Abstract
Quality of chronic obstructive pulmonary disease (COPD) care is thought to be an important intermediate process to improve the well-being of patients admitted to hospital for exacerbation. We sought to examine the quality of inpatient COPD care and the associations with readmission and mortality. We performed a cohort study of 2,364 veterans aged over 40 and hospitalized for COPD between 2005 and 2011 at five Department of Veterans Affairs hospitals. We examined whether patients received six guideline recommended care items including short-acting bronchodilators, corticosteroids, antibiotics, positive-pressure ventilation (in cases of acute hypercarbic respiratory failure), chest imaging, and arterial blood gas measurement. Our primary outcome was all-cause hospital readmission or death within 30 days. Overall quality of care was not significantly associated with readmission or death (acute care aOR 0.98; 95% CI 0.87–1.11; ICU aOR 0.89; 95% CI 0.71–1.13). Delivery of corticosteroids and antibiotics was associated with reduced odds of readmission and death (aOR 0.77; 95% CI 0.61–0.92). Few patients received all of the recommended care items (18% of acute care, 38% of ICU patients). Quality of care did not vary by race or sex but did vary significantly across sites and did not improve over time. Our composite measure of COPD care quality was not associated with readmission or death. Further efforts are needed to improve care delivery to patients hospitalized with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) exacerbations are a frequent cause of hospital readmissions (Citation1), and account for approximately 50% of costs generated by patients with COPD, now estimated at $3 billion annually (Citation2). In response to these costs, the Center for Medicare and Medicaid Services (CMS) implemented a penalty for hospital systems with excess hospital readmission rates (Citation3). However, whether the delivery of high quality COPD care by hospitals affects readmission or patient-centred outcomes such as mortality is unknown.
Several international organizations have produced clinical practice guidelines and strategy documents for the management of COPD exacerbations (Citation4–6). Collectively, guideline panels consistently recommended delivery of key therapies and processes for the management of acute exacerbations of COPD. These items include diagnostic evaluation with chest imaging and arterial blood gas; treatment with supplemental oxygen; short-acting bronchodilators; systemic corticosteroids; respiratory antibiotics; and in cases of acute respiratory acidosis, the use of invasive or noninvasive positive-pressure ventilation (NIPPV). Several of these measures, such as chest imaging and treatment with supplemental oxygen, are based on expert opinion (Citation6–8). Others, such as treatment with systemic corticosteroids (Citation9–11), antibiotics (Citation12, Citation13), and NIPPV amongst patients with acute hypercarbic respiratory failure (Citation14, Citation15) have been shown to have various benefits in studies of COPD exacerbations. These benefits have largely focussed on in-hospital measures, such as treatment failure. More recently, use of antibiotics in conjunction with corticosteroids, has been associated with reductions in readmission and mortality (Citation12).
A single prior study done more than a decade ago showed that only two thirds of patients receive these recommended care items during hospitalization for an acute exacerbation of COPD, but was unable to examine how quality of care changed over time or how quality was associated with patient outcomes (Citation16). Understanding the relationship of care quality with readmission and survival could advance understanding between quality of care indicators and outcomes as well as lead to development of novel approaches to intervention design that improve outcomes for patients with COPD. The primary aim of this study was to understand the association between quality of COPD care on primary outcome of 30-day readmission or mortality.
Methods
Data sources
We collected information from the Veterans Integrated Service Network (VISN)-20 data warehouse (VISN20DW) that contains inpatient discharge diagnoses, diagnostic testing, laboratory values, and outcomes, including dates of death. The VISN20DW also contains a comprehensive reflection of medical services including detailed pharmacy records of drug name, drug class and dosage both in the hospital or outpatient setting.
Study population
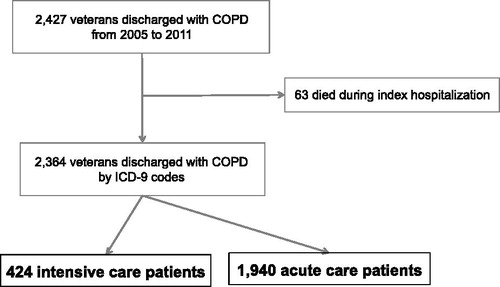
We identified patients admitted to the hospital for severe exacerbations of COPD from 2005 to 2011 in five centres that make up VISN-20 of the Veterans Affairs (VA) in the Pacific Northwest region of the United States with ICU-level care available. We identified patients 40 years of age or older and were discharged with a primary diagnosis of COPD by ICD-9 codes (491.xx Chronic Bronchitis, 492.xx Emphysema, 493.2x, and 496.xx Chronic Airway Obstruction) (Citation17–19). The CMS penalty includes these patients, and also those with a primary diagnosis of acute respiratory failure and an additional diagnosis of COPD at the exact second position (Citation20). We did not include this second group of patients to reduce the possibility of misclassification of COPD with other conditions. We excluded patients who died during the index hospitalization. The first admission during the study period was considered. Our study was approved by the Institutional Review Board at the VA Puget Sound Health Care System (#00461).
Measuring care quality for COPD
Our primary exposure of interest was the proportion of recommended care received during inpatient treatment for a COPD exacerbation during the index hospitalization. We developed a proportional measure of care quality based on the seven total inpatient recommended care items from clinical practice guidelines produced by the American College of Physicians (ACP) and the Global Initiative for Obstructive Lung Disease (GOLD) strategy (Citation5, Citation6, Citation16). We assumed that all patients received oxygen therapy based on the prior literature (Citation16) and the automatic inclusion of ‘as needed’ oxygen to correct hypoxemia in admission orders within our healthcare system. This resulted in five quality measures for patients admitted to acute care, and six quality measures for those admitted to an ICU who qualified for NIPPV or invasive mechanical ventilation. Each admission was allocated the appropriate denominator of eligible care items based on admission to the ICU versus acute care floor. The proportion, or fraction, of care received was then calculated into an appropriate care quality score.
To calculate the proportion of guideline recommended care received, we collected data regarding in-hospital treatments and tests delivered during the index stay. We considered various types of systemic steroids, and included prednisone, prednisolone, methylprednisolone, hydrocortisone, and triamcinolone for analysis. We assumed that corticosteroids delivered were indicated for treatment of a COPD exacerbation if the dose, regardless of route, was equal to or greater than the equivalent of 20 mg of prednisone (Citation10) daily. We selected antibiotics if it provided plausible respiratory pathogen coverage, including penicillins, cephalosporins, fluoroquinolones, macrolides, tetracyclines, sulfonamides, vancomycin, clindamycin, carbapenems, and aminoglycosides. We recorded treatment with short-acting beta-agonists and muscarinic antagonists via metered dose inhaler or nebulization. Chest imaging included chest radiographs and computed tomography (CT). We identified arterial blood gas measurements (including point of care testing) from laboratory results including pH and PaCO2. We then determined whether acute hypercarbic respiratory failure was present (pH ≤ 7.35 and PaCO2 ≥ 45 mmHg). To evaluate for the appropriate use of NIPPV and/or mechanical ventilation, we recorded the use of NIPPV or mechanical ventilation at any time point in the index hospitalization. We gave a score of ‘one’ in our composite measure of quality of positive-pressure ventilation was administered in patients with respiratory acidosis on ABG. If positive-pressure ventilation was administered without an ABG or without respiratory acidosis, we assigned a score of ‘one’ in our measure. If respiratory acidosis was present, but positive-pressure ventilation was not applied, a score of ‘zero’ was assigned.
Primary outcome
We defined the primary outcome as a composite measure of mortality or readmission from any cause within 30 days of discharge from index hospitalization (Citation21). The discharge date was set as the index date.
Patient and hospital characteristics
We examined a range of individual characteristics including: demographics (age, sex, race), body mass index (BMI), and smoking status. We collected smoking status via validated methods from health factors data that is collected annually at minimum (Citation22, Citation23). We assessed overall COPD severity through the prescription of inhaled controller medications (such as long-acting muscarinic antagonists, long-acting β2 agonists), and prescription of systemic steroids in the previous year (Citation24). Spirometry results were not available for this cohort.
Five sites had inpatient hospital services including ICU-level care. We categorized these as academic if they were affiliated with a University programme. Three of these sites had an academic affiliation.
Statistical analysis
We used two-tailed t-tests for means, Mann-Whitney nonparametric tests for medians, and chi-square tests for proportions for bivariate analyses, as appropriate. We used one-way analysis of variance (ANOVA) to test for differences in mean care scores by year and by facility site. We built logistic regression models to assess for associations between both the acute care and ICU quality scores and our outcome. We added known risk factors for COPD exacerbations and increased disease severity and mortality into the final adjusted model, including patient age, smoking status (Citation25), and BMI (Citation26). We also added receipt of systemic corticosteroids in the year prior to admission as a marker of COPD severity and exacerbation history. We adjusted for comorbidities that may confound the delivery of COPD care items, and included obstructive sleep apnea (OSA), diabetes mellitus (DM), and congestive heart failure (CHF). We used Stata 14 (College Station, TX) software for all analyses.
Sensitivity analysis
To address concerns about potential of misclassification of COPD exacerbations, we excluded patients who did not receive either corticosteroid or antibiotic therapy during the index hospitalization. Given the additional possibility of misclassification of COPD exacerbations by pneumonia, we also excluded those with concomitant discharge diagnosis for pneumonia by ICD-9 code. We also examined the receipt of corticosteroids and antibiotics, separate from the proportional measure of care quality, as these therapies have been shown to be beneficial when examining these outcomes. In a post-hoc analysis, we collected data on discharge therapies provided to patients after COPD exacerbation. Data regarding tobacco cessation was collected from orders for nicotine replacement therapy, varenicline, and bupropion. Referrals for pulmonary rehab were obtained from the VISN20DW.
Results
We identified 2,427 patients who were eligible for entry into our cohort (). Sixty-three patients (3.6%) died during the index hospitalization. Of the 2,364 remaining in the cohort, 97.0% were males, 82.7% were white, and 18.0% (n = 424) were admitted to the ICU. Half of patients were active smokers (51.0%, n = 1,206) and treated with long-acting bronchodilators (36.3% n = 859). Readmission or death occurred amongst 16.2% (n = 382) of patients within 30 days following discharge from index hospitalization. Ninety-six patients (4.1%) died within 30 days, with a median length of survival of 14.7 days (IQR 6.9–22.9).
The majority of the cohort received chest imaging (89.1%, n = 2,107), short-acting bronchodilator therapy (85.6%, n = 2,025), systemic corticosteroids (84.8%, n = 2,005), and antibiotics (74.3%, n = 1,756) (). Fluoroquinolone monotherapy was the most common antibiotic regimen. A minority of patients had at least one arterial blood gas analysis (38.1%, n = 905). Of those who underwent ABG analysis, 299 patients (33.0%) had acute respiratory acidosis, and 200 (22.1%) went on to receive either NIPPV or mechanical ventilation. There were 182 patients with respiratory acidosis that did not receive positive-pressure ventilation. Overall, 11.8% (n = 279) of the cohort received positive-pressure therapy either invasively or noninvasively. Few patients (3.3%, n = 79) received positive-pressure ventilation and did not have an ABG measurement. Amongst those that receive positive-pressure ventilation, 48% were obese (n = 134), 15% had a diagnosis of OSA (n = 42), and 20% had a diagnosis of pneumonia (n = 57).
Figure 2. Performance data for the five hospitals. The line in the middle of each box represents the median performance percentile and the box extends to the interquartile range (IQR). The whisker lines emerging from the boxes extend to the maximum and minimum values. The ideal acute care composite measurement indicates receiving all of the following five therapies recommended by guidelines: short-acting bronchodilators, steroids, antibiotics, chest radiography and arterial blood gas measurement. The ideal ICU care composite measurement indicates receiving all of the five therapies from acute care in addition to the appropriate use of positive-pressure ventilation for acute hypercarbic respiratory failure. ABG = arterial blood gas. SABD = short-acting bronchodilators.
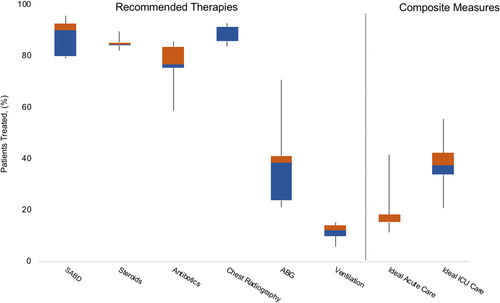
Less than one in five of the 1,940 acute care patients (18.1%, n = 351) received all five recommended care items with a mean score of 3.61 (SD 1.05). A greater proportion of patients received all six items in the ICU (38.2%, n = 162) with a mean score of 5.05 (SD 0.97) (). Amongst those patients who received an ICU score of five (n = 164), 66.4% (n = 109) did not receive recommended positive-pressure ventilation, 23.8% (n = 39) did not receive antibiotic therapy, and 22.6% (n = 37) did not receive arterial blood gas analysis.
Amongst patients admitted to the ICU, those older than 70 years received fewer services when compared to younger patients in the ICU (4.92 versus 5.12, p value .04). Patients older than 70 years of age (n = 1,008) received chest imaging (90.7%, n = 901), ABG analysis (36.1%, n = 358), SABA (85.2%, n = 846), and antibiotics (75.1%, n = 746) similarly to younger patients. However, older patients received fewer treatments with corticosteroids (82.9%, n = 823) and NIPPV and/or mechanical ventilation (8.9%, n = 90). ICU and acute care scores did not vary significantly by race or sex.
In a post-hoc analysis of discharge therapies prescribed to patients, 14.8% (n = 179) of current smokers were prescribed medications for tobacco cessation (including nicotine replacement, bupropion, and varenicline). Six patients (<1%) were referred to pulmonary rehab. Addition of these metrics to our acute and ICU care scores did not significantly change our results.
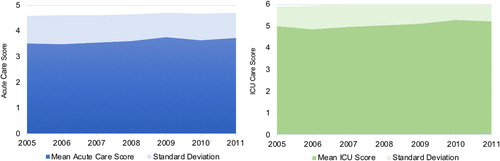
Care quality did vary significantly by site for both levels of care (acute care score range 3.40–4.06, F-statistic 18.1, p value < .001; ICU score range 4.65–5.48, F-statistic 7.2, p value < .001). When comparing academic and nonacademic sites, there was significant variation favouring academic sites in ICU-level care (4.75 versus 5.12; p value < .001) but did not differ for acute level care (3.60 versus 3.61; p value .96). When examining the six individual components of the score, the delivery of short-acting bronchodilators and systemic corticosteroids did not vary by site. However, the use of chest imaging and arterial blood gas analysis, in addition to treatment with antibiotics and positive-pressure ventilation did vary significantly by site (). The quality of COPD care did not change over time () when analyzing the mean score by year for both acute care (F-statistic 1.85, p value .06) and ICU care (F-statistic 1.26, p value .27).
In a multivariable logistic regression model, the quality score was not associated with readmission or death for either acute care patients (aOR 0.98; 95% CI 0.87–1.10) or those admitted to the ICU (aOR 0.92; 95% CI 0.72–1.17) in adjusted analysis (). However, administration of dual corticosteroid and antibiotics therapy during the index stay was significantly associated with a reduction in readmission and mortality (aOR 0.77; 95% CI 0.61–0.96). There were 151 patients (6.3%) who did not receive either corticosteroid or antibiotic therapy. Their exclusion for our sensitivity analysis did not significantly alter our findings. The results were also similar when excluding patients with pneumonia.
Table 1. Inpatient quality care scores are not associated with patient outcomes.
Discussion
We found that the majority of patients admitted to the hospital for a COPD exacerbation do not receive all care processes recommended by clinical practice guidelines. However, our composite measure of care quality was not associated with readmission and/or mortality. Amongst those measures known to improve outcomes in COPD exacerbations, we found that receipt of both antibiotics and corticosteroids was associated with significantly lower odds of readmission and death. We also found significant variation in care quality across medical facilities, although collectively, our measures of quality of care did not change over time.
There are a number of explanations for our findings. First, our measure of quality may not truly reflect the actual quality of care delivered to patients, since it may not adequately reflect all care processes that lead to outcomes such as hospital readmission. Measures of inpatient quality are constructed focussing on components of recommended inpatient services, and therefore will not include those care processes including outpatient care and social determinants of health (Citation27, Citation28), that are highly likely to affect outcomes (Citation29). Second, our quality measure encompassed diagnostic and therapeutic variables and each was weighted equally. When examining the individual components of our quality measure, we did find that treatment with steroids and antibiotics was associated with reduced odds of readmission and death. Development of a quality process measure should include receipt of therapies for COPD exacerbations and focus on improvement of patient outcomes. Our findings bolster the argument against the CMS penalty for COPD in that there is a lack of evidence to support that readmissions are a function of the quality of inpatient COPD care.
Older patients did not receive as many care items as their younger counterparts when examining patient-level factors in the ICU. The lower mean care score is explained by the differing use of positive-pressure ventilation, whether noninvasive or invasive, in this older age group. Lower rates of use in this age group may not reflect poor delivery of care but may instead be explained by patient preference in the setting of older age and critical illness. There was no mean difference in care quality scores in older patients in the acute care setting, where positive-pressure ventilation is not an available treatment. Future design of quality metrics in COPD care should consider patient preferences and could explain our findings.
Our study found that only 12% of hospitalized patients with COPD received positive-pressure ventilation therapy. Positive-pressure ventilation was likely underused in this cohort of patients with severe COPD exacerbations. This therapy has been shown to have a beneficial effect on the outcome of in-hospital mortality in multiple studies (Citation30). Our study did not assess in-hospital mortality, but a new study has demonstrated the benefit of NIV on longer-term outcomes such as admission-free survival in patients suffering from severe COPD exacerbations and persistent hypercapnia when discharged with NIV (Citation31). It is possible that increasing the use of NIV or mechanical ventilation both in the hospital and home settings would improve patient outcomes and should be the subject of future study.
Care quality varied widely by site in our study, with significant variation in the delivery of antibiotics. Previous studies have described such variability but were either under-powered or did not find the variability in quality to be explained by hospital type (Citation16, Citation32). We did find that care varied by academic affiliation in the ICU setting. This likely represents a local pattern reflective of the availability of critical care and pulmonary specialists at the three academic sites in our region. Overall, site variation offers an opportunity to further examine health systems and structure to improve care delivery at the site-level.
Our study found that the quality of COPD care did not change over time. This cohort predates the 2014 CMS penalty for excess readmissions for COPD and future studies should examine for changes around the time of the penalty. Additionally, the studies published regarding the benefit of antibiotics in severe COPD exacerbations were mostly published after the time of this cohort (Citation12, Citation33), and prior guidelines recommended conditional use in those with purulent sputum. Antibiotic delivery may change with increased knowledge dissemination of the benefit of antibiotics and recent guideline and strategy document revisions for severe exacerbations of COPD. Broad organizational efforts have led to the implementation of programmes that have improved quality for heart failure measures and a decline in CHF-related mortality (Citation34). A similar organizational emphasis does not exist for COPD, but may be similarly beneficial and improve COPD-related care quality in the future.
Our study has limitations. First, this study utilized clinical administrative data and we did not have symptoms or spirometry to discern aspects of COPD severity and confirm diagnosis via airflow obstruction. We have previously found that although the sensitivity of a primary discharge diagnosis was poor, the specificity for airflow obstruction was found to be 99% (Citation17). Using our inclusion criteria by diagnosis codes also provides a ‘real world’ examination of a COPD population, especially since the CMS penalty does not require spirometric confirmation. This study was solely within the Department of Veterans Affairs, which serves predominantly men of lower socioeconomic means. This limitation, however, should not impact patterns of care delivery.
This study had a number of important strengths. First, the VISN20CDW allows for the assessment of other important markers of disease severity, including outpatient inhaler use and the history of prior exacerbations. Second, we had a complete assessment of all hospital admissions for a COPD exacerbation across a large geographic region, and across community and academic facilities, enhancing generalizability and making idiosyncratic practices less likely to impact our findings. Finally, we had the ability to examine practice patterns over time, which has never been previously reported.
Conclusion
Our composite measure of care quality was not associated with outcomes of readmission or death, but the delivery of antibiotics and corticosteroids did show benefit. Future efforts are needed to improve delivery of therapies for COPD exacerbations in the hospital setting. Future study is needed to identify and develop quality process measures that are associated with improved patient outcomes in COPD.
Disclosure statement
D.H.A reports personal fees from Novartis for service on a data monitoring committee, personal fees from American Board of Internal Medicine for service on the exam writing committee, and personal fees from Annals of the American Thoracic Society for service as a deputy editor, outside the submitted work. L.J.S. reports grant support from NIH NHLBI T32HL007287-38 and F32HL142125-01, during the conduct of the study. L.M.D. reports grant support from NIH NHLBI T32HL007287-38, during the conduct of the study. M.F.G. reports grant support from NIH NHLBI T32HL007287-38, during the conduct of the study. L.C.F reports grants from NIH K23 HL111116, grants from American Lung Association, grants from Veteran's Health Administration, outside the submitted work.
Additional information
Funding
References
- Association AL. 2015 July 29. COPD Fact Sheet. Available from: www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact_Sheet.html, accessed 2017 July 29.
- Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214–228.
- Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634–639.
- Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, Curren K, Balter MS, Bhutani M, Camp PG, et al. Prevention of acute exacerbations of COPD: American college of chest physicians and Canadian thoracic society guideline. Chest. 2015;147(4):894–942.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD): global strategy for the diagnosis, management and prevention of COPD. Available from: www.goldcopd.org, accessed 2018 May 20.
- Wedzicha JAEC-C, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, Criner GJ, Papi A, Rabe KF, Rigau D, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3): 1–16.
- Bach PB, Brown C, Gelfand SE, McCrory DC, American College of Physicians-American Society of Internal Medicine, American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001;134(7):600–620.
- McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest. 2001;119(4):1190–1209.
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456–460.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359–2367.
- Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014(9):CD001288.
- Stefan MS, Rothberg MB, Shieh MS, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82–90.
- Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257.
- Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822.
- Bott J, Carroll MP, Conway JH, Keilty SE, Ward EM, Brown AM, Paul EA, Elliott MW, Godfrey RC, Wedzicha JA, et al.. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341(8860):1555–1557.
- Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903.
- Cooke CR, Joo MJ, Anderson SM, Lee TA, Udris EM, Johnson E, Au DH. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37.
- Ginde AA, Tsai CL, Blanc PG, Camargo CA, Jr. Positive predictive value of ICD-9-CM codes to detect acute exacerbation of COPD in the emergency department. Jt Comm J Qual Patient Saf. 2008;34(11):678–680.
- Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6(5):388–394.
- Centers for Medicare and Medicaid Services (CMS), HHS. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and Fiscal Year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status. Final rules. Fed Regist. 2013;78(160):50495–51040.
- Drye EE, Normand SL, Wang Y, Ross JS, Schreiner GC, Han L, Rapp M, Krumholz HM. Comparison of hospital risk-standardized mortality rates calculated by using in-hospital and 30-day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;156(1 Pt 1):19–26.
- McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239.
- Melzer AC, Feemster LC, Collins MP, Au DH. Predictors of pharmacotherapy for tobacco use among veterans admitted for COPD: the role of disparities and tobacco control processes. J Gen Intern Med. 2016;31(6):623–629.
- Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM, Anto JM, Estudi del Factors de Risc d'Aguditzacio de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105.
- Au DH, Bryson CL, Chien JW, Sun H, Udris EM, Evans LE, Bradley KA. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24(4):457–463.
- Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, Kosnik M, Anker SD, Suskovic S. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–86.
- Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9(3):216–226.
- Gershon AS, Hwee J, Victor JC, Wilton AS, To T. Trends in socioeconomic status-related differences in mortality among people with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(8):1195–1202.
- Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62(7):1650–1671.
- Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:CD004104.
- Criner GJ, Dreher M, Hart N, Murphy P. COPD home oxygen therapy and home mechanical ventilation: improving admission-free survival in persistent hypercapnic COPD. Chest. 2018;153(6):1499–1500.
- Sandhu SK, Chu J, Yurkovich M, Harriman D, Taraboanta C, Fitzgerald JM. Variations in the management of acute exacerbations of chronic obstructive pulmonary disease. Can Respir J. 2013;20(3):175–179.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303(20):2035–2042.
- Rinne ST, Liu CF, Wong ES, Hebert PL, Heidenreich P, Bastian LA, Au DH. Organizational structure for chronic heart failure and chronic obstructive pulmonary disease. Am J Manag Care. 2016;22(3):e82–7.