Abstract
Pulmonary rehabilitation is an important treatment for patients with chronic obstructive pulmonary disease (COPD). Although this intervention leads to large and clinically meaningful improvements in exercise capacity and quality of life, the effect of pulmonary rehabilitation on physical activity is controversial. Physical activity is lower in patients with COPD as compared to healthy age-matched controls and it is related to important health outcomes (e.g. increased risk of mortality and hospitalization). It is an important goal for rehabilitation programs to enhance physical activity to more normal levels in order to achieve the ultimate goal of rehabilitation ‘to improve adherence to health enhancing behaviors’. This review discusses the role of physical activity in the context of pulmonary rehabilitation and possible ways to embed interventions geared to behavior change (i.e. to enhance physical activity) and exercise training (i.e. to enhance physical fitness) into comprehensive rehabilitation programs for patients with COPD.
Introduction
Patients with chronic obstructive pulmonary disease (COPD) engage in less physical activity compared to healthy age matched controls (Citation1). At a group level, inactivity worsens over time (Citation2) and with disease severity (but at the individual patient level, this correlation is at best weak) (Citation3). Airflow limitation and hyperinflation, pathological changes present early in the disease, lead to unpleasant symptoms of dyspnea when performing activities. This is believed to lead to further avoidance of physical activity, consequent exercise limitations and decline in functional status (Citation4). This adaptive decrease in physical activity was already observed in (ex-) smoking subjects with an undiagnosed airflow obstruction (Citation5). The vicious cycle of inactivity accelerates when patients experience exacerbations (Citation4). Physical activity levels acutely drop as the result of moderate and severe exacerbations (Citation6, Citation7) and our group showed that even one month after a hospital admission recovery of physical activity remains limited (Citation8, Citation9). The impact of exacerbations may even be longer lasting since the yearly decline in physical activity was faster in patients with two moderate exacerbations per year (−753 steps/year) and those patients with at least one severe exacerbation per year (−705 steps/year), when compared to stable patients (Citation10).
Adherence to physical activity guidelines is related with substantial health benefits in healthy as well as chronic diseased populations (Citation11). In healthy subjects, large worldwide trials showed the relationship between physical activity and mortality. Lear et al. (Citation12) investigated the effect of physical activity on mortality and cardiovascular disease in 17 countries. Higher levels of physical activity (>150 min per week of moderate intense activity) resulted in a reduction of the mortality risk compared to low physical activity levels (<150 min per week moderate intense activity). Furthermore, physical inactivity plays an important role in the risk to develop risk factors such as insulin resistance, hypertension and dyslipidemia, which can lead to chronic diseases such as coronary heart disease and type 2 diabetes (Citation11, Citation13).
In patients with COPD, physical activity is also an important prognostic factor. Patients with COPD have a higher risk to develop comorbidities (Citation14). Many of these comorbidities are linked to a lack of physical activity. Osteoporosis, type II diabetes, hypertension, cardiovascular disease and depression are more prevalent in patients with COPD compared to control subjects (Citation5, Citation15). Our group showed that physical inactivity (rather than the presence of airflow obstruction) was an important predictor for the development of comorbidities in early COPD. In patients with COPD, a population-at-risk, promoting physical activity is now recognized as a crucial part of the disease management (Citation16). A systematic review on outcomes associated with physical activity in patients with COPD showed a positive and consistent relation between higher physical activity levels and a lower risk of mortality and exacerbations (Citation17). Moreover, physical activity is associated with health status and quality of life (Citation18). Symptoms of depression and anxiety relate to physical activity both in cross sectional and longitudinal studies (Citation19, Citation20). Unfortunately, to the best of our knowledge, no studies have so far shown the direct consequences of enhancing physical activity on health outcomes in COPD. Such studies are needed to underpin the evidence to enhance physical activity based on objective theories.
Experiencing exacerbations and having symptoms of dyspnea during physical activity resulting in loss of functional status are reasons to refer patients to a pulmonary rehabilitation program. Patients referred to a rehabilitation program perceive functional limitations during daily-life activities and would potentially benefit of an increased physical activity (Citation21). By acting on exercise capacity, symptoms and muscle strength, pulmonary rehabilitation aims to interrupt the vicious cycle of inactivity described above and (as included in the definition) to obtain long-term health enhancing behaviors, including an increased physical activity. By this, the negative consequences of physical inactivity can (in theory) be reversed. However, information on the specific role of physical activity in and after a rehabilitation program is limited. The present chapter will describe:
Possible benefits of increasing physical activity in the context of a pulmonary rehabilitation program
The effect of exercise training program on physical activity
Other strategies to improve physical activity
Future directions on implementing physical activity in context of pulmonary rehabilitation
The possible benefits of enhancing physical activity during and after rehabilitation
Enhancing physical activity is an important treatment goal in itself in patients with COPD (Citation16). This is particularly true for patients referred to pulmonary rehabilitation as these are selected to be patients with impaired functional status and persistent symptoms of dyspnea during daily life activities (Citation21), which is often related with physical inactivity (Citation4, Citation22). In this context, increasing physical activity can have several potential benefits.
Exercise training is an efficient way to counteract the negative influences of inactive behavior (muscle wasting, exercise intolerance, comorbidities), but does not act on the inactivity itself. Attention towards physical activity in a context of pulmonary rehabilitation gives the opportunity to act on the inactive behavior itself and not only on its consequences. This is certainly true because physiological effects of exercise training tend to wear off after the end of the program (Citation23), which is possibly due to non-adherence to suggested maintenance programs or the occurrence of exacerbations and comorbidities (Citation21). It is tempting to speculate that increased physical activity (as an alternative to a maintenance exercise program) could be sufficient to counteract the loss in training effects seen after cessation of an exercise training program. Unfortunately, there is no evidence yet to support this hypothesis. Integration of both concepts (exercise training and behavior intervention) might result in a long-term behavior change, an improved attitude towards physical activity and a conservation of training effects and functional status on the long run.
In qualitative research, patients reported that a large proportion of daily-life physical activities are affected due to disease progression. Reported activities range from difficulties during stair walking and uphill walking (which are metabolically demanding tasks), to household activities and even self-care activities (activities that barely require more energy expenditure compared to resting) (Citation24). These perceived difficulties require adaptations in daily life and result into an increased dependency. This underlines the importance of physical activity from a patient’s point of view and the relevance in the context of re-habilitation. The burden of functional impairments in daily life due to the disease is one of the reasons for patients to engage in a pulmonary rehabilitation program (Citation25). Pulmonary rehabilitation aims to improve the functional impairments and helps with the process of accepting limitations in daily life. So, from a patient‘s perspective, pulmonary rehabilitation is closely related to enhancing the daily life activities and disease burden. By addressing not only physiological responses of pulmonary rehabilitation, but also by looking at physical activity, more attention is going to re-engagement in daily life, which may potentially lead to an even higher improvement in health-related quality of life compared to pulmonary rehabilitation alone (Citation18).
A last and appealing argument is that patients who enhance their physical activity after rehabilitation have a lower risk for hospital admission (Citation26). However, so far, it remains unclear whether there is a causal relation between the change in physical activity and the risk reduction on comorbidities, hospital admission and mortality.
The effect of conventional exercise training on physical activity
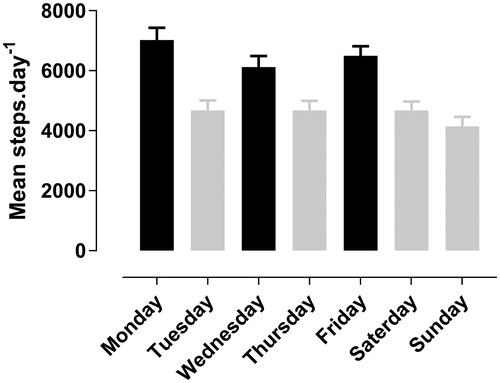
First, following an outpatient pulmonary rehabilitation program has an immediate effect on the physical activity level of patients with COPD. Due to their participation in the program, patients are ‘forced’ to be active by transportation to the rehabilitation center, walking from the car or public transport to the rehabilitation facility as well as taking part in the exercise sessions. Based on 11 consecutive patients wearing a step counter during their rehabilitation course in our center (total of 641 patient-days), we see that physical activity is 2098 steps (43%) higher on rehabilitation days compared to non-rehabilitation days (6594 ± 2763 steps ±SD versus 4608 ± 3063 steps ±SD respectively, P = 0.003) (). This is a structured, somewhat artificial, increase in physical activity, this is not thought to result in an increase in physical activity in daily life outside these planned moments. On the contrary, when we asses physical activity on a week where the exercise training is interrupted, patients do not show an enhanced physical activity level (Citation27) in absence of the ‘obligation’ to go to the pulmonary rehabilitation program.
Figure 1. Mean (SE) steps per day of 11 patients (641 patient-days), measured with waist-worn step counter (iChoice). Black bars represent training days, gray bars show non-training days; significant difference in physical activity between training and non-training days (P = 0.003).
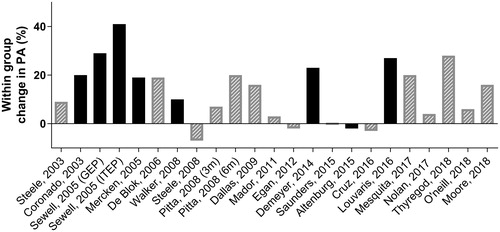
Second, following a conventional exercise training program might, by increasing the exercise capacity and lowering symptoms of dyspnea, result in an increased physical activity. Evidence on the changes in physical activity due to pulmonary rehabilitation with an emphasis on exercise training is summarized in . While some studies showed an increase in physical activity after engaging in a pulmonary rehabilitation program, other studies failed to show a significant or meaningful change. Whereas taking part in exercise training undoubtedly increases exercise tolerance and physical fitness, an exercise training program by itself does not automatically result in more daily-life activities in patients with COPD. Overall, the effect of exercise training on physical activity is small, with a weighted mean of 350 steps/day. However, recent data suggest that, whereas the overall mean changes in physical activity after 3 months of pulmonary rehabilitation were small, patients with a higher exercise capacity at baseline were more likely to have a clinically relevant increase in physical activity after the pulmonary rehabilitation program. The proportion of patients improving physical activity to a clinical significant extent (>1000 steps/day) after the program was significantly higher in the patients with a better exercise capacity (6MWD > 350m) as compared to patients with lower baseline 6MWD (38% versus 16%, P < 0.001) (Citation28). Nevertheless, the translation to enhance daily-life physical activity levels remains controversial (Citation29, Citation30).
Figure 2. Overview of physical activity within group changes from baseline (objectively measured) after following an exercise training program in different studies (adapted from Troosters, Eur Resp Rev. 2010 (Citation61) and Spruit, AJRCCM, 2015 (Citation62)). Gray bars: no statistically significant change in physical activity reported in original research, black bars: statistically significant change in physical activity reported in original research (P < 0.05). GEP = general exercise program; ITEP = individually targeted exercise program.
There are several reasons why physical activity does not improve after following a training program.
First, as reported by patients, certain barriers such as beliefs, social support and environmental factors hinder them from being active after following a rehabilitation program (Citation31, Citation32). Although a rehabilitation program aims to educate about the importance of being active, low self-efficacy and fear of dyspnea are important barriers to be active. The main reported barrier though was falling into old (inactive) habits. This may be partly genetically driven or it can be the result of the progressive and slow adaptation towards an inactive lifestyle over the years. Roberts et al. showed that after 9 generation of selective breeding in rats, there was a significant lower physical activity in the low voluntary rats compared to the original population (P < 0.001) (Citation33). Therefore, returning to old inactive habits may (at least partly) be difficult to counter. Participating in a rehabilitation program improves the social support and interaction with peers. The professional contacts are of high value for patients with COPD, resulting in a secure and pleasant atmosphere to be active (Citation31). The termination of the program with a sudden drawback of social group support can be a barrier for patients to remain more active after terminating the program. Environmental factors, such as owning a dog, taking care of grand children or the activity levels of spouses, also affects physical activity behavior (Citation34, Citation35). Therefore, the involvement of family members towards the end of the program might be beneficial to counter the loss of support.
Second, exercise training is designed to improve the physiological outcomes by using specific training principles (Citation36, Citation37), leading to enhanced exercise capacity and muscle force and decreased symptoms of dyspnea for a given exercise. However, exercise training in itself is not designed to change the behavior of patients. Physical activity is a complex, multifactorial behavior that needs specific interventions aiming at changing the inactive behavior itself. Other strategies, aiming to change physical activity behavior are discussed below.
Other strategies to improve physical activity
Other strategies to improve physical activity have been investigated in patients with COPD. These interventions can be added to the rehabilitation program or can be organized outside the program.
Adding a behavior intervention in the rehabilitation program
Since one can argue that exercise training alone is not the right intervention to change physical activity, several studies explored the effects of combining exercise training and a behavior intervention into a pulmonary rehabilitation program ().
Figure 3. Change in steps per day (objectively measured) for the intervention group in black bars (exercise training + behavior intervention) and control group in gray bars (exercise training). *Significant differences between intervention and control group in the original research (P < 0.05), ns: between group differences not significant in the original research. The gray box indicates the mean baseline 6-minute walking distance (6MWD) of the intervention group for each study. An estimation of the 6MWD was calculated based on the incremental shuttle walk test (ISWT) data reported in the paper of Nolan et al. (Citation41). This estimation was based on the correlation between ISWT and 6MWD reported by Rosa et al. (Citation63). The dotted line indicates the minimal important difference for physical activity in the context of pulmonary rehabilitation (Citation26).
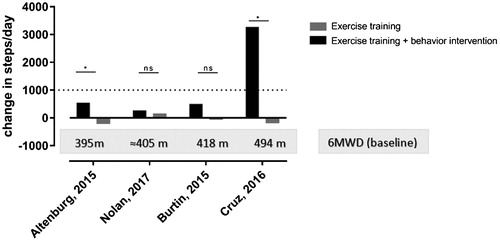
All studies displayed in implemented behavioral strategies such as goal setting, action planning, education, self-belief and feedback (Citation38) and investigated this intervention on top of exercise training. Only one study could show an improvement in physical activity in the intervention group that was clinically relevant (i.e. exceeding the MID) and concluded a significant addition of the behavioral intervention on top of exercise training (Citation39). The three other studies could not show this increase (Citation27, Citation40, Citation41). However, in patients outside the context of pulmonary rehabilitation, comparable behavior interventions based on principles of goal setting, education and feedback led to significant and clinically relevant changes in physical activity in the majority of trials (Citation40, Citation42–44).
The discrepancies between studies could be explained as follows:
First, one study gave indirect feedback using weekly reports coming from the Sensewear armband, rather than real-time feedback (Citation27), whereas the other studies used a step counter to give real-time feedback on physical activity. The use of direct, real-time feedback is superior as a behavior change strategy. Despite the use of direct feedback using step counters, Nolan et al. did not find significant differences between the intervention group (exercise training plus behavior intervention) and the control group (exercise training only) on physical activity (P = 0.99) (Citation41). These authors even found a larger improvement on health-related quality of life (fatigue, mastery and total CRQ score) in the control group compared to the intervention group. The researchers speculate that this might be explained by the added burden of using a step counter on top of the rehabilitation program. Therefore, as a second reason of failure, the timing of both interventions, at the start of the rehabilitation might not be the best. A comprehensive pulmonary rehabilitation program induces large amount of fatigue and motivating patients to be more active on the days outside rehabilitation might be a too high burden (Citation41). In addition, the timing of the physical activity assessment immediately after termination of the rehabilitation program could have partly masked the result as this is a period where patients may be ‘taking is easy’ or ‘rest’ from the efforts they did by coming to the rehabilitation program. A possible solution for the high burden of patients while combining both interventions might be to start the behavior intervention in a later phase, when patients are used to engage in the rehabilitation program, exercise capacity is increased and symptom burden reduced.
As indicated above, one study (Citation39) did show a significant and clinically relevant increase in physical activity (+4200 steps/day, P < 0.05) when adding a behavior intervention to an exercise training program. We hypothesize that this large improvement can be explained by the patient characteristics at baseline. The well-preserved exercise capacity (6MWD = 494 m) may have provided patients with sufficient ability to cope with enhanced physical activity. Baseline exercise capacity is indeed an independent baseline predictor for change in physical activity after a 3-month rehabilitation program (Citation28). We also reported that the effect of a 12-week tele coaching intervention on physical activity in patients with COPD outside a rehabilitation program was larger in patients with a better exercise capacity (6MWD > 450 meter) at baseline compared to those with lower exercise capacity (Citation42). When functional capacity is larger, and patients have thus sufficient functional reserve, it becomes easier to implement a more physically active lifestyle.
In general, we believe that a behavioral intervention can be successfully implemented in the context of a rehabilitation program, when using a step counter to provide direct feedback and when the intervention starts when patients have sufficient functional reserve (e.g. in the second phase of the rehabilitation program).
b. Exercise interventions outside the rehabilitation | |||||
Community-based exercise interventions, designed to improve exercise capacity and physical activity outside a well-equipped rehabilitation room, might facilitate the translation to behavior change in daily life compared to the conventional exercise program. One hour of Nordic walking in group three times a week over three months led to significant improvements in exercise capacity and quality of life, but also increased the time spent standing and walking and decreased sedentary time as compared to the control group (P < 0.01) (Citation45). These changes remained significant even after termination of the intervention period when, importantly, a majority of patients remained engaged with the Nordic walking group. Varas et al. showed a significant effect on exercise capacity and a clinical meaningful improvement of physical activity (+3361 steps/day, P < 0.01) by combining a community-based exercise training program with behavior interventions (e.g. education about physical activity, individualized goal setting, direct feedback provided by step counter, weekly feedback via phone call) (Citation46). Another study showed an increase in time spent walking (self-reported) (+32 min/day) by providing urban walking circuits and regular follow-up visits after a 3-month training program (Citation47).
While above-mentioned community-based studies focused on exercise training as well as physical activity, another recent study conducted in Spain, assessed the efficacy of a community-based self-paced walking program with behavioral components. A significant improvement in steps per day (+ 957 steps/day, P < 0.05) was found in the patients who did not reported unwillingness or non-adherence to the intervention (42% of initial study population) as compared to the control group (Citation48). On the average, however, no significant differences on physical activity were observed suggesting that adherence remains a key issue in these patients and interventions should focus on enhancing adherence or finding a strategy in individual patients that is of their preference. As previously indicated, behavioral interventions using a step counter and goal setting outside the rehabilitation context, could increase physical activity, with a weighted mean (1138 steps/day) exceeding the minimal important difference (Citation40, Citation42–44, Citation49, Citation50).
Community-based exercise interventions have potential to improve exercise capacity as well as physical activity, however, without behavior interventions, effect on physical activity remains limited (Citation51). It should be said that, so far successful initiatives were merely conducted in countries where the climate allows for outdoor, community-based walking programs.
Future directions on implementing physical activity in context of pulmonary rehabilitation
A standardized assessment that makes comparisons possible
A standardized assessment of physical activity is needed to get a better insight in the possible behavior interventions to enhance physical activity in the context of pulmonary rehabilitation. The use of a comparable outcome that can be measured by different devices (for example mean steps per day) is suggested to allow comparison between studies. Additionally, more standardization is needed in the way physical activity is analyzed. Demeyer et al. proposed to exclude weekend days and use a minimum of 4 weekdays as a valid physical activity assessment (Citation52). Likewise, behavior interventions should assess the subjective patients’ perspective on physical activity to create an accurate insight in the broader perspective of physical activity (including both an objective and subjective outcome) (Citation53).
Next to a standardized assessment and measurement of physical activity, standardization in the behavior intervention and importantly the description of it is needed for a proper comparison between studies. Clear insights in the set up and the form of behavior interventions is mandatory (Citation54). Direct feedback, goal setting, graded tasks, problem solving, action plans and support seem important and crucial components of behavioral interventions (Citation38).
Phasing the approach to optimize exercise capacity and behavior
Both exercise training and behavior interventions have an important place in the management of COPD. One cannot substitute the other and both interventions have their own different purposes. Exercise training along with optimal pharmacologic management is designed to alter the exercise physiology of patients with COPD, resulting in fewer symptoms of dyspnea for a given activity (Citation36) by increasing the exercise capacity and muscle force. Physical activity interventions on the other hand intend to change behavior aspects and do not change exercise capacity to a clinical relevant magnitude (Citation42). Combining exercise training with a behavior intervention, next to optimal medical therapy, resulted in less difficulties in daily life physical activity compared to a behavior intervention alone (Citation55). However, merging these interventions in the management of patients with COPD appears to be complex. Although studies show conflicting results, it should be possible to make these interventions work together.
The baseline exercise capacity has shown to be an important predictor of success and should be sufficiently high. If patients lack the exercise capacity to engage in a more active lifestyle, the first focus should likely be on interventions enhancing exercise capacity, such as optimized pharmacotherapy, exercise training or (in patients that qualify) lung volume reduction surgery. In a later phase, when capacity is optimized, a behavior intervention to translate these gains towards an active lifestyle is in its place. This strategy was already proposed by others (Citation56). Timing is important since overloading patients with a behavior intervention next to a comprehensive exercise program can lead to a too high burden, as suggested by Nolan et al (Citation41).
Duration of the intervention
Capacity and timing are not the only important elements when looking into behavior change interventions. To ensure a change in behavior, an exercise program with longer duration is needed to ensure translation in daily life (>12 weeks) (Citation29). The diminished support at the end of an exercise training program could be overcome by switching to a (tele) coaching intervention, where patients are (remotely) coached to improve physical activity after the end of a training program. By doing so, the change of pulmonary rehabilitation to daily life is less abrupt and patients still have professional support. Proper support is needed during as well as after the rehabilitation program. Even in patients with well-preserved exercise capacity (6MWT > 450 m), a coaching intervention with limited contact time between the coach and the patient and relatively static goals, did not increase physical activity (Citation49). Patients reported that the contact time between the researcher and the patient as one of the most important things in the tele coaching intervention (Citation57).
Volume and/or intensity of physical activity?
Most of the above-mentioned interventions focused on increasing the volume of physical activity (steps per day) by using a step counter. Although this is easily applicable and well understandable for patients, it does not take the concept of intensity of physical activity into account. Contemporary guidelines on physical activity in the healthy population focus on volume as well as intensity of daily life activities (Citation58).
Studies on physical activity interventions nowadays show generally limited effect on exercise capacity. The lack of attention to the appropriate intensity of physical activity might be a missing link here. More research is needed to see how these interventions can be combined to achieve long-term health benefits. In addition, it is currently unclear whether the concept of intensity should be interpreted in absolute terms (e.g. > 3 metabolic equivalents (METs)) or relative to the peak performance of patients (Citation59).
Use of technology with buy in of patients and long-term use
To ensure long-term effects, the adherence to the intervention is crucial. However, this is often difficult to achieve (Citation40, Citation48, Citation60). With the growing market of step counters and activity trackers, attention towards patients’ preferences on the use of these devices might be an important aspect in improving the long-term adherence to behavior change for many patients. More insight in these preferences and its role in long-term adherence is needed.
Conclusion
Physical activity is important for all patients with COPD. While this is probably especially the case for patients referred to pulmonary rehabilitation, evidence on physical activity in context of pulmonary rehabilitation is limited. Both exercise training as well as physical activity interventions should be an integral and complementary part of pulmonary rehabilitation and are important assets to the management of patients with COPD. Both have distinct goals; exercise training aims to enhance fitness, physical activity programs aim at behavior change towards a more active lifestyle. We suggest that exercise training is needed in the first phase of a pulmonary rehabilitation program to increase exercise tolerance and general physical fitness. Due to the type of intervention, not much change in physical activity is expected when an exercise training program is offered alone. When functional reserve is sufficiently large, behavioral interventions can be introduced to translate the physiological gains into daily-life activities. Further research is needed to answer the remaining questions concerning optimal timing, duration, intensity, patients’ preferences and patient selection when combining both interventions to achieve the long-term health enhancing behavior in these patients, which is the ultimate goal of pulmonary rehabilitation (Citation21).
Declaration of Interest Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–7.
- Waschki B, Kirsten AM, Holz O, Mueller KC, Schaper M, Sack AL, et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(3):295–306. doi: 10.1164/rccm.201501-0081OC.
- Troosters T, Sciurba F, Battaglia S, Langer D, Valluri SR, Martino L, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005–11.
- Ramon MA, Ter Riet G, Carsin AE, Gimeno-Santos E, Agusti A, Anto JM, et al. The dyspnoea-inactivity vicious circle in COPD: development and external validation of a conceptual model. Eur Respir J. 2018;52(3).
- Van Remoortel H, Hornikx M, Langer D, Burtin C, Everaerts S, Verhamme P, et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(1):30–8.
- Alahmari AD, Kowlessar BS, Patel AR, Mackay AJ, Allinson JP, Wedzicha JA, et al. Physical activity and exercise capacity in patients with moderate COPD exacerbations. Eur Respir J. 2016;48(2):340–9.
- Ehsan M, Khan R, Wakefield D, Qureshi A, Murray L, Zuwallack R, et al. A longitudinal study evaluating the effect of exacerbations on physical activity in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(6):559–64. doi: 10.1513/AnnalsATS.201304-100OC.
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–44.
- Hornikx M, Demeyer H, Camillo CA, Janssens W, Troosters T. The effects of a physical activity counseling program after an exacerbation in patients with chronic obstructive pulmonary disease: a randomized controlled pilot study. BMC Pulm Med. 2015;15:136.
- Demeyer H, Costilla-Frias M, Louvaris Z, Gimeno-Santos E, Tabberer M, Rabinovich RA, et al. Both moderate and severe exacerbations accelerate physical activity decline in COPD patients. Eur Respir J. 2018;51(1).
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–211.
- Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–54.
- Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev. 2017;97(4):1351–402. doi: 10.1152/physrev.00019.2016.
- Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–35.
- Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest. 2011;139(3):648–57.
- From the Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018 report. 2018.
- Gimeno-Santos E, Frei A, Steurer-Stey C, de Batlle J, Rabinovich RA, Raste Y, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–9.
- Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, Perez-Izquierdo J, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292–300. doi: 10.1183/09031936.00021409.
- Duenas-Espin I, Demeyer H, Gimeno-Santos E, Polkey MI, Hopkinson NS, Rabinovich RA, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287–95.
- Durr S, Zogg S, Miedinger D, Steveling EH, Maier S, Leuppi JD. Daily physical activity, functional capacity and quality of life in patients with COPD. COPD. 2014;11(6):689–96. doi: 10.3109/15412555.2014.898050.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64.
- Demeyer H, Duenas-Espin I, De Jong C, Louvaris Z, Hornikx M, Gimeno-Santos E, et al. Can health status questionnaires be used as a measure of physical activity in COPD patients? Eur Respir J. 2016;47(5):1565–8. doi: 10.1183/13993003.01815-2015.
- Jacome C, Marques A. Short- and long-term effects of pulmonary rehabilitation in patients with mild COPD: a comparison with patients with moderate to severe COPD. J Cardiopulm Rehabil Prev. 2016;36(6):445–53.
- Dobbels F, de Jong C, Drost E, Elberse J, Feridou C, Jacobs L, et al. The PROactive innovative conceptual framework on physical activity. Eur Respir J. 2014;44(5):1223–33. doi: 10.1183/09031936.00004814.
- Meis JJ, Bosma CB, Spruit MA, Franssen FM, Janssen DJ, Teixeira PJ, et al. A qualitative assessment of COPD patients' experiences of pulmonary rehabilitation and guidance by healthcare professionals. Respir Med. 2014;108(3):500–10.
- Demeyer H, Burtin C, Hornikx M, Camillo CA, Van Remoortel H, Langer D, et al. The minimal important difference in physical activity in patients with COPD. PLoS One. 2016;11(4):e0154587.
- Burtin C, Langer D, van Remoortel H, Demeyer H, Gosselink R, Decramer M, et al. Physical activity counselling during pulmonary rehabilitation in patients with COPD: a randomised controlled trial. PLoS One. 2015;10(12):e0144989. doi: 10.1371/journal.pone.0144989.
- Osadnik CR, Loeckx M, Louvaris Z, Demeyer H, Langer D, Rodrigues FM, et al. The likelihood of improving physical activity after pulmonary rehabilitation is increased in patients with COPD who have better exercise tolerance. Int J Chron Obstruct Pulmon Dis. 2018;13:3515–27. doi: 10.2147/COPD.S174827.
- Mantoani LC, Rubio N, McKinstry B, MacNee W, Rabinovich RA. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48(1):69–81.
- Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2016;11:3121–36.
- Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
- Kosteli MC, Heneghan NR, Roskell C, Williams SE, Adab P, Dickens AP, et al. Barriers and enablers of physical activity engagement for patients with COPD in primary care. Int J Chron Obstruct Pulmon Dis. 2017;12:1019–31. doi: 10.2147/COPD.S119806.
- Roberts MD, Brown JD, Company JM, Oberle LP, Heese AJ, Toedebusch RG, et al. Phenotypic and molecular differences between rats selectively bred to voluntarily run high vs. low nightly distances. Am J Physiol Regul Integr Comp Physiol. 2013;304(11):R1024–35.
- Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, Benet M, Borrell E, Dadvand P, et al. Socio-environmental correlates of physical activity in patients with chronic obstructive pulmonary disease (COPD). Thorax. 2017;72(9):796–802. doi: 10.1136/thoraxjnl-2016-209209.
- Mesquita R, Nakken N, Janssen DJA, van den Bogaart EHA, Delbressine JML, Essers JMN, et al. Activity levels and exercise motivation in patients with COPD and their resident loved ones. Chest. 2017;151(5):1028–38.
- Casaburi R, Porszasz J, Burns MR, Carithers ER, Chang RS, Cooper CB. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(5):1541–51.
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigare R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. doi: 10.1164/rccm.201402-0373ST.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. doi: 10.1007/s12160-013-9486-6.
- Cruz J, Brooks D, Marques A. Walk2Bactive: a randomised controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron Respir Dis. 2016;13(1):57–66.
- Altenburg WA, ten Hacken NH, Bossenbroek L, Kerstjens HA, de Greef MH, Wempe JB. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med. 2015;109(1):112–21. doi: 10.1016/j.rmed.2014.10.020.
- Nolan CM, Maddocks M, Canavan JL, Jones SE, Delogu V, Kaliaraju D, et al. Pedometer step count targets during pulmonary rehabilitation in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med. 2017;195(10):1344–52.
- Demeyer H, Louvaris Z, Frei A, Rabinovich RA, de Jong C, Gimeno-Santos E, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax. 2017;72(5):415–23. doi: 10.1136/thoraxjnl-2016-209026.
- Moy ML, Collins RJ, Martinez CH, Kadri R, Roman P, Holleman RG, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest. 2015;148(1):128–37. doi: 10.1378/chest.14-1466.
- Mendoza L, Horta P, Espinoza J, Aguilera M, Balmaceda N, Castro A, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J. 2015;45(2):347–54. doi: 10.1183/09031936.00084514.
- Breyer MK, Breyer-Kohansal R, Funk GC, Dornhofer N, Spruit MA, Wouters EF, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res. 2010;11:112.
- Varas AB, Cordoba S, Rodriguez-Andonaegui I, Rueda MR, Garcia-Juez S, Vilaro J. Effectiveness of a community-based exercise training programme to increase physical activity level in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Physiother Res Int. 2018;23(4):e1740. doi: 10.1002/pri.1740.
- Pleguezuelos E, Perez ME, Guirao L, Samitier B, Ortega P, Vila X, et al. Improving physical activity in patients with COPD with urban walking circuits. Respir Med. 2013;107(12):1948–56.
- Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, Balcells E, Benet M, Borrell E, et al. Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J. 2018;52(4).
- Vorrink SN, Kort HS, Troosters T, Zanen P, Lammers JJ. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur Respir J. 2016;48(4):1019–29.
- Wan ES, Kantorowski A, Homsy D, Teylan M, Kadri R, Richardson CR, et al. Promoting physical activity in COPD: Insights from a randomized trial of a web-based intervention and pedometer use. Respir Med. 2017;130:102–10. doi: 10.1016/j.rmed.2017.07.057.
- Wootton SL, Hill K, Alison JA, Ng LWC, Jenkins S, Eastwood PR, et al. Effects of ground-based walking training on daily physical activity in people with COPD: A randomised controlled trial. Respir Med. 2017;132:139–45. doi: 10.1016/j.rmed.2017.10.008.
- Demeyer H, Burtin C, Van Remoortel H, Hornikx M, Langer D, Decramer M, et al. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest. 2014;146(2):318–27. doi: 10.1378/chest.13-1968.
- Gimeno-Santos E, Raste Y, Demeyer H, Louvaris Z, de Jong C, Rabinovich RA, et al. The PROactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur Respir J. 2015;46(4):988–1000. doi: 10.1183/09031936.00183014.
- Leidy NK, Kimel M, Ajagbe L, Kim K, Hamilton A, Becker K. Designing trials of behavioral interventions to increase physical activity in patients with COPD: insights from the chronic disease literature. Respir Med. 2014;108(3):472–81.
- Troosters T, Maltais F, Leidy N, Lavoie KL, Sedeno M, Janssens W, et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(8):1021–32. doi: 10.1164/rccm.201706-1288OC.
- Singh S. Physical activity and pulmonary rehabilitation - A competing agenda? Chron Respir Dis. 2014;11(4):187–9. doi: 10.1177/1479972314552999.
- Loeckx M, Rabinovich RA, Demeyer H, Louvaris Z, Tanner R, Rubio N, Frei A, De Jong C, Gimeno-Santos E, Rodrigues FM, Buttery SC, Hopkinson NS, Büsching G, Strassmann A, Serra I, Vogiatzis I, Garcia-Aymerich J, Polkey MI, Troosters T. Smartphone-Based Physical Activity Telecoaching in Chronic Obstructive Pulmonary Disease: Mixed-Methods Study on Patient Experiences and Lessons for Implementation. JMIR Mhealth Uhealth. 2018 Dec 21;6(12):e200. epub. doi: 10.2196/mhealth.9774.
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–8.
- van Remoortel H, Camillo CA, Langer D, Hornikx M, Demeyer H, Burtin C, et al. Moderate intense physical activity depends on selected Metabolic Equivalent of Task (MET) cut-off and type of data analysis. PLoS One. 2013;8(12):e84365. doi: 10.1371/journal.pone.0084365.
- Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, et al. Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res. 2016;18(8):e215. doi: 10.2196/jmir.5622.
- Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. Eur Respir Rev. 2010;19(115):24–9. doi: 10.1183/09059180.00007809.
- Spruit MA, Pitta F, McAuley E, ZuWallack RL, Nici L. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):924–33. doi: 10.1164/rccm.201505-0929CI.
- Rosa FW, Camelier A, Mayer A, Jardim JR. Evaluating physical capacity in patients with chronic obstructive pulmonary disease: comparing the shuttle walk test with the encouraged 6-minute walk test. J Bras Pneumol. 2006;32(2):106–13.
