Abstract
Exercise can improve walking capacity in persons with chronic obstructive pulmonary disease (COPD). However, most endurance training programs use cycle ergometers. The objectives of this study were: (i) to evaluate the feasibility of a randomized controlled trial (RCT) comparing outdoor walking training (OWT) to cycle ergometer training (CT) during inpatient rehabilitation in persons with severe COPD; (ii) to estimate the effect of OWT and CT on health-related quality of life, physical capacity and physical activity; and (iii) to estimate the required sample size for a RCT. A single-blind randomized controlled feasibility trial was conducted with three months' follow-up in the rehabilitation center in Walenstadtberg, Switzerland. Sixteen patients were included in the study, which had a recruitment rate of 33% (16/48). Patients were allocated to an OWT (n = 8) or CT (n = 8) group. Participants completed 75% of scheduled training and the follow-up rate was 75%. All participants in the OWT group were satisfied with the training. The OWT group had better health-related quality of life after three weeks' training compared to the CT group (p = 0.042, 95% confidence interval (95% CI) 1.06–49.94, effect size (d)=1.19). No exacerbations occurred in the OWT group, but three occurred in the CT group after three months’ follow-up. There was no significant difference in the other outcomes. In conclusion, the study design and the OWT are feasible. Health-related quality of life improved in the OWT group compared to the CT group after three weeks' inpatient rehabilitation. A minimum of 46 participants is needed for a RCT.
Trial registration: www.who.int/trialsearch DRKS00010977
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive life-threatening condition that causes dyspnea, initially with exertion, and predisposes to exacerbations and serious illness (Citation1). Patients with COPD are physically less active and spend less time walking per day compared to healthy people (Citation2,Citation3). Inactivity is a high-risk factor for exacerbation of COPD and hospital admissions (Citation4). Furthermore, dyspnea is a limiting symptom that leads to reduced physical activity and reduced exercise tolerance (Citation5). According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), COPD is graded as GOLD stages I–IV (Citation6).
Endurance training in persons with COPD (pwCOPD) GOLD stages III–IV improves exercise tolerance and skeletal muscle function by reducing dyspnea and increasing health-related quality of life (HRQoL) (Citation7,Citation8). Walking training also improves functional exercise capacity and HRQoL in pwCOPD (Citation9–11). However, more than other training modalities, walking has a higher relevance for participating in activities of daily living, as specified in the International Classification of Function, Disability and Health (ICF) (Citation12). Training in pwCOPD generally uses cycle ergometers (CE) (Citation13) or indoor walking, often performed on a treadmill (Citation9,Citation10). However, cycle ergometer and treadmill training might not be continued independently by patients after the rehabilitative programs; therefore outdoor walking may be the most effective option.
Compliance with endurance training in pwCOPD is reduced by severe dyspnea, especially during continuous training sessions (Citation14). Interval training allows better recovery from dyspnea, thereby improving compliance (Citation15,Citation16).
There is a need for evidence to show that outdoor walking training (OWT) is an equivalent training modality compared to cycle ergometer training (CT). OWT is instructed under supervision during inpatient rehabilitation and continued independently by the participants. OWT could be an inexpensive option applicable to patients in their daily life, and may increase physical activity at home and improve health-related outcomes.
The objectives of this study were to evaluate: (i) the feasibility of OWT for pwCOPD GOLD III–IV; (ii) the feasibility and effect of a RCT comparing OWT with indoor CT to estimate the effect on HRQoL, physical capacity and physical activity; and (iii) to estimate the required sample size for a RCT.
Methods
Study design
Single-blind randomized controlled feasibility trial with three months’ follow-up performed at the Rehabilitation Center Walenstadtberg, Switzerland. From July to December 2016 patients with COPD were screened for inclusion. All participants received either OWT or CT as part of an individually tailored rehabilitation program. The study was carried out in accordance with the Declaration of Helsinki, the guidelines of Good Clinical Practice (GCP), and was approved by the local ethics committee Ostschweiz (EKOS2016-00666) and registered at www.who.int/trialsearch (DRKS00010977).
Participants
Inpatients assigned to rehabilitation who had a definite diagnosis of COPD GOLD stages III (FEV1 30–50% of the predicted reference value) and IV (FEV1<30% of the predicted reference value) (Citation6) were screened for inclusion. Participants were included if they were able to walk 150 m, cycle at 25 Watt power for 30 minutes, and read and speak German. During the study participants were excluded if they developed acute exacerbation of COPD, uncontrolled arterial hypertension, cardiac insufficiency or limiting musculoskeletal disorders. All participants had physician clearance, were informed about the study and gave written consent before the study started.
Recruitment and randomization
A concealed block randomization procedure was carried out and participants were allocated to the OWT group or CT, using COPD severity (GOLD stages III and IV) as stratification factor. An independent examiner screened patients on the day after admission and evaluated patients’ ability to walk 150 m during a Six Minute Walk Test (6MWT). Candidates underwent cycle ergometry to determine maximum heart rate (HRmax) on the second day.
Interventions
Study interventions started on day three after admission. Two physiotherapists, who were experienced in pulmonary rehabilitation, supervised the training sessions. Training in both groups comprised six sessions of 30 minutes training per week over three weeks, based on the GOLD recommendations (Citation17). OWT and CT both started with 5 minutes' warming-up followed by 20 minutes' alternating one-minute intervals of high- and low-intensity, and ended with 5 minutes' cooling-down. Patients were instructed to rate dyspnea on the modified 0–10 Borg scale (Citation18–22) and were informed that very strong dyspnea (7/10) was expected during high-intensity intervals, and moderate dyspnea (3/10) during low-intensity intervals. Oxygen saturation was monitored with a portable flinger-clip oximeter (OXYMAX N-65 Technologie Nellcor, Pleasanton, CA, USA). Patients used additional oxygen as prescribed by the physician if their oxygen saturation was <90% (Citation23,Citation24).
Outdoor walking training (OWT)
OWT was based on the COPD guidelines and training concept published by Merz (Citation6,Citation7,Citation25). Training was monitored via the maximum performance values achieved by the participants during graded exercise testing (GDX) (HRpeak and maximum workload (Wmax)). The GDX results were adjusted by increasing 10 heart beats for the OWT due to orthostatic reactions from sit to stand (Citation26,Citation27).
Heart rate (HR) was monitored with a HR monitor (Polar A360, Polar Electro Oy, Oulu, Finland). High-intensity walking intervals were set at 50–70% (GOLD IV) or 70–80% of HRmax (GOLD III).
Participants were free to use walking aids and could choose between an even track or one with ascents and descents. Participants who used an oxygen cylinder transported it on a walker or in a backpack.
Cycle ergometer training (CT)
GDX was performed on a cycle ergometer (Ergoline Ergoselect 200, Bitz, Germany) at entry and discharge to determine HRpeak. HRpeak was defined as the highest HR value achieved in the test. Workload was continuously ramp-type increased by 5–10 Watts per minute to ensure voluntary exhaustion within 8–12 minutes after the start of testing or reaching symptom-limitation of the participant. HR was monitored with the Polar A360.
Indoor CT (Kardiomed Comfort Cycle, Proxomed, Alzenau, Germany) training intensity was heart rate-monitored according to Wmax achieved in GDX. High-intensity cycling intervals were set at 50–70% (GOLD IV) and at 70–80% of Wmax (GOLD III).
Analysis
Feasibility
Feasibility was determined according to the Consolidated Standards of Reporting Trials (CONSORT) statement and its extension for feasibility studies (Citation28). The study coordinator (EG) kept a study diary documenting feasibility issues. All reasons for exclusion, recruitment rate, and reasons for drop-outs were recorded. Satisfaction with treatment was evaluated at the end of rehabilitation with a customized questionnaire. Five questions with Likert-scaled and dichotomous answers addressed feasibility, training intensity and improvements in OWT. Physiotherapists reported their opinion about feasibility and satisfaction with OWT on a standardized questionnaire. Open questions covered the strengths of the intervention, problems occurring during execution, and any needs for improvement. To monitor the intensity of OWT, HRpeak was recorded during training sessions. The study coordinator recorded all reasons for missing outcome data. Based on the results, the required sample size for a future full study with G*power 3.1.9.2 was estimated (Citation29).
Efficacy and follow-up
The treatment outcomes were HRQoL, physical capacity, physical activity and exacerbations.
HRQoL was measured with the Chronic Respiratory Questionnaire (CRQ) (Citation30,Citation31) at admission, after three weeks, and at three-month follow-up.
Physical capacity was measured with the Six Minute Walk Test (6MWT), performed according to the American Thoracic Society Guidelines (Citation32) at admission and after three weeks, by an independent assessor (Citation33–35).
Physical activity (PA) was assessed with an activity tracker (Fitbit One, Fitbit Inc., San Francisco, CA, USA) (Citation36). Patients were instructed to use the tracker during waking hours for five days at the end of rehabilitation and again at three months’ follow-up.
Adverse events
Exacerbations and other adverse events were recorded in a logbook during rehabilitation. Exacerbations of COPD were defined as an acute worsening of the patient’s respiratory symptoms that result in additional therapy (Citation37). Exacerbations were evaluated by phone at three months’ follow up.
Statistics
Data were checked for normality using the Kolmogorov-Smirnov test. Explorative analyses for parametric data (CRQ total score, 6MWT, Fitbit One) included the unpaired t-test for independent variables. Between-group differences in changes in HRQoL and physical capacity were analyzed with unpaired t-tests. Changes in physical capacity were expressed as percentages. All results are reported with p-values, 95% confidence interval (95% CI) and Cohen’s d effect size (defined as small if >0.2, medium if >0.5 and large if >0.8) (Citation38). Significance was set at p = 0.05. All statistical analysis was conducted as intention to treat (ITT) using IBM SPSS version 24.0.
For data missing at random (MAR) an expectation-maximization (EM) algorithm was used (Citation37,Citation38). The EM algorithm is a maximum-likelihood-based method (Citation39).
The sample size for a full RCT was estimated using the between-group differences in the changes in HRQoL at three months' follow-up with G*power 3.1.9.2 (29) and ITT data (Citation38). Therefore, raw data were used without the EM algorithm and Cohen’s d formula (Citation38). Due to the small sample size a sensitivity analysis with different assumptions regarding the effect was performed.
Results
Recruitment, baseline characteristics and compliance
Between July and December 2016, 48 patients were screened for study participation and 16 (33%) were included in the study. Groups showed no differences at baseline, except for physical capacity in the 6MWT, which was higher in CT (p = 0.001, 95% CI 56.1–170.1, effect size (d)=2.13). Overall 75% of the patients completed the interventions according to the study protocol (n = 12). Four patients were not treated according to protocol and were listed as drop-outs. The follow-up rate was 75% (n = 12). During the intervention no adverse events occurred. shows the study flow-chart. The baseline characteristics of the participants are reported in .
Figure 1. Flow diagram of process according to the Consolidated Standards of Reporting Trials (CONSORT) statement. The figure shows the progress of the parallel randomized feasibility trial of the two groups; i.e. screening, enrollment, allocation, results after three weeks and three month follow-up. CRQ, chronic respiratory questionnaire; COPD: chronic obstructive pulmonary disease; GOLD: global initiative for chronic obstructive lung disease.
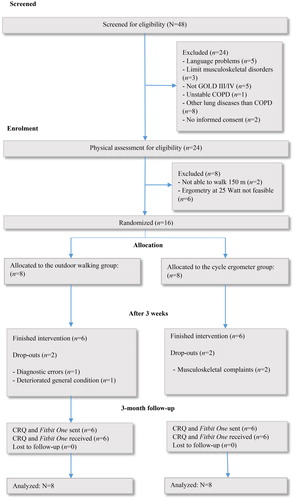
Table 1. Study participants’ baseline characteristics.
Feasibility of OWT
Participants in the CT group were able to monitor training intensity using the Watt levels; however, HR-levels determined by GDX were too low for the OWT, as shown by the modified Borg scale and the training protocol. Participants in the OWT group attended all training sessions despite occasional rainy weather. Training at the prescribed intensity was feasible for all participants. The position of the wrist, while holding the walker in dorsal flexion, disturbed the signal detection via the HR monitor. A chest strap resolved this problem. Handling the HR monitor was difficult for 70% of the patients during OWT. Participants were unable to concentrate on breathing, walking and following the prescribed time intervals. On uneven parts of the road, the walker rolled away when patients checked the HR monitor. Difficulties using the HR monitor were resolved by using the modified Borg scale instead to guide training intensity. Using GOLD stages to prescribe training intensity (50–70% of HRpeak for GOLD IV and 70–80% of HRpeak for GOLD III) was inappropriate. In 80% of the patients, training intensity had to be increased or decreased, guided by dyspnea rated on the Borg scale. Box 1 summarizes the results concerning feasibility.
Patients' satisfaction with interventions
Twelve patients completed the interventions according to the study protocol and completed the questionnaire. Satisfaction with both OWT and CT was very high (n = 8), high (n = 3), or moderate (n = 1). Training intensity was experienced as severe (n = 10) or moderately severe (n = 2) in both groups. All patients (n = 12) reported improved walking ability; for example, walking distance in daily life. Patients in the OWT were not familiar with interval training before the study and considered the interval training helpful and easy to perform at home, as outdoor walking does not require special equipment. In the CT group 87% of the patients (n = 7) stated that they expected not to continue the training at home because the equipment was not available to them.
Physiotherapists’ satisfaction with OWT
Physiotherapists reported OWT to be feasible. They emphasized the relevance of patients taking responsibility for controlling the intensity of training. This was in line with the patients’ feedback showing that, although the training was perceived as very intensive, it was experienced as effective and valuable. During each training session, patients completed repetitions of high-intensity intervals, inducing severe dyspnea. During the subsequent low-intensity intervals, they experienced recovery from dyspnea and increased confidence. Physiotherapists recommend the modified Borg scale as feasible for application at the different training intensities
Missing data
Sixteen percent of the data were missing. The use of activity trackers was difficult and led to 31% (n = 5) missing data at the end of rehabilitation and 37% (n = 6) missing data after three months' follow-up. Quality of life questionnaires were not completed by 19% (n = 3) and 25% (n = 4) of the patients respectively.
Adverse effects
No adverse effects occurred during rehabilitation. During follow-up three patients in the CT group and none in the OWT group experienced exacerbation of COPD symptoms (Box 1).
Sample size
The between-group effect size for the CRQ after three months' follow-up of inpatient rehabilitation was large (d = 0.85; 95% CI 0.511–1.189). A total of 46 pwCOPD, GOLD stages III–IV, would be needed to detect a difference this size between groups, with a two-tailed α of 0.05 and a (1–β) of 0.80 for a comparison of two independent proportions (Box 1). Between-group effect sizes are usually smaller with longer duration of follow-up. Assuming an effect size of d = 0.54 resulted in a total required sample size of 128 patients.
The results are summarized in Box 1.
Treatment outcome
shows the secondary outcomes HRQoL, physical capacity and PA at baseline, three weeks after rehabilitation and at three-month follow-up. There was a significant difference in HRQoL (CRQ) after three weeks’ rehabilitation between the groups in favor of the OWT group. There was no significant difference in the improvement in physical capacity in the 6MWT after three weeks' rehabilitation, as well as in the HRQoL (CRQ) and PA (Fitbit One) after three months' follow-up between the groups.
Table 2. Health-related quality of life (chronic respiratory questionnaire; CRQ), physical capacity (six minute walk test; 6MWT)) after three weeks’ rehabilitation and three months’ follow-up.
Discussion
This is the first randomized controlled feasibility trial to compare two different training modalities in COPD. The results suggest that, with predefined training intensities, OWT is feasible in severe and very severe pwCOPD. The recruitment rate (33%) was adequate and the inclusion criteria were chosen well. Participants in the OWT and CT group were satisfied with the training. None of participants in the OWT group showed exacerbations of COPD symptoms during the training sessions. The interval method avoided frustration in participants during endurance training for reasons of burden of physical limitation, such as dyspnea, and was well tolerated by all participants (Citation15,Citation39). There is evidence to show that interval training has psychological advantages in improving patients’ motivation (Citation40). Also, in the current study, participants in both groups showed a willingness to participate in the study procedures including the follow-up (75%).
Inclusion and exclusion criteria led to a recruitment rate of 33%. Future research will include patients able to walk 25 m instead of 150 m and will not use cycle ergometry for inclusion. This may improve the recruitment rate by 50%.
During inpatient rehabilitation this study showed that, compared with CT, OWT resulted in significantly improved HRQoL after three weeks. However, participants did not maintain this improvement during follow-up. PA decreased by 53% after three months, indicating that a continuation of OWT at home may help to maintain quality of life as well as levels of physical activity. These results are in line with the study of Egan et al. which also showed no changes in physical activity after rehabilitation (Citation41). Based on these results, it must be assumed that the OWT group stopped the training, although this was not assessed in this study.
Reasons for persons not continuing with outpatient pulmonary rehabilitation found previously were: disruption to the daily routine, influence of the referring doctors, poor access to transport, and lack of perceived benefit (Citation42). Adding a behavioral intervention could help overcome these barriers (Citation43,Citation44). Future research will include tele-rehabilitation, linking expert healthcare providers with patients at home. This has been shown to be feasible, reinforce motivation and self-esteem, improve health behavior and maintain PA in pwCOPD (Citation45,Citation46).
The training outcomes of this study showed no significant differences between the groups after three weeks' inpatient rehabilitation and three months' follow-up. This was probably due to the exercise intensities being too low to enable participants to reach their target HR. Future research will determine individual walking training intensity with a shuttle run test instead of cycle ergometry. For self-monitoring of training intensity a HR sensor and the display of a smartphone, worn on the lower arm or fixed to the rollator, will be used instead of the HR watch, which had poor usability in this study.
Overall, OWT was comparable with CT and is an effective training method for increasing HRQoL in severe pwCOPD. Fewer exacerbations of COPD were observed in the patients in the OWT group compared to those in the CT group. These findings are relevant, as exacerbations impact negatively on the patient's course of disease (Citation47).
Furthermore, mortality significantly increases with the frequency of severe exacerbations, in particular if the patients require hospitalization (Citation47). In addition, COPD exacerbations account for the greatest proportion of the (COPD-related) cost of healthcare (Citation6). In order to limit these costs, there is a need for an efficient intervention, with OWT being one option.
All participants were able to achieve the determined training intensity with the modified Borg scale. The scale enables pwCOPD to exercise with adequate training intensity (Citation19), and this could be an efficient option for the patients to continue their training at home. However, future trials must consider stricter and more assessable intensity controls: here adjustments of the training intensities were mainly performed with self-reported performance measurements of fatigability. Thus, assessment strategies that include objective sensor-based (pedometers, heart rate monitors or actigraphs) as well as subjective questionnaire-based measurements and activity logs are advised to comprehensively register changes in activity behavior following the training intervention. Since this study was performed over several seasons, the results regarding physical activity at home should be interpreted with caution. Depending on the weather conditions, patients were more or less active, especially during the winter when snow covered the roads. These findings are supported by a study by Sewell et al. in 2010, which also showed seasonal variations in the levels of daily physical activity (Citation48). For a future full RCT, we recommend recruiting patients with severe and very severe COPD during all seasons in a single year.
Limitations
This study has some limitations. First, the sample size was too small for testing hypotheses regarding effectiveness. Second, the statistical results only tend to an impression of the expected direction of results. In addition, the groups were heterogeneous, leading to bias in the comparison analysis. Third, results show 16.1% missing data, which is less than in other RCTs, but should be supported by ITT analysis (Citation49). However, the levels of missing data for physical activity were high, which impacts negatively on the internal and external validity of this trial (Citation50). This must be taken into account in the design of future studies. Fourth, GOLD stages III or IV were poorly related to patients’ training performance, as has been reported by others (Citation51). The ABCD assessment tool evaluates the impact of COPD on an individual patient (Citation37). Stages A-–D display the increasing impact of COPD on patients’ lives, based on airflow restriction (GOLD stages), symptoms (dyspnea, evaluated with a modified MRC dyspnea scale) and history of exacerbations (ABCD stages) (Citation37). In a future trial, patients should be stratified according to their physical capacity and not according to the GOLD stages. Fifth, the higher respiratory capacity during OWT was underestimated, as shown in the simultaneous increase in HR. It is known that pwCOPD exhibit higher respiratory responses during cycle exercises compared to treadmill walking, but here no significant differences in HR were observed (Citation27). We assume that the higher HR is due to the specific conditions of the walking track (gradients and unevenness of the road). Finally, no specific walking test was performed, and intensity controls were limited to self-reported performance measurements of participants’ fatigability. Reliable determination and verification of the applied training intensity for OWT are essential. OWT trials should include a symptom-limiting, incremental field walking assessment designed for pwCOPD, similar to a standardized shuttle walking test, performed at baseline and study end to individually tailor the applied training program (Citation52,Citation53).
Strengths
This study was the first to investigate the feasibility of OWT in pwCOPD GOLD stages III–IV. The study was planned as a randomized controlled feasibility trial that monitored all relevant aspects. The study procedures were well chosen and were characterized by good compliance and satisfaction among the participants. Blinded randomization was used and validated outcome measures were included. Thus this study was of high quality.
Conclusion
In conclusion, the study design and the OWT were feasible and a future RCT can be carried out. All participants were satisfied with the OWT. Participants who completed the three-week intervention also completed the final follow-up assessment. OWT increased HRQoL and led to fewer exacerbations after three weeks’ in-patient rehabilitation. OWT can be recommended for use as a supplement to inpatient rehabilitation for pwCOPD. However, a larger RCT is needed that includes additional behavioral change techniques during follow-up.
Declaration of interest statement
The authors state that they have no financial, consulting or personal relationships to people or organizations that could influence the authors' work. There are no conflicts of interests.
Acknowledgments
The authors would like to thank the rehabilitation team at the rehabilitation center Walenstadtberg for their valuable cooperation. Special thanks go to Philippe Merz who made his concept available for this study.
Winner of the ENPHE Master Thesis Award 2017
Funding
This study was financially supported by Valens Clinics.
Box 1 Feasibility of outdoor walking training (OWT) and study design
Study design
Adaptations for a future RCT
Use a walking test (instead of a cycle ergometer test) to determine HRmax for OWT.
Total sample size of 128 patients.
Extended recruitment period of three years.
OWT
All participants performed the training at the determined intensities.
High compliance rate for the training sessions despite changing weather conditions.
70% of the patients in OWT had difficulty handling the heart rate monitor and were instructed to use the modified Borg scale instead.
It is not possible to classify the patients into performance groups according to the GOLD stages.
Patients and physiotherapist were satisfied with OWT.
Patients intended to continue OWT at home.
Physiotherapists described OWT as effective, feasible and meaningful.
Low exacerbation rate after three-month follow-up in the OWT: n = 0, CT group: n = 3.
Box 1 shows the feasibility of OWT and the study design. In summary, OWT is feasible in patients with chronic obstructive pulmonary disease (COPD), the study design needs some adaptions for a future RCT. RCT; randomized controlled trial, HRmax; maximal heart rate, OWT; outdoor walking training, GOLD; global initiative for chronic obstructive lung disease, CT; cycle ergometer training
References
- WHO. Chronic obstructive pulmonary disease (COPD) [Internet]. Geneva, Switzerland: World Health Organization; 2017 [updated 2017 Nov; cited 2018 Apr 2]. Available from: http://www.who.int/mediacentre/factsheets/fs315/en/.
- Pitta F, Troosters T, Probst VS, et al. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134(2):273–80. doi:10.1378/chest.07-2655.
- Vorrink SN, Kort HS, Troosters T, et al. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12:33.
- Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–5.
- O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–4. doi:10.1513/pats.200508-093DO.
- GOLD. Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. 2017.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(5 Suppl):42–4.
- Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(1):19–38. doi:10.1164/rccm.200408-1109SO.
- Leung RW, Alison JA, McKeough ZJ, et al. Ground walk training improves functional exercise capacity more than cycle training in people with chronic obstructive pulmonary disease (COPD): a randomised trial. J Physiother. 2010;56(2):105–12. doi:10.1016/S1836-9553(10)70040-0.
- Wootton SL, Ng LW, McKeough ZJ, et al. Ground-based walking training improves quality of life and exercise capacity in COPD. Eur Respir J. 2014;44(4):885–94. doi:10.1183/09031936.00078014.
- Breyer MK, Breyer-Kohansal R, Funk GC, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res. 2010;11:112.
- WHO. International CLassification of Functioning, Disability and Health (ICF) [Internet]. Geneva, Switzerland: World Health Organisation; 2001 [updated 2017 Jan 27 cited 2017 Mar 4]. Available from: http://www.who.int/classifications/icf/en/.
- Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev. 2013;22(128):178–86. doi:10.1183/09059180.00000513.
- Maltais F, LeBlanc P, Jobin J, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(2):555–61. doi:10.1164/ajrccm.155.2.9032194.
- Puhan MA, Busching G, Schunemann HJ, et al. Interval versus continuous high-intensity exercise in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2006;145(11):816–25.
- Beauchamp MK, Nonoyama M, Goldstein RS, et al. Interval versus continuous training in individuals with chronic obstructive pulmonary disease-a systematic review. Thorax. 2010;65(2):157–64. doi:10.1136/thx.2009.123000.
- GOLD. Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. GOLD Report: Global Initiative for Chronic Obstructive Lung Disease. 2016.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
- Horowitz MB, Littenberg B, Mahler DA. Dyspnea ratings for prescribing exercise intensity in patients with COPD. Chest. 1996;109(5):1169–75.
- Gosselink R, Langer C, Burtin C, et al. KNGF clinical practice guideline for physical therapy in patients with COPD. Dutch J Phys Ther. 2008;118(4):64.
- Hareendran A, Leidy NK, Monz BU, et al. Proposing a standardized method for evaluating patient report of the intensity of dyspnea during exercise testing in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:345–55.
- Meyer FJ, Borst MM, Buschmann HC, et al. [Exercise testing in respiratory medicine]. Pneumologie. 2013;67(1):16–34. doi:10.1055/s-0032-1325901.
- Russi EW, Karrer W, Brutsche M, et al. Diagnosis and management of chronic obstructive pulmonary disease: the Swiss guidelines. Respiration. 2013:160–74. doi:10.1159/000346025.
- Guell Rous R. Long-term oxygen therapy: are we prescribing appropriately? Int J Chron Obstruct Pulmon. 2008;3(2):231–7. doi:10.2147/COPD.S1230.
- Merz P. Training von Lungenpatienten. In: Gesundheit B, editor. Lungenmobil Merz. BasStadt, Switzerland. 2015. p. 20.
- Medicine ACoS. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia (PA): Lippincott Williams & Wilkins; 2013.
- Mahler DA, Gifford AH, Waterman LA, et al. Mechanism of greater oxygen desaturation during walking compared with cycling in patients with COPD. Chest. 2011;140(2):351–8. doi:10.1378/chest.10-2415.
- Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:32–1.
- Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. doi:10.3758/BF03193146.
- Reda AA, Kotz D, Kocks JW, et al. Reliability and validity of the clinical COPD questionniare and chronic respiratory questionnaire. Respir Med. 2010;104(11):1675–82. doi:10.1016/j.rmed.2010.04.023.
- Puhan MA, Behnke M, Laschke M, et al. Self-administration and standardisation of the chronic respiratory questionnaire: a randomised trial in three German-speaking countries. Respir Med. 2004;98(4):342–50.
- ATS. American Thoracic Society Statement: guidelines for the 6-minutes walking test. Respir Crit Care Med. 2002;166:111–7.
- Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–78. doi:10.1183/09031936.00150414.
- Jenkins SC. 6-Minute walk test in patients with COPD: clinical applications in pulmonary reahbilitation. Physiotherapy 2007(93):175–82. doi:10.1016/j.physio.2007.02.001.
- Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–46. doi:10.1183/09031936.00150314.
- Takacs J, Pollock CL, Guenther JR, et al. Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport. 2014;17(5): 496–500. doi:10.1016/j.jsams.2013.10.241.
- GOLD. Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. GOLD Report. 2018.
- Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–82. doi:10.4300/JGME-D-12-00156.1.
- Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J. 2002;20(1):12–9. doi:10.1183/09031936.02.01152001.
- Bartlett JD, Close GL, MacLaren DP, et al. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci. 2011;29(6):547–53. doi:10.1080/02640414.2010.545427.
- Egan C, Deering BM, Blake C, et al. Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med. 2012;106(12):1671–9. doi:10.1016/j.rmed.2012.08.016.
- Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8(2): 89–99 doi:10.1177/1479972310393756.
- Leidy NK, Kimel M, Ajagbe L, et al. Designing trials of behavioral interventions to increase physical activity in patients with COPD: insights from the chronic disease literature. Respir Med. 2014;108(3):472–81. doi:10.1016/j.rmed.2013.11.011.
- Cavalheri V, Straker L, Gucciardi DF, et al. Changing physical activity and sedentary behaviour in people with COPD. Respirology. 2016;21(3):419–26. doi:10.1111/resp.12680.
- Holland AE, Hill CJ, Rochford P, et al. Telerehabilitation for people with chronic obstructive pulmonary disease: feasibility of a simple, real time model of supervised exercise training. J Telemed Telecare. 2013;19(4):222–6. doi:10.1177/1357633x13487100.
- Stickland M, Jourdain T, Wong EY, et al. Using Telehealth technology to deliver pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Can Respir J. 2011;18(4):216–20. doi:10.1155/2011/640865.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–31. doi:10.1136/thx.2005.040527.
- Sewell L, Singh SJ, Williams JE, et al. Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J Cardiopulm Rehabil Prev. 2010;30(5):329–33. doi:10.1097/HCR.0b013e3181e175f2.
- Hussain JA, White IR, Langan D, et al. Missing data in randomized controlled trials testing palliative interventions pose a significant risk of bias and loss of power: a systematic review and meta-analyses. J Clin Epidemiol. 2016;74:57–65. doi:10.1016/j.jclinepi.2015.12.003.
- Dong YR, Peng CYJ. Principled missing data methods for researchers. Springerplus. 2013;2: 17–1.
- Casanova C, de Torres JP, Aguirre-Jaime A, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med. 2011;184(9):1015–21. doi:10.1164/rccm.201105-0831OC.
- Singh SJ, Morgan MDL, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–24. doi:10.1136/thx.47.12.1019.
- Holland AE, Spruit MA, Singh SJ. How to carry out a field walking test in chronic respiratory disease. Breathe. 2015;11(2):129–39.
