Abstract
Chronic obstructive pulmonary disease (COPD) and coronary artery disease (CAD) are leading causes of morbidity and mortality. There are conflicting results regarding the association between COPD and CAD. We sought to measure the association between COPD and angiographically diagnosed CAD in a population-based cohort. We performed a retrospective analysis using data from the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH), a prospectively collected registry capturing all patients undergoing coronary angiography in Alberta, Canada, since 1995. We included adult patients who had undergone coronary angiogram between April 1, 2007 and March 31, 2014. CAD was present if at least one coronary artery had a significant stenosis ≥50%. COPD was present if the patient had a documented COPD history and was prescribed bronchodilators or inhaled steroids. We evaluated the association between COPD and CAD using univariable and multivariable logistic regression. There were 26,137 patients included with a mean age of 63.3 ± 12.2 years, and 19,542 (74.8%) were male. The crude odds ratio (OR) of having CAD was 0.83 (95% CI 0.74–0.92) for patients with COPD compared to those without COPD. The adjusted OR was 0.75 (95% CI 0.67–0.84) after controlling for age, sex, smoking history, body mass index, hypertension, diabetes, hyperlipidemia, peripheral artery disease and cardiac family history. In patients undergoing coronary angiography, COPD was negatively associated with CAD with and without the adjustment for classic risk factors. COPD patients should be properly examined for heart disease to reduce premature mortality.
Background
Chronic Obstructive Pulmonary Disease (COPD) is a common disease that is characterized by persistent and progressive airflow limitation and is associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and the lung (Citation1). Both COPD and Coronary Artery Disease (CAD) are leading causes of morbidity and mortality globally, and result in a significant societal burden (Citation2, Citation3). In a 30 years surveillance study in the United States, COPD affected 5%–10% of the population (Citation4), while CAD caused more than 116 per 100,000 deaths, and accounted for 50% of all cardiovascular related deaths in the United States in 2009 (Citation5). COPD and CAD share many risk factors such as smoking and age. Campo et al. summarized the main possible mechanisms for the co-existence of COPD and CAD were smoking habit, hypoxia, systematic inflammation, platelet reactivity and arterial stiffness (Citation6). COPD patients have various concomitant diseases and are especially prone to cardiovascular disease (Citation1, Citation7). In patients with ischemic heart disease, Nishiyama et al. have demonstrated that COPD is an independent risk factor for long-term cardiac mortality (Citation8). COPD severity is independently associated with the severity of coronary calcification, a noninvasive marker of CAD risk (Citation9), and some have found that COPD is independently associated with the severity of CAD measured by diagnostic coronary angiography (Citation10). However, one systemic review found that only five of the nine relevant studies reported an increased risk of CAD in patients with COPD (Citation11). The review authors also pointed out that some negative studies tended to have smaller sample sizes (Citation11). And in a case-control study, it was found that COPD was not associated with CAD when adjusting for classic cardiovascular risk factors (Citation12). Therefore, there remains some doubt as to whether COPD is truly an independent risk factor for CAD. Confirming an association between COPD and CAD is important, as it may have implications for the management of the co-existence of these two diseases (Citation11). Therefore, we sought to determine if there is an independent association between COPD and angiographically diagnosed CAD by analyzing a large population-based cohort of patients undergoing coronary angiography.
Methods
We obtained data from the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH). APPROACH is a prospective clinical collection registry capturing information from patients undergoing cardiac intervention and revascularization in the province of Alberta, Canada, since 1995 (Citation13). The research files in APPROACH are routinely merged with Administrative health data. This registry contains detailed clinical information including demographics, common cardiac risk factors, co-morbidities, procedure indications and coronary anatomy (Citation14). All the angiograms were evaluated by an independent cardiologist blinded to the patients’ clinical data. Alberta has a population of about 3.6 million, and there are in total 3 cardiac catheterization laboratories located in 2 large cities in Alberta (Citation15).
This is a retrospective cohort study design. This study was approved by the University of Alberta Health Research Ethics Board. We included patients aged >18 years who had undergone coronary angiogram for any reason between April 1, 2007 and March 31, 2014 (7 fiscal years). A patient was considered to have CAD if at least 1 coronary artery (left main [LM], left anterior descending artery [LAD], right coronary artery [RCA] or left circumflex [LCX]) had a significant stenosis ≥50%. Multivessel diseases were defined as more than one coronary artery having significant stenosis ≥ 50%. COPD was present if the patient had a documented COPD history and was on a pharmacological therapy (i.e. bronchodilators or inhaled steroids). This definition has a very high diagnostic validity (Specificity = 99.2%, sensitivity = 54.9%, positive predictive value = 90.8%, negative predictive value = 92.2%) according to a validation study (Citation16). This is also a very common approach in the literature (Citation17–19). Former and current cigarette smokers were all defined as smokers. Diabetes mellitus was defined as a history of diabetes mellitus diagnosed and/or treated by a physician. If a patient had typical symptoms of claudication or had prior corrective surgery, angioplasty or amputation to the extremities, it was diagnosed with peripheral artery disease. Hypertension and hyperlipidemia were defined according to Canadian Medical Association Guideline 2000 (Citation20, Citation21). A patient’s Body Mass Index (BMI) was calculated as the patient’s weight in kilograms divided by the patient’s height in meters squared. The family history of heart disease was presented if the immediate family of the patient had a cardiac history at the age of fewer than 60 years.
Statistical analyses
Continuous and categorical variables were expressed as mean ± standard deviation (SD) and frequency (percentage), respectively. Independent samples t-test and chi-square test were used for the comparison between groups. A 2-tailed p-value < 0.05 was considered statistically significant. Univariable logistic regression models were first conducted, followed by a multivariable logistic regression model to evaluate the association between CAD and COPD adjusting for classic cardiovascular risk factors including age, sex, cigarette smoking, cardiac family history, diabetes mellitus, hypertension, hyperlipidemia, BMI and peripheral artery disease. Odds ratios (OR) and their 95% confidence intervals were used and reported to measure the effect of association. The interaction between smoking history and COPD was tested, and Hosmer-Lemeshow goodness of fit test was examined. We also conducted pre-specified subgroup analyses by fitting the multivariable logistic regression model in each fiscal year. All statistical analyses were performed with statistical software STATA version 13.1 (Stata Corp., Houston, Texas, USA).
Results
There were 26,137 patients included in the analysis, with a mean age of 63.3 ± 12.2 years and 74.8% of which were male (). COPD was identified in 3523 patients (13.5%). The prevalences of CAD in patients with COPD and without COPD were 86.6% and 88.7%, respectively. The patients in the COPD group were significantly older than the patients without COPD (67.7 ± 11.3 years vs. 62.6 ± 12.2 years, p < 0.001). There were significant differences between the two groups in terms of smoking history, co-morbidities of diabetes mellitus, hypertension, hyperlipidemia, peripheral vascular disease and family history of heart attack. In contrast, the average BMI showed no statistical difference between the two groups (p = 0.725).
Table 1. Baseline characteristics of the study population.
shows the angiographic presentation of the study population. Significant coronary artery stenosis was more likely to be observed in the COPD group than in the non-COPD group in the RCA, LCX and LM (p < 0.001). Half of the subjects with COPD had multivessel diseases, which was significantly greater than the proportion in the non-COPD group (45.1%, p < 0.001).
Table 2. Angiographic characteristics of the study population.
The crude OR of having CAD was 0.83 (95% CI 0.74–0.92) for patients with COPD compared to those without COPD in the univariable logistic regression analysis (). After controlling for age, sex, smoking history, BMI, hypertension, diabetes, hyperlipidemia, peripheral artery disease and cardiac family history in the multivariable logistic regression, the OR was 0.75 (95% CI 0.67–0.84). No interaction was found between smoking history and COPD (p = 0.75). The p-value for the Hosmer-Lemeshow goodness of fit test was 0.36, which indicates that this model fits the data well.
Table 3. Univariable and multivariable logistic regression analysis to determine the presence of ≥50% stenosis in 1 or more coronary arteries.
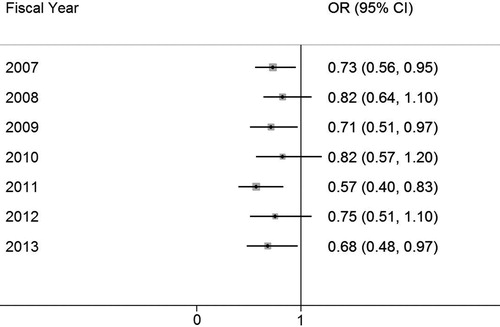
shows the OR of having CAD in the COPD group compared to the non-COPD group and its corresponding 95% CIs in each fiscal year from 2007 to 2013. The OR of having CAD in the COPD group ranged from 0.57 to 0.82 across 7 fiscal years, despite the non-significant findings in 2008, 2010 and 2012.
Discussion
We found that COPD was negatively associated with CAD, with or without controlling for classic cardiovascular risk factors. This negative association between COPD and CAD was roughly consistent across 7 fiscal years. However, those COPD patients with CAD had significantly more coronary lesions in the RCA, LCX and LM at the segment level, compared with the CAD patients without COPD. Higher proportions of multivessel lesions were also found in the COPD group compared to the non-COPD group.
It is important to investigate the COPD-CAD relationship. Studies have shown that the prevalence of patients with multiple chronic diseases is considerably greater: more than two-thirds (68%) have ≥2 chronic diseases, and 14% have ≥6 chronic diseases (Citation22). And many clinical trials often exclude patients with co-morbidities, limiting the potential to generalize the findings to a larger population (Citation23). Therefore, more real-world evidence studying patients with both COPD and CAD are warranted to complement the limitation of clinical trials.
In our study, the crude OR of having CAD was 0.83 for the COPD group in univariable logistic regression analysis, which was different from the findings in . The reason was that the COPD group had a significantly higher probability of having multi-vessel lesions than the non-COPD group, and the analysis of angiographic characteristics was on a segment level (RCA, LAD, LCX and LM).
In the literature, the adjusted relative risk of CAD between patients with and without COPD varies between 0.7 and 6.8, and one systemic review demonstrated that only five of the nine relevant studies reported an increased risk of CAD for patients with COPD (Citation11), among which some negative studies tended to have smaller sample sizes (Citation11). However, we found an inverse relationship between CAD and COPD before and after adjustment for risk factors in a large population-based cohort, so this negative association cannot be explained by a small sample size limitation. This association was also showed to be relatively consistent in the subgroup analyses that the point estimates of the OR for CAD in each year were all smaller than one with 5 out of 8 95% CIs being significant (). This kind of inconsistent findings in the literature may attribute to the differences in the populations studied as well as study designs, including differences in how CAD is ascertained as well as which risk factor was controlled.
A study comparing carotid intima-media thickness to angiography in patients suspected of CAD have indicated that, in COPD patients, coronary lesions were more frequently seen in LM, and LCX, which is similar to our findings (Citation24). Both Almagro et al. (Citation25) and Liang et al. (Citation26) have also demonstrated similar findings that the patients with COPD have more coronary vessels affected.
The underlying mechanism for a COPD-CAD association is unknown. Some advocates of positive associations between COPD and CAD believe that low-grade systemic inflammation may contribute to the higher prevalence of cardiovascular complication in COPD patients (Citation27). However, there are conflicting findings regarding the inflammation hypothesis for atherothrombosis. Ridker et al. found that anti-inflammatory therapy targeting the interleukin-1β innate immunity pathway with canakinumab significantly reduced the rate of recurrent cardiovascular events compared to the placebo group (Citation28). In contrast, several randomized controlled trials of testing anti-inflammatory agents in patients with CAD have not demonstrated any benefit. For example, a randomized controlled trial of using statins as a potential anti-inflammatory agent showed a daily dose of Simvastatin did not affect the exacerbation rate or the time to the first exacerbation in COPD patients with high risk of exacerbation (Citation29). Another randomized control trial found that almonds, which reduce cardiovascular risk via suppressing inflammation, do not significantly impact vascular function in CAD patients (Citation30). In addition, colchicine, known for its anti-inflammatory effects, failed to improve the inflammatory profiles or reduce the infarct size in patients admitted for ST-segment elevation myocardial infarction (Citation31). Hypoxia is one of the common manifestations in patients with COPD. It has been shown that hypoxia could improve the outcome of CAD patients, which may partly support the inverse relationship between COPD and CAD. Liang etc. found that long-term remote hypoxic preconditioning improved the endothelial function in patients with CAD (Citation32). A systematic review summarizing the remote ischemic conditioning-related effected on myocardial injury biomarkers also demonstrated that remote preconditioning appeared to reduce ischemia and long-term clinical events (Citation33). This systematic review implies that hypoxia could have a protective effect on CAD, which could be a possible mechanism to explain the paradoxical negative associations between COPD and CAD. An alternative explanation for our findings is the existence of the competing risk of death. Patients with mild COPD (Global Initiative for Obstructive Lung Disease [GOLD] B type), which have more prevalent ischemic heart disease and previous myocardial infarction, are reported to be associated with higher mortality than patients with severe COPD (GOLD C type) (Citation34). It has recently been shown that using the GOLD standard instead of the lower limits of normal (LLN, 5th percentile of a healthy, nonsmoking population) (Citation35) has the potential to over-diagnose COPD in older people with cardiovascular disease (Citation36, Citation37). The GOLD standard uses a fixed ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity of <0.7, which declines with age and differs between the sexes and by ethnicity (Citation38). The COPD B type was probably misdiagnosed as COPD by using the GOLD criteria for defining airflow obstruction instead of the LLN criteria. The under-represented mild COPD patients may die before their coronary arteries being examined, which could explain the negative COPD-CAD association. It also suggests that COPD patients should be properly examined for the presence of cardiac disease to reduce premature mortality.
Smoking is a shared risk factor for COPD and CAD. Smoke and other inhaled noxious particles are considered the key factors in the inflammatory responses of the lung and arterial wall, which induce airway obstruction and promote atherosclerosis (Citation6). In the multivariable analysis, controlling for the smoking effect revealed a more negative relationship between COPD and CAD, confirming that smoking is an important exposure for the development of CAD. The mechanism for an inverse association between COPD and CAD is not clear, but our data do not support the statement that COPD-associated inflammation contributes to the development of CAD.
Our study has a number of strengths. First of all, this large provincial retrospective cohort study design has enabled us to reduce possible selection bias and random error by capturing all the patients undergoing coronary catheterization in Alberta, Canada, when compared with single-center regional studies. Secondly, by using coronary angiography, which is the gold standard for diagnosing CAD, the information bias in terms of the outcome of interest has been extensively minimized. In addition, multivariable logistic regression was used to control the confounding effects of classic risk factors for CAD, including the share risk factors age and smoking between CAD and COPD.
There are also some limitations in this study. Firstly, due to the nature of the observational study design, we were unable to adjust for all possible confounders, including diet and physical activities. However, we treated BMI as a proxy in the regression model, since BMI is highly related to diet and physical activities. Secondly, a number of COPD patients may have been misclassified as non-COPD patients by the definition we used (Sensitivity = 54.9%), which may bias the OR estimate. However, since severer cases of COPD can be more easily identified through administrative data than mild cases, if we assume that COPD has a biological dose-response effect on the CAD, our results may underestimate the effect size, a perfect classification of the COPD patients will only result in an estimate that is further away from null. As a result, this limitation is unlikely to change the direction of the COPD-CAD relationship found in this study. Thirdly, for a similar degree of coronary artery stenosis, COPD patients with mild CAD may have less chance to trigger angina than the non-COPD group due to less physical activity restricted by dyspnea. This could bias toward fewer COPD patients with mild CAD going forward to coronary angiogram and those who had angiography tend to have severe coronary lesions, which may explain our findings of more severe CAD in COPD subjects but with a lower overall probability of CAD being present in COPD. Lastly, in this cohort of patients suspected of having CAD, the prevalence of CAD in patients with COPD was 86.6%, which was higher than the general COPD population, which ranges from 4.7% to 60% (Citation11). Thus, our findings may not apply to the general population of COPD directly.
Conclusion
In patients undergoing coronary angiography, COPD was negatively associated with CAD with and without adjustment for classic risk factors. COPD patients should be properly examined for heart disease to reduce premature mortality.
Declaration of interest
The authors report no conflict of interest.
Additional information
Funding
References
- Vestbo J, Hurd SS, Rodriguez-Roisin R. The 2011 revision of the global strategy for the diagnosis, management and prevention of COPD (GOLD)-why and what? Clin Respir J. 2012;6(4):208–14.
- Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608.
- Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46(4):517–92.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. Respir Care. 2002;47(10):1184–99.
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220.
- Campo G, Pavasini R, Malagu M, Mascetti S, Biscaglia S, Ceconi C, Papi A, Contoli M. Chronic obstructive pulmonary disease and ischemic heart disease comorbidity: overview of mechanisms and clinical management. Cardiovasc Drugs Ther. 2015;29(2):147–57. doi:10.1007/s10557-014-6569-y.
- Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, Rutten EP, Op 't Roodt J, Wouters EF, Franssen FM. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–35. doi:10.1164/rccm.201209-1665OC.
- Nishiyama K, Morimoto T, Furukawa Y, Nakagawa Y, Ehara N, Taniguchi R, Ozasa N, Saito N, Hoshino K, Touma M. Chronic obstructive pulmonary disease-an independent risk factor for long-term cardiac and cardiovascular mortality in patients with ischemic heart disease. Int J Cardiol. 2010;143(2):178–83.
- Rasmussen T, Køber L, Pedersen JH, Dirksen A, Thomsen LH, Stender S, Brodersen J, Groen J, Ashraf H, Kofoed KF. Relationship between chronic obstructive pulmonary disease and subclinical coronary artery disease in long-term smokers. Eur Heart J Cardiovasc Imaging. 2013;14(12):1159–66. doi:10.1093/ehjci/jet057.
- Topsakal R, Kalay N, Ozdogru I, Cetinkaya Y, Oymak S, Kaya MG, Dogan A, Inanc MT, Ergin A. Effects of chronic obstructive pulmonary disease on coronary atherosclerosis. Heart Vessels. 2009;24(3):164–8. doi:10.1007/s00380-008-1103-4.
- Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):1163–78. doi:10.1378/chest.12-2847.
- Izquierdo JL, Martinez A, Guzman E, de Lucas P, Rodriguez JM. Lack of association of ischemic heart disease with COPD when taking into account classical cardiovascular risk factors. Int J Chron Obstruct Pulmon Dis. 2010;5:387–94.
- Shavadia J, Norris CM, Graham MM, Verma S, Ali I, Bainey KR. Symptomatic graft failure and impact on clinical outcome after coronary artery bypass grafting surgery: Results from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease registry. Am Heart J. 2015;169(6):833–40. doi:10.1016/j.ahj.2015.02.022.
- Kaila KS, Norris CM, Graham MM, Ali I, Bainey KR. Long-term survival with revascularization in South Asians admitted with an acute coronary syndrome (from the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease Registry). Am J Cardiol. 2014;114(3):395–400. doi:10.1016/j.amjcard.2014.04.051.
- Bresee LC, Knudtson ML, Zhang J, et al. Likelihood of coronary angiography among First Nations patients with acute myocardial infarction. CMAJ. 2014;186(10):E372–80. doi:10.1503/cmaj.131667.
- Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–41. doi:10.1111/j.1475-6773.2007.00822.x.
- Vozoris NT, Wang X, Austin PC, O'Donnell DE, Aaron SD, To TM, Gershon AS. Incident diuretic drug use and adverse respiratory events among older adults with chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2018;84(3):579–89.
- McGuire K, Avina-Zubieta JA, Esdaile JM, Sadatsafavi M, Sayre EC, Abrahamowicz M, Lacaille D. Risk of incident chronic obstructive pulmonary disease (COPD) in rheumatoid arthritis: a population based cohort study. Arthritis Care Res (Hoboken). 2017. doi:10.1002/acr.23410. [Epub ahead of print]
- Lee TM, Tu K, Wing LL, Gershon AS. Identifying individuals with physician-diagnosed chronic obstructive pulmonary disease in primary care electronic medical records: a retrospective chart abstraction study. NPJ Prim Care Respir Med. 2017;27(1):34.
- Fodor JG, Frohlich JJ, Genest JJ, McPherson PR. Recommendations for the management and treatment of dyslipidemia Report of the Working Group on Hypercholesterolemia and Other Dyslipidemias. Can Med Assoc J. 2000;162(10):1441–7.
- Feldman RD, Campbell N, Larochelle P, Bolli P, Burgess ED, Carruthers SG, Floras JS, Haynes RB, Honos G, Leenen FH. 1999 Canadian recommendations for the management of hypertension. Can Med Assoc J. 1999;161(12 suppl):S1–S17.
- Lochner KA, Goodman RA, Posner S, Parekh A. Multiple chronic conditions among Medicare beneficiaries: state-level variations in prevalence, utilization, and cost, 2011. Medicare Medicaid Res Rev. 2013;3(3).
- Arnett DK, Goodman RA, Halperin JL, Anderson JL, Parekh AK, Zoghbi WA. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. J Am Coll Cardiol. 2014;64(17):1851–6. doi:10.1016/j.jacc.2014.07.012.
- Koseoglu C, Kurmus O, Ertem AG, Colak B, Bilen E, Ipek G, Durmaz T, Keles T, Bozkurt E. Association between carotid intima-media thickness and presence of coronary artery disease in chronic obstructive pulmonary disease patients. Anatol J Cardiol. 2016;16(8):601–7.
- Almagro P, Lapuente A, Pareja J, Yun S, Garcia ME, Padilla F, Heredia JL, De la Sierra A, Soriano JB. Underdiagnosis and prognosis of chronic obstructive pulmonary disease after percutaneous coronary intervention: a prospective study. Int J Chron Obstruct Pulmon Dis. 2015;10:1353.
- Liang B, Xu Z, Yi Q, Ou X, Feng Y. Association of chronic obstructive pulmonary disease with coronary artery disease. Chin Med J. 2012;126(17):3205–8.
- Sin DD, Man SP. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? Circulation. 2003;107(11):1514–9. doi:10.1161/01.CIR.0000056767.69054.B3.
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
- Criner GJ, Connett JE, Aaron SD, Albert RK, Bailey WC, Casaburi R, Cooper JA Jr, Curtis JL, Dransfield MT, Han MK. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201–10.
- Chen CY, Holbrook M, Duess MA, Dohadwala MM, Hamburg NM, Asztalos BF, Milbury PE, Blumberg JB, Vita JA. Effect of almond consumption on vascular function in patients with coronary artery disease: a randomized, controlled, cross-over trial. Nutr J. 2015;14:61.
- Akodad M, Lattuca B, Nagot N, et al. COLIN trial: value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis. 2017;110(6–7):395–402. doi:10.1016/j.acvd.2016.10.004.
- Liang Y, Li YP, He F, Liu XQ, Zhang JY. Long-term, regular remote ischemic preconditioning improves endothelial function in patients with coronary heart disease. Braz J Med Biol Res. 2015;48(6):568–76. doi:10.1590/1414-431X20144452.
- Le Page S, Bejan-Angoulvant T, Angoulvant D, Prunier F. Remote ischemic conditioning and cardioprotection: a systematic review and meta-analysis of randomized clinical trials. Basic Res Cardiol. 2015;110(2):11.
- Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, Nordestgaard BG. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–81. doi:10.1164/rccm.201207-1299OC.
- Culver BH. How should the lower limit of the normal range be defined? Respir Care. 2012;57(1):136–45; discussion 43–5.
- Miller MR, Levy ML. Chronic obstructive pulmonary disease: missed diagnosis versus misdiagnosis. BMJ. 2015;351:h3021.
- Miller MR, Haroon S, Jordan RE, Sitch AJ, Dickens AP, Enocson A, Fitzmaurice DA, Adab P. Clinical characteristics of patients newly diagnosed with COPD by the fixed ratio and lower limit of normal criteria: a cross-sectional analysis of the TargetCOPD trial. Int J Chron Obstruct Pulmon Dis. 2018;13:1979–86. doi:10.2147/COPD.S146914.
- Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–51.