Altman mean was close to zero (0.7) and the IC 95% (−8.7–10.1 points). Silva et al. (Citation12) observed IC narrower for the CAT in COPD (95% CI 0.4–4.6 to 5 points); however, a smaller number of individuals were evaluated. Our results show that the CAT has small variability, considering the mean difference between the tests. Additionally, 70% of the studied patients had the difference between CAT-1 and CAT-2 under the minimal clinically important difference, which is four points for COPD (Citation15) and three for other respiratory diseases (Citation29).
The CAT questionnaire is well established for use in COPD patients. For bronchiectasis patients, CAT has been successfully used to detect the healthy status after chest physiotherapy treatment (Citation29, Citation30). Additionally, this questionnaire has demonstrated to be responsive to changes at exacerbation, which might be useful in monitoring the patients (Citation31). However, based on our knowledge, only Lee et al. (Citation16) studied the CAT validity on patients with bronchiectasis. This is the first time that a multidimensional assessment was used to investigate the CAT’s validity in patients with bronchiectasis.
Study limitations
This study has some limitations. Despite the inclusion of a large sample with a wide variability of clinical and severity presentation, caution is recommended when the results are extrapolated to other populations and we suggest that further studies be performed to corroborate the use of CAT in bronchiectasis. No disease-specific HRQL questionnaire for bronchiectasis was used in this study. The choice to use the SGRQ instead of Qol-B based on the more widespread use of the first questionnaire in the studies focused on this disease. The BHQ had not been published at the time of conducting this study.
Conclusions
We concluded that after assessing multiple methods to evaluate functional capacity, quality of life, exercise, dyspnea, and physical activity in daily life with the CAT, it is a valid and reproducible instrument for measuring the impact of bronchiectasis on the patients’ life. Additionally, the categorised CAT scores were able to differentiate clinical and functional conditions of patients. Therefore, the CAT is an easy-to-use, easy-to-understand, quick, and a useful tool for assessment of the impact of bronchiectasis on patients’ life and may play an important role both daily medical practice and clinical trial settings.
Acknowledgements
This study research was conducted at Universidade Nove de Julho-UNINOVE.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Swigris J, Kuschner W, Jacobs S, Wilson S, Gould M. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax 2005;60(7):588–94. doi:10.1136/thx.2004.035220.
- Ozalp O, Inal-Ince D, Calik E, Vardar-Yagli N, Saglam M, Savci S, Arikan H, Bosnak-Guclu M, Coplu L. Extrapulmonary features of bronchiectasis: muscle function, exercise capacity, fatigue, and health status. Multidiscip Respir Med. 2012;7(3):1–6. doi:10.1186/2049-6958-7-3.
- Guan W, Gao Y, Xu G, Lin ZY, Tang Y, Li H, Lin Z, Zheng J, Chen R, Zhong N. Six-minute walk test in Chinese adults with clinically stable bronchiectasis: association with clinical indices and determinants. Curr Med Res Opin. 2015;31(4):843–52. doi:10.1185/03007995.2015.1013625.
- Bradley J, Wilson J, Hayes K, Kent L, McDonough S, Tully M, Bradbury I, Kirk A, Cosgrove D, Convery R, et al. Sedentary behaviour and physical activity in bronchiectasis: a cross-sectional study. BMC Pulm Med. 2015;13(61):1–10. doi:10.1186/s12890-015-0046-7.
- Wilson C, Jones P, O’Leary C, Cole P, Wilson R. Validation of the St. George’s respiratory questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):536–41. doi:https://doi.org/10.1164/ajrccm.156.2.9607083.
- Vodanovich D, Bicknell T, Holland A, Hill C, Cecins N, Jenkins S, McDonald C, Burge A, Thompson P, Stirling R, et al. Validity and reliability of the chronic respiratory disease questionnaire in elderly individuals with mild to moderate non-cystic fibrosis bronchiectasis. Respiration 2015;90(2):89–96. doi:https://doi.org/10.1159/000430992.
- Murray M, Turnbull K, MacQuarrie S, Pentland J, Hill A. Validation of the Leicester cough questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34(1):125–31. doi:10.1183/09031936.00160508.
- Quittner A, Marciel K, Salathe M, O’Donnell A, Gotfried M, Ilowite J, Metersky M, Flume P, Lewis S, McKevitt M, et al. A preliminary quality of life questionnaire-bronchiectasis: a patient-reported outcome measure for bronchiectasis. Chest. 2014;146(2):437–48. doi:10.1378/chest.13-1891.
- Spinou A, Siegert R, Guan W, Patel A, Gosker H, Lee K, Elston C, Loebinger M, Wilson R, Garrod R, et al. The development and validation of the bronchiectasis health questionnaire. Eur Respir J. 2017;49(5):1601532. doi:10.1183/13993003.01532-2016.
- Jones P, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–54. doi:https://doi.org/10.1183/09031936.00102509.
- CAT - COPD Assessment Test [homepage on the Internet]. Middlesex: GlaxoSmithKline Services Unlimited. Available from: www.catestonline.org.
- Silva G, Morano M, Viana C, Magalhães C, Pereira E. Portuguese-language version of the COPD assessment test: validation for use in Brazil. J Bras Pneumol. 2013;39(4):402–408. doi:https://doi.org/10.1590/S1806-37132013000400002.
- Jones P, Brusselle G, Dal Negro R, Ferrer M, Kardos P, Levy M, Perez T, Soler Cataluña J, van der Molen T, Adamek L, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi:10.1183/09031936.00177210.
- Global Initiative for Chronic Obstructive Lung Disease - GOLD [homepage on the Internet]. GOLD; Available from: www.goldcopd.org.
- Jones P, Harding G, Wiklund I, Berry P, Tabberer M, Yu R, Leidy N. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142(1):134–140. doi:10.1378/chest.11-0309.
- Lee B, Lee S, Lee J, Song J, Lee S, Jang S, Jung K, Hwang Y, Oh Y. Validity and reliability of CAT and dyspnea-2 in bronchiectasis and tuberculous destroyed lung. Tuberc Respir Dis (Seoul). 2012;72(6):467–74. doi:10.4046/trd.2012.72.6.467.
- Amalakuhan B, Maselli D, Martinez-Garcia M. Update in bronchiectasis 2014. Am J Respir Crit Care Med. 2015;192(10):1155–6. doi:10.1164/rccm.201505-0926UP.
- American Thoracic Society (ATS), European Respiratory Society (ERS). Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American thoracic society and European respiratory society. Am J Respir Crit Care Med. 1999;159(4 Pt 2):S1–S40. doi:10.1164/ajrccm.159.supplement_1.99titlepage.
- Pereira CA. Espirometria. J Pneumol, 2002;28(3):S1–S82.
- Souza T, Jardim J, Jones P. Validação do questionário do hospital Saint George na doença respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Pneumol. 2000;26(3):119–25. doi:10.1590/S0102-35862000000300004.
- Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, Lebargy F, Deslee G. The modified medical research council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012;12:61. doi:10.1186/1471-2466-12-61.
- Martinez-Garcia M, Athanazio R, Girón R, Máiz-Carro L, de la Rosa D, Oliveira C, de Garcia J, Vendrell M, Prados-Sánchez C, Gramblicka G, et al. Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J Chron Obstruct Pulmon Dis. 2017;12:275–284. doi:10.2147/COPD.S121943.
- Athanazio R, Pereira M, Gramblicka G, Cavalcanti-Lundgren F, de Figueiredo MF, Arancibia F, Rached S, de la Rosa D, Máiz-Carro L, Girón R, et al. Latin America validation of FACED score in patients with bronchiectasis: an analysis of six cohorts. BMC Pulm Med. 2017;17(1):73. doi:10.1186/s12890-017-0417-3.
- Chalmers J, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, Poppelwell L, Salih W, Pesci A, Dupont LJ, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–85. doi:10.1164/rccm.201309-1575OC.
- Singh S, Morgan M, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax.1992;47(12):1019–24. doi:10.1136/thx.47.12.1019.
- Probst V, Hernandes N, Teixeira D, Felcar J, Mesquita R, Golçalves C, Hayashi D, Sing S, Pitta F. Reference values for the incremental shuttle walking test. Respir Med. 2012;106(2):243–248. doi: 10.1016/j.rmed.2011.07.023.
- Tudor-Locke C, Bassett DR J. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi:10.2165/00007256-200434010-00001.
- Dodd J, Hogg L, Nolan J, Jefford H, Grant A, Lord V, Falzon C, Garrod R, Lee C, Polkey M, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax. 2011;66(5):425–9. doi:https://doi.org/10.1136/thx.2010.156372.
- Kon S, Clark A, Dilaver D, Canavan J, Patel M, Polkey M, Man W. Response of the COPD assessment test to pulmonary rehabilitation in unselected chronic respiratory disease. Respirology. 2013;18(6):974–7. doi:10.1111/resp.12084.
- Nicolini A, Cardini F, Landucci N, Lanata S, Ferrari-Bravo M, Barlascini C. Effectiveness of treatment with high-frequency chest wall oscillation in patients with bronchiectasis. BMC Pulm Med. 2013;13(21):21–8. doi: 10.1186/1471-2466-13-21.
- Brill S, Patel A, Singh R, Mackay AJ, Brown J, Hurst J. Lung function, symptoms and inflammation during exacerbations of non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Res. 2015;16:16. doi:10.1186/s12931-015-0167-9.