Abstract
The effectiveness of the tiotropium Respimat® formulation in routine clinical practice is still an open issue due to concern about the generalizability of the Tiotropium Safety and Performance in Respimat® (TIOSPIR) trial findings. Our aim was to compare the incidence of acute respiratory events between new users of tiotropium Respimat® and HandiHaler®. The study population comprised patients aged ≥45 years resident in two Italian regions who received a first tiotropium prescription (HandiHaler® or Respimat®) between 1 July 2011 and 30 November 2013. The cohort was identified within the database of drug prescriptions reimbursed by the Italian National Health Service. Clinical outcomes were obtained from hospital records. The primary outcome was the first hospitalization for respiratory events, including chronic obstructive pulmonary disease (COPD) exacerbation, respiratory failure, hypoxemia/hyperventilation and pneumonia, during the exposure period. The hazard ratios were estimated for the propensity score matched groups with Cox regression. After matching, 31,334 patients with incident tiotropium prescriptions were included. Similar incidence rates of the primary outcome between the Respimat® and HandiHaler® users were identified (adjusted hazard ratio 0.95, 95% CI 0.84–1.07). No differences emerged in the subgroup analyses conducted according to the baseline characteristics of the tiotropium users. This study confirms the findings observed in the TIOSPIR trial in a more heterogeneous population that included patient subgroups with severe respiratory disease and unstable COPD.
Introduction
Tiotropium is available in the market in two different formulations, i.e., HandiHaler® and Respimat®. The Respimat® formulation has been presented as having several advantages from the patient’s perspective (Citation1). The standard pressurized metered-dose inhaler enables easier usage, the delivered dose is independent of inspiratory capacity, and the delivery method reduces oropharyngeal deposition and increases lung deposition. These factors suggest that a lower dose of tiotropium is needed with the Respimat® device than with the HandiHaler® (Citation2).
However, the Respimat® formulation has been subjected to scrutiny by the European Medicines Agency (EMA) due to a signal of increased cardiovascular and all-cause mortality (Citation3). To provide additional evidence, the marketing authorization holder sponsored the “Tiotropium Safety and Performance in Respimat®” (TIOSPIR) trial, which enrolled a large population of patients with chronic obstructive pulmonary disease (COPD). This study highlighted the comparable efficacy and safety profiles of the two formulations (Citation4). Subsequent observational studies conducted in clinical practice were also reassuring but only in terms of the safety findings (Citation5,Citation6).
Accumulating evidence has demonstrated that only a minority of COPD patients treated with tiotropium in “real life” could have met the criteria for participation in the TIOSPIR trial (Citation7). In clinical practice, tiotropium users were found to have more severe symptoms than those who were included in the TIOSPIR trial (Citation8); specifically, the trial excluded patients with severe respiratory disorders and those with a recent COPD exacerbation (unstable disease). The majority of patients who were enrolled in the TIOSPIR trial were already experienced with tiotropium and may have exhibited higher tolerance to anticholinergic effects and thus responded differently to treatments with tiotropium Respimat® or HandiHaler®.
The low representativeness of the RCT’s (randomized clinical trial) population is not uncommon in the COPD setting (Citation9–11). These observations cast doubt on the generalizability of the TIOSPIR trial findings and call for a proper effectiveness evaluation of the tiotropium Respimat® in the general patient population.
For these reasons, we conducted an observational cohort study with the aim of comparing the effectiveness of the two tiotropium formulations, Respimat® and HandiHaler®, in a large patient population in a real-life setting. The objective of this study was to compare the incidence of hospitalization for respiratory events, including COPD exacerbation, respiratory failure, hypoxemia/hyperventilation and pneumonia, between new users of Respimat® and HandiHaler®.
Methods
We conducted a retrospective cohort study among incident users of the tiotropium Respimat® and tiotropium HandiHaler® who were aged ≥45 years. The cohorts were identified through the drug prescription monitoring system of the National Health Service (NHS) in two Italian Regions, i.e., Lombardy and Umbria (covering almost 11 million inhabitants), in the period from July 2010 to December 2013. Incident users (as a proxy of tiotropium-naïve users) were defined as subjects with the first prescription of tiotropium between 1 January 2011 and 31 December 2013. The semester July–December 2010 was used as a wash out period to guarantee a minimum of six months without prescription of tiotropium. Users of tiotropium were excluded if they received both formulations at the first prescription. The index date was the date of the first prescription of tiotropium.
For each formulation, the duration of use (current exposure) was defined according to the expected duration of the prescription (in DDDs), as the total time that the patient was taking the medication, i.e. from the date of first prescription, and of all consecutive prescriptions dispensed, plus two weeks of grace period. If the gap between two consecutive prescriptions was greater than two weeks, the exposure period ended and the subjects could not be re-enrolled in the study.
The demographic information (age and sex) of the users, information about potential confounders and clinical outcomes were obtained through deterministic record linkage; the regional database of NHS enrollees, drug prescriptions and hospital discharges were linked through the anonymized patient code.
Subjects were characterized at baseline with regard to other medications (defined as at least two prescriptions in the same category, including other respiratory drugs) and/or hospitalizations in the 12 months preceding the index date (Supplementary Table 1).
The study events were defined as the first hospitalization occurring during the current exposure period for: COPD exacerbation (ICD-9 codes 490; 491; 492; 493.22; 496); acute respiratory failure (ICD-9 codes 518.81; 518.82; 518.84; 799.1); hypoxemia/hyperventilation (ICD-9 codes 799.0; 786.0); and pneumonia (ICD-9 codes 480-486). Events were retrieved from the archive of hospital discharges using the principal diagnosis (Supplementary Table 2). The respiratory events were analyzed as both single and composite outcomes. Each subject was followed from the first tiotropium prescription to the earliest of the following dates: the end of the current exposure, a change in the formulation (switch), hospitalization for a study event in the current exposure, in-hospital death for any cause, and the end of the study (31 December 2013).
The following potential confounders were taken into account: age; gender; cardiomyopathy; heart failure; diabetes mellitus; neoplasms; respiratory diseases, excluded COPD; COPD; operations on vessels of heart; cerebrovascular diseases; glaucoma; beta blockers (Anatomical Therapeutic Chemical classification, ATC code C07); antihypertensives and/or diuretic (C02, C03); non-dihydropyridine CCB (C08D); angiotensin receptor blockers and ACE-I (C09); NSAIDs (M01A); ICS (R03BA); SAMA (R03BB); LABA (R03AC12, R03AC13, R03AC18); SABA (R03AC02, R03AC03, R03AC04); adrenergics in combination with corticosteroids or other drugs, excl. anticholinergics (R03AK); corticosteroids for systemic use (H02); antibacterials (J01).
Respimat® users were matched to HandiHaler® users (1:1) based on propensity score. Propensity score was fitted by a logistic regression model in which the dependent variable was the exposure to Respimat®, as opposed to HandiHaler® and the independent variables were the confounding variables mentioned above. The nearest neighbor matching algorithm was used (caliper width 0.01 of standard deviation of the logit score).
The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox-proportional hazards regression model for unmatched cohort (unadjusted HR) and for the two matched groups of tiotropium users, i.e., the propensity score-matched cohort (adjusted HR). For each patient, the observation was censored at the end of the follow-up period.
Analyses by patient characteristics at baseline and by specific subgroups of high-risk patients who were less represented in the TIOSPIR trial were conducted to evaluate the HRs in selected patient populations. Subgroups were defined on the basis of hospitalizations and drug prescriptions in the 12 months preceding the index date as: subjects with severe respiratory disease (at least one prescription of antibacterials and at least one hospitalization for respiratory disease or pneumonia); frail populations (subjects with age >75 years and at least one hospitalization for acute myocardial infarction or at least two prescriptions of anticoagulants); users of multiple respiratory drugs (at least two prescriptions of different respiratory drugs: ICS, SAMA, LABA, SABA, leukotriene receptor antagonists, xanthines, adrenergics in combination with corticosteroids or other drugs, adrenergics in combination with anticholinergics, antiallergic agents, other systemic drugs for obstructive airway diseases, corticosteroids for systemic use). A sensitivity analysis that involved extending the grace period from 15 to 30 days following the exposure period was conducted.
All statistical tests were two sided. The STATA software (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP) was used to perform the analysis. The study was approved by the ethics committee of the Italian National Institute of Health (Prot. PRE-C-17/15). After submitting the study protocol to the Health Authorities of the Lombardy and Umbria Regions, the authors received anonymized raw data on drug prescriptions (restricted to the subjects who received at least one prescription of tiotropium during the study period) and on hospital discharges (restricted to the same subjects). When the study protocol was submitted, one of the authors (MV) was an employee of the Health Authority of Lombardy Region.
Results
The study population included 69,025 subjects who received a first prescription of tiotropium between 2011 and 2013 (15,937 treated with Respimat® and 53,088 with HandiHaler®). The mean duration of use was 83 days for Respimat® and 95 days for HandiHaler®. The baseline characteristics of the two tiotropium groups were substantially different. The Respimat® users were younger and had lower prevalence of comorbidities and concomitant drug use than the HandiHaler® users. After 1:1 propensity score matching, 31,334 tiotropium users (15,667 in each cohort) were analyzed; the two cohorts were balanced with respect to baseline characteristics (Supplementary Table 3).
Overall, 1035 events occurred in the matched cohorts, including 477 in the Respimat® group and 558 in the HandiHaler® group (). The hospitalization rates for the primary outcome (which included COPD exacerbation, respiratory failure, hypoxemia/hyperventilation and pneumonia) were comparable between the Respimat® and HandiHaler® propensity score matched cohort (adjusted HR 0.95, 95% CI 0.84–1.07) (Table 1, Supplementary Figure 1). No differences in risk were observed when the individual outcomes were analyzed separately ().
Table 1. Hazard ratios for the primary outcomes of patients treated with Respimat® versus HandiHaler®.
The findings were similar in the specific subgroups of patients who were excluded or less represented in the TIOSPIR trial: patients with previous respiratory disease (adjusted HR 0.98, 95% CI 0.79–1.21); frail population (adjusted HR 1.04, 95% CI 0.85–1.28) and previous users of other respiratory drugs (adjusted HR 1.00, 95% CI 0.83–1.21) (). Moreover, subgroup analyses of the primary outcome according to the potential risk factors did not reveal any differences from the results of the overall analysis (, Supplementary Table 4).
Figure 1. Hazard ratios for the primary outcome of patients treated with Respimat® versus HandiHaler® according to risk factors (patient characteristics and comorbidities; only risk factors with at least 40 events per group were included in the graph, with the exception of region). HR: Hazard Ratio; CI: Confidence Interval; COPD: Chronic Obstructive Pulmonary Disease.
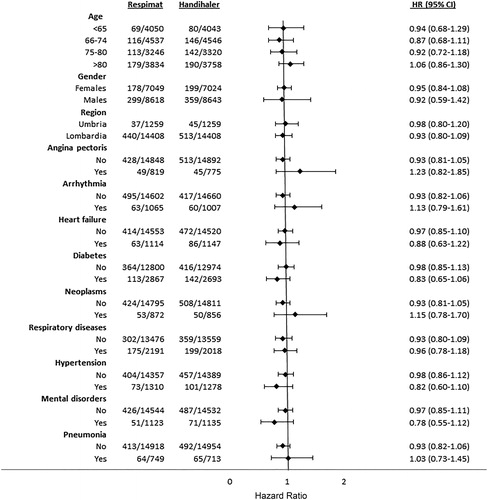
Table 2. Hazard ratios of the primary outcomes for the specific subgroups of patients at higher risk treated with Respimat® versus HandiHaler®.
Extending the “grace” period from 15 to 30 days after the duration of the prescription did not change the main findings (adjusted HR 0.94, 95% CI 0.85–1.05). A total of 301 in-hospital deaths (for any cause) were observed during the current period: 131 in the Respimat® group and 170 in the HandiHaler® group (adjusted HR =0.89, 95% CI 0.71–1.12).
Discussion
Principal statement of the findings
In this observational cohort study conducted in a large sample of tiotropium users in routine clinical practice, we found no differences between the incident users of the two formulations (Respimat® versus HandiHaler®, adjusted HR 0.95, 95% CI 0.84–1.07) in terms of respiratory events, including COPD exacerbation, respiratory failure, hypoxemia/hyperventilation and pneumonia. Similar effectiveness profiles were confirmed in the multiple subgroup analyses that were designed to evaluate the occurrence of events in patients who were excluded or less represented in the TIOSPIR trial.
Comparison with other studies
Our study strengthens the findings of the TIOSPIR trial by providing effectiveness data regarding the use of the Respimat® formulation by the general population. In addition, we found that the tiotropium Respimat® was as effective as the tiotropium HandiHaler® in a population of naïve users, at least with regard to the first episodes of exacerbation. The TIOSPIR trial included a large patient population that was already receiving treatment with a tiotropium formulation (HandiHaler®), which may have ultimately resulted in a higher tolerance to the anticholinergic effects. A post hoc analysis of the TIOSPIR patients who were naïve to anticholinergics revealed similar efficacy profiles for both formulations (Citation12); however, this analysis adopted a relatively weak definition of naiveté (i.e., no use of anticholinergics in the two months preceding enrollment), whereas our study used a more conservative definition and included only patients without prescriptions for anticholinergics in the six months preceding inclusion in the cohort.
Unlike the post hoc analysis from the TIOSPIR trial, our study investigated the comparative effectiveness of the two formulations not only in terms of exacerbation but also with consideration of other robust outcomes, such as respiratory failure and severe pneumonia. There were no differences between the tiotropium Respimat® and HandiHaler® considering all the outcomes, both single and composite events. Moreover, the population enrolled in our study did not exclude patients with severe respiratory disorders or those with recent COPD exacerbations (which are considered indicative of “unstable disease”). We also demonstrated that the effectiveness of the two formulations did not change when evaluated in specific patient subgroups. The present confirmation of the results of the TIOSPIR trial and extension of its findings to subgroups of patients who were excluded from the trial despite being representative of the general COPD population (Citation7,Citation9) supports the generalizability of the TIOSPIR trial’s findings.
A systematic review that was conducted to evaluate the pharmacokinetic, efficacy and safety data from comparative clinical trials of the tiotropium Respimat® and HandiHaler® in patients with COPD suggested that these formulations produce similar clinical outcomes in terms of FEV1 (forced expiratory volume in the 1st second), FVC (forced vital capacity), COPD exacerbation, death and quality of life (Citation13). These results prompted the authors to suggest that physicians can base their decision regarding tiotropium inhalers on factors other than efficacy and safety.
Strengths and weaknesses of the study
Our study included the entire population of patients who were treated in two Italian regions that covered almost 11 million inhabitants. Several pre-specified subgroup and sensitivity analyses were performed, and substantial consistency in the results was observed.
Many potential confounders were identified through the linkage of multiple databases, which allowed for a thorough ascertainment of the underlying comorbidities and co-medications. We had no information about the actual diagnoses for which the concomitant drugs were used; the prescriptions and the hospitalizations in the 12 months preceding the index date were adopted as proxy indicators of a variety of underlying conditions (other than COPD).
We were not able to control for some potential confounding factors in our estimates that may be relevant to the choice of specific tiotropium formulation, such as smoking status, BMI, socioeconomic status and dosage/posology. Moreover, direct clinical information about the time of first COPD diagnosis and the severity of COPD (i.e., the GOLD stage or FEV1 status) was not available; thus, we used indirect measures of COPD severity that were based on the use of respiratory medications and hospitalization for COPD exacerbation or pneumonia as previously published elsewhere (Citation6,Citation8).
A further potential limitation of the study is the relatively short mean duration of tiotropium use (i.e., 83 days for Respimat® and 95 days for HandiHaler®); however, the temporal relationships of the exposures to Respimat® and HandiHaler® with the primary outcome indicated that the majority of the primary outcomes (>90%) occurred within a three month-period, which fits with the mean time of tiotropium use of the current cohort (Citation14). The Kaplan-Meier curves for the primary outcome (Supplementary Figure 1) also revealed a rapidly increasing pattern of events immediately after the initiation of tiotropium use (for both formulations) and a stabilization after one year of follow-up. These patterns are suggestive of a confounding by indication; however, this potential confounding should have equally affected both formulations.
As with other observational studies based on routinely collected data, exposure misclassifications may have occurred if all the tiotropium packages that were prescribed to (and received by) the patients were not subsequently administered. However, such misclassification would be expected to occur at a similar rate in both groups. Regarding other studies that have used prescription databases, one limitation of our analysis is that the identification of the incident users was based on pharmacy records, and we were unable to confirm naiveté based on the clinical records.
We did not evaluate events not requiring hospital admission, and in general less serious events. However, exacerbation requiring hospitalization can be considered as a useful outcome for the real life evaluation of respiratory drugs since it is robust, less subject to misclassification when compared to more surrogate ones (such as FEV1), and highly generalizable to the general population.
We did not have access to the information on patients dying outside the hospital. However, the in-hospital death rates were similar in the two cohorts (adjusted HR = 0.89, 95% CI 0.71–1.12) and there is no reason to expect that death rates occurring after hospital discharge might be different between the two formulations.
Conclusions
In this large cohort study, the patients who were new users of the tiotropium Respimat® and HandiHaler® exhibited similar effectiveness profiles. This study confirms the findings observed in the TIOSPIR trial in a more heterogeneous population that included patient subgroups with severe respiratory disease and unstable COPD.
Author contributions
GT, FT and SSA conceived the study; RDC, MR, MV, GT and FT designed the study; RDC, SSA and FT analyzed the data; and SSA and FT wrote the manuscript. All authors contributed to the discussion and reviewed the manuscript. GT will act as the guarantor of the paper. All authors saw, commented on and approved the final version of the paper.
Supplemental Material
Download PDF (460 KB)Acknowledgments
We wish to thank Maja Rajevic (formerly of the “La Sapienza” University of Rome, Italy) and Valentino Conti (formerly of the Lombardy Region, Milan, Italy) for their valuable support during the protocol writing phase.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
Only public employees of the National or Regional Health Authorities were involved in conceiving, planning, and conducting this study. Partial financial support was received from the Umbria Regional Health Authority. The corresponding author had full access to all study data and approved the manuscript for publication.
References
- Ram FS, Carvallho CR, White J. Clinical effectiveness of the Respimat inhaler device in managing chronic obstructive pulmonary disease: evidence when compared with other handheld inhaler devices. Int J Chron Obstruc Pulmon Dis. 2011;6:129–139. doi:10.2147/COPD.S8092.
- Keating GM. Tiotropium Respimat® Soft Mist™ inhaler: a review of its use in chronic obstructive pulmonary disease. Drugs 2014;74(15):1801–1816. doi:10.1007/s40265-014-0307-4.
- European Medicines Agency. Pharmacovigilance Risk Assessment Committee (PRAC). Minutes of the meeting- 7–10 January 2013 [accessed 2018 Apr 24]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Minutes/2013/02/WC500139251.pdf. .
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, Dusser D, Joseph E, Kattenbeck S, Koenen-Bergmann M, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369:1491–1501. doi:10.1056/NEJMoa1303342.
- Verhamme KMC, Afonso A, Romio S, Stricker BC, Brusselle GGO, Sturkenboom MCJM. Use of tiotropium Respimat Soft Mist Inhaler versus HandiHaler and mortality in patients with COPD. Eur Respir J. 2013;42:606–615. doi:10.1183/09031936.00005813.
- Trotta F, Spila Alegiani S, Da Cas R, Rajevic M, Conti V, Venegoni M, Rossi M, Traversa G. Cardiovascular safety of tiotropium Respimat® vs HandiHaler in the routine clinical practice: a population-based cohort study. PLoS One. 2017;2(4): e0176276. doi:10.1371/journal.pone.0176276.
- Schmiedl S, Fischer R, Ibanez L, Fortuny J, Thürmann P, Ballarin E, Ferrer P, Sabaté M, Rottenkolber D, Gerlach R, et al. Tiotropium Respimat vs. HandiHaler: real-life usage and TIOSPIR trial generalizability. Br J Clin Pharmacol. 2016;81:379–388. doi:10.1111/bcp.12808.
- Trotta F, Da Cas R, Rajevic M, Rossi M, Traversa G. Risk factors influencing the prescription of tiotropium Respimat formulation: a population-based cohort study. BMJ Open. 2015;5: e006619. doi: 10.1136/bmjopen-2014-006619.
- Scichilone N, Basile M, Battaglia S, Bellia V. What proportion of chronic obstructive pulmonary disease outpatients is eligible for inclusion in randomized clinical trials? Respiration. 2014;87:11–17. doi:10.1159/000355082.
- Kruis AL, Ställberg B, Jones RCM, Tsiligianni IG, Lisspers K, van der Molen T, Kocks JWH, Chavannes NH. Primary care COPD patients compared with large pharmaceutically-sponsored COPD studies: an UNLOCK validation study. PLoS One. 2014;9: e90145. doi:10.1371/journal.pone.0090145.
- Battaglia S, Scichilone N. Eligibility of real-life patients with COPD for inclusion in RCTs: a commentary. Resp Res. 2017;18:5. doi:10.1186/s12931-016-0494-5.
- Wise R, Calverley PMA, Dahl R, Dusser D, Metzdorf N, Müller A, Fowler A, Anzueto A. Safety and efficacy of tiotropium Respimat versus HandiHaler in patients naive to treatment with inhaled anticholinergics: a post hoc analysis of the TIOSPIR trial. NPJ Prim Care Respir Med. 2015;25:15067. doi:10.1038/npjpcrm.2015.67.
- Dahl R, Kaplan A. A systematic review of comparative studies of tiotropium Respimat® and tiotropium HandiHaler® in patients with chronic obstructive pulmonary disease: does inhaler choice matter? BMC Pulm Med. 2016;16:135. doi:10.1186/s12890-016-0291-4.
- Singh S, Loke YK, Enright P, Furberg CD. Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax. 2013;68:114–116. doi:10.1136/thoraxjnl-2011-201275.
