Abstract
Although recently introduced in the pharmacological treatment algorithm of chronic obstructive pulmonary disease (COPD), there is a need for more data supporting the use of blood eosinophil counts as a biomarker to guide inhaled corticosteroids (ICS) therapy. The aim of this study was to evaluate the risk of moderate and/or severe exacerbations and all-cause mortality in a large primary care population after withdrawal of ICS compared to continued users stratified by elevated blood eosinophil counts. In this population based cohort study, we used data from the Clinical Practice Research Datalink (CPRD) in the United Kingdom. We included subjects’ aged 40 years or more who had a diagnosis of COPD. We excluded subjects with a history of asthma, pulmonary fibrosis, cardiac arrhythmia and bronchiectasis, COPD exacerbations occurring within 6 weeks prior to index date, or with a myocardial infarction within 3 months prior to index date. Continuous users were subjects who received their most recent ICS prescription within 3 months before the start of an interval. ICS withdrawals were those who discontinued ICS for more than 3 months. We evaluated the risk of moderate and/or severe exacerbations and all-cause mortality among subjects with various blood eosinophil thresholds who withdrew from ICS compared to continuous ICS users with elevated blood eosinophil levels using Cox regression analysis adjusted for potential confounders. We identified 48,157 subjects diagnosed with COPD between 1 January 2005 to 31 January 2014. Withdrawal of ICS was not associated with an increased risk of moderate-to-severe exacerbations among subjects with absolute blood eosinophil counts ≥0.34 × 109 cells/L [adjusted hazard ratio (adj. HR) 0.72; 95% confidence interval (CI) 0.63–0.81] or relative counts ≥ 4.0% (adj. HR 0.72; 95% CI: 0.66–0.78). Similarly, withdrawal of ICS was not associated with an increased risk of severe exacerbations among subjects with absolute blood eosinophil ≥0.34 × 109 cells/L (adj. HR 0.82; 95% CI: 0.61–1.10) or relative blood eosinophil counts ≥4.0% (adj. HR 0.80; 95% CI: 0.61–1.04). No increased risk of all-cause mortality was observed among subjects who withdrew from ICS irrespective of elevated absolute or relative blood eosinophil counts. In a real-world primary care population, we did not observe an increased risk of moderate and/or severe COPD exacerbations or all-cause mortality among subjects with eosinophilia who withdrew their use of ICS.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide and is defined by the presence of chronic respiratory symptoms and persistent airflow limitation (Citation1). Exacerbations play a central role in the pathophysiology of COPD as they are related to lung function decline, poor health status and increased mortality (Citation2). While bronchodilators are the cornerstone of pharmacological management of COPD, patients with frequent exacerbations are often additionally treated with inhaled corticosteroids (ICS) (Citation3). While non-response to ICS therapy is common (Citation4), potential side effects of ICS include fractures and pneumonia (Citation3).
Clinical data have suggested that elevated blood eosinophil counts, which is present in up to 40% of COPD patients (Citation5), is a promising biomarker of response to ICS (Citation6–9). Eosinophilic airway inflammation has been associated with an increased risk of exacerbations, and patients with eosinophilic inflammation responded better to ICS therapy than non-eosinophilic patients (Citation6, Citation10). The role of eosinophils in guiding ICS therapy is a growing area of research. Pascoe et al. (Citation6) performed a post hoc analysis of data and found that across all doses of ICS, fluticasone furoate and vilanterol reduced exacerbations by 29% compared with vilanterol alone in patients with eosinophil counts ≥2%, and by 10% in patients with eosinophil counts <2%. Analysis of data from the Withdrawal of Inhaled Steroids during Optimized Bronchodilator Management (WISDOM) trial showed a reduction of the rate of moderate and severe exacerbations in patients with relative eosinophil counts of 4% or more, or absolute eosinophil counts of 300 cells/µL or higher when ICS was continued compared to patients with the same eosinophil counts who tapered off ICS use. Therefore, it has been suggested that withdrawal of ICS in patients with eosinophil counts ≥4% and a history of exacerbations may increase the risk of COPD exacerbations (Citation7). Although the Global initiative for Obstructive Lung Disease (GOLD) recently introduced blood eosinophils in the pharmacological treatment algorithm of the disease (Citation11), long-term effects of blood eosinophil-guided ICS withdrawal are currently unexplored and data in patients treated in real-life clinical setting are scarce (Citation12). In this study, we use the word “eosinophilia” to refer to elevated blood eosinophil count based on our defined thresholds.
Therefore, aim of this study was to evaluate the risk of moderate and/or severe exacerbations and all-cause mortality in a large primary care population in UK after withdrawal of ICS compared to continued use of ICS among COPD subjects stratified by elevated relative or absolute eosinophil counts. A priori, we hypothesised that ICS withdrawal is associated with increased risk of exacerbations and mortality in subjects with blood eosinophilia.
Methods
Data source
This study was conducted with data obtained from the Clinical Practice Research Datalink (CPRD), providing detailed information on drug prescriptions, clinical events, demographics, specialist referrals, hospital admissions, and electronic lab link data of subjects from 674 general practices, who are representative for 7% of the total British population (Citation13, Citation14). Data collection started on 1 January 2005, corresponding to the introduction of the Quality and Outcomes Framework (QOF) in April 2004, which improved routine recording of various diseases, including COPD (Citation15). Routinely collected historical data was available, dating back to 1987. Previous studies with the CPRD have shown a high level of validity of recording of COPD (Citation13) and COPD exacerbations (Citation16). The independent scientific advisory committee (ISAC) for Medicines and Healthcare products Regulatory Authority (MHRA) database research approved the study protocol (number18_036R).
Study population
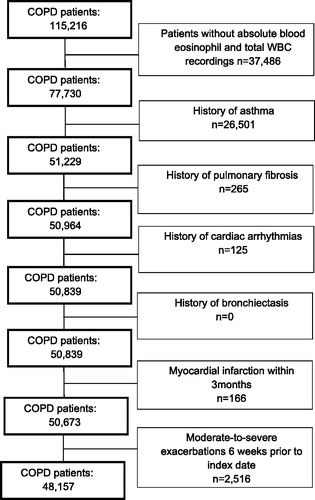
We selected subjects aged 40 years and older with a diagnosis of COPD recorded by a read code during valid data collection (1 January 2005 to 31 January 2014). Subjects were followed from the date of COPD diagnosis (index date) until their dates of transfer out of database, the end of the study period (31 January 2014), death, or until the outcome of interest occurred, whichever occurred first. Only subjects with at least one blood eosinophil measurement were included. We excluded subjects with a history of asthma, pulmonary fibrosis, cardiac arrhythmia and bronchiectasis, COPD exacerbations occurring within 6 weeks prior to index date, or with a myocardial infarction within 3 months prior to index date. We did not exclude patients with prior exposure to ICSs.
Outcomes and exposure
The primary outcome of interest was the first moderate-to-severe exacerbation of COPD. The secondary outcomes included first severe exacerbation and all-cause mortality. We used validated definitions of moderate-to-severe exacerbations and severe exacerbations of COPD using the clinical and referral files (Citation17) as listed in Supplementary material. Exposure to ICS was determined time-dependently during follow-up. Each patient’s follow-up time was divided into fixed intervals of 90 days, starting at index date. Prior to the start of each interval, ICS exposure was determined based on the most recent date ICS prescription, and classified as current, past or never use (subjects without ICS prescriptions). For this study, current ICS users were defined as continuous users; these were subjects who received their most recent ICS prescription within 3 months before the start of an interval. Past users were those who withdrew from ICS for more than 3 months. While in the Study to Understand the Safety and Efficacy of ICS Withdrawal from Triple Therapy in COPD (SUNSET) trial ICS withdrawal effects were seen after 4 weeks, the early effects were comparable with the longer-term effects, suggesting that a different time interval, for example 1 month versus the current 3 months, would not result in different results regarding these outcomes (Citation18). Moreover, as exacerbations and mortality were the primary outcomes in our study, we believe that a discontinuation of ICS beyond 3 months is more appropriate. Also in SUNSET trial, exacerbations were evaluated after 26 weeks. This threshold (3 months) have be found to be robust in evaluating the risk of exacerbations in patients withdrawn from ICS use (Citation7, Citation9). During follow-up subjects could move between different exposure categories. Continuous ICS users and those who had withdrawn ICS use were stratified by absolute eosinophil count (using 0.34 × 109 cells/L as a cut-off value) or relative eosinophil counts (using 4% as a cut-off value) (Citation7).
Covariates
Potential confounders were assessed time-dependently with the exception of gender, smoking status, alcohol use and body mass index, which were determined at baseline. The following covariates were considered as potential confounders, and identified at the start of each interval: a history of congestive heart failure, ischemic heart disease, anxiety, chronic liver disease, cancer excluding non-melanoma skin cancer, stroke, rheumatoid arthritis, diabetes mellitus, hypertension, inflammatory bowel disease, solid organ transplant, atopic dermatitis, renal dialysis, human immunodeficiency virus or osteoporosis. In addition, the use of the following drugs within 6 months prior to the start of an interval were considered as potential confounders: antihistamines, proton pump inhibitors, antipsychotics, or antidepressants (Citation19–22). We statistically adjusted our analyses for proxy indicators of the severity of obstructive airway disease, as previously defined as use of short- and long-acting β-agonists, short- and long-acting anti-muscarinic agents, xanthine derivatives or oral corticosteroids (Citation23, Citation24)
Statistical analysis
We evaluated the risk of moderate-to-severe exacerbations, severe exacerbations and all-cause mortality among subjects with elevated eosinophil counts who withdrew from ICS versus continuous ICS users with elevated eosinophil counts using Cox regression analysis (SAS 9.4) (SAS Inc., Cary, NC). The difference between each eosinophil count stratum was tested using Wald’s test. To avoid immortal time bias, never ICS users were incorporated into the statistical model. We assessed the presence of risk factors by reviewing subjects’ records prior to each 90-day interval. Our initial analysis was adjusted for age and sex. All potential confounders were tested and adjusted for in the final model if they changed the β-coefficient for continuous ICS exposure by at least 5% or when consensus about inclusion existed within the team of researchers, supported by clinical evidence from literature, or both.
Sensitivity analyses
Two sensitivity analyses were conducted. In the first analyses we stratified ICS users (continuous use and withdrawal groups) by absolute eosinophil count cuff-off (0.15 × 109 cells/L, 0.40 × 109 cells/L) and relative eosinophil cut-off (2.0 or 6.0%) (Citation7) (see Supplementary material). Secondly, for moderate-to-severe exacerbations as an outcome we censored subjects on any hospitalisations/A&E visit unrelated to COPD for absolute eosinophil count or relative eosinophil counts cut-off defined in our primary analysis ().
Results
Of subjects, 213,561 were identified with COPD, of whom 48,157 met the inclusion criteria (). shows the baseline characteristics of the COPD cohort, which consisted of 46% females, with a mean age of 68.4 ± 10.9 years. Most subjects were current (43%) or former smokers (47%). At baseline, elevated blood eosinophil counts (defined as absolute blood eosinophil counts ≥0.34 × 109 cells/L) were present in 18% (n = 8671) of all subjects. The overall population were either overweight (32%) or obese (34%) and 12% suffered from diabetes mellitus. About 28% had been prescribed any ICS (alone or in fixed combinations) at baseline.
Table 1. Baseline characteristics of COPD patients by blood eosinophil counts
Moderate-to-severe exacerbations
shows that among COPD subjects with an elevated blood eosinophil count, withdrawal of ICS was not associated with an increased risk of moderate-to-severe exacerbations as compared to continuous ICS users, yielding adjusted hazard ratios (adj. HR) of 0.72; 95% confidence interval (CI) 0.63–0.81 (eosinophilia defined by absolute values) and 0.73; 95% CI: 0.66–0.82 (eosinophilia defined by relative values). We found a decreased risk of moderate-to-severe exacerbations with low relative or absolute blood eosinophil counts among patients withdrawn from ICS. Supplementary Tables S2 and S5 show that these results were not considerably different when we used different absolute or relative threshold values to define eosinophilia among subjects who withdrew from ICS. Higher absolute cut-off of ≥0.40 × 109 cells/L yielded a fully adj. HR of 0.70; 95% CI: 0.62–0.80 for the risk of moderate-to-severe exacerbations, while a lower cut-off value ≥0.15 × 109 cells/L showed (adj. HR 0.74; 95% CI: 0.69–0.79). Results were not substantially different for higher (≥6%) or lower (≥2%) thresholds to define relative eosinophilia.
Table 2. Risk of moderate-to-severe exacerbations with ICS use stratified by absolute and relative eosinophil counts among COPD patients.
Severe exacerbations
We found that withdrawal of ICS was not associated with an increased risk of severe exacerbations among subjects with absolute blood eosinophil ≥0.34 × 109 cells/L (adj. HR 0.82; 95% CI: 0.61–1.10) compared to continuous ICS users with elevated absolute blood eosinophil counts (). shows a decreased risk of severe exacerbations with low relative or absolute blood eosinophil counts among patients withdrawn from ICS. For relative blood eosinophil counts ≥4.0%, there was no significant difference in the risk of severe exacerbations (adj. HR 0.80; 95% CI: 0.61–1.04) compared to continuous ICS users with elevated relative blood eosinophil counts. We found no increased risk of severe exacerbations when we used different absolute or relative threshold values to define eosinophilia. Absolute cut-off value of ≥0.40 × 109 cells/L resulted in (adj. HR 0.65; 95% CI: 0.48–0.89), while a lower cut-off value ≥0.15 × 109 cells/L showed (adj. HR 0.59; 95% CI: 0.50–0.69) Results were similar for higher (≥6%) or lower (≥2%) threshold values to define eosinophilia (Supplementary Tables S3 and S6).
Table 3. Risk of severe exacerbations with ICS use stratified by absolute and relative eosinophil counts among COPD patients.
All-cause mortality
shows that withdrawal of ICS was not associated with an increased risk of all-cause mortality among subjects with absolute blood eosinophil ≥0.34 × 109 cells/L (adj. HR 1.08; 95% CI: 0.96–1.22), compared to continuous ICS users with elevated absolute blood eosinophil counts. At relative blood eosinophil counts ≥4.0% there no increased risk of all-cause mortality (adj. HR 1.08; 95% CI: 0.97–1.21) compared to continuous ICS users with elevated relative blood eosinophil counts. Results were not significantly different when we adopted different absolute or relative eosinophil counts (Supplemetnary Table S4 and S7). Higher absolute cut-off value of ≥0.40 × 109 cells/L resulted in no increased risk of all-cause mortality (adj. HR 0.96; 95% CI: 0.85–1.10) .
Table 4. Risk of all-cause mortality with ICS use stratified by absolute and relative eosinophil counts among COPD patients.
Sensitivity analysis
shows the second sensitivity analyses for moderate-to-severe exacerbations, following censoring for any hospitalisations/A&E visit unrelated to COPD for absolute or relative blood eosinophil counts the results was similar to .
Table 5. Risk of moderate-to-severe exacerbations with ICS use stratified by absolute and relative eosinophil counts among COPD patients censoring at any hospitalisation/A&E visit.
Discussion
In this study conducted in a real-world primary care population, we did not observe an increased risk of moderate and/or severe COPD exacerbations or all-cause mortality among patients with relative or absolute peripheral blood eosinophilia after withdrawal from ICS, in comparison to continuous users with blood eosinophilia. Opposite to our hypothesis, risk of exacerbations was significantly lower in patients withdrawn from ICS compared to those with continued use with blood eosinophilia. In addition, no increased mortality risk was observed in patients with blood eosinophilia withdrawn from ICS compared to continuous ICS users with blood eosinophilia.
Our results are consistent with the WISDOM trial which found no difference in the risk of exacerbations among COPD patients withdrawn from ICS therapy (Citation25). However, a decreased risk of severe exacerbations was seen in some sub-groups in our study. More recently, in a Study to Understand Mortality and Morbidity (SUMMIT) trial, which enrolled more than 16,000 COPD patients with heightened cardiovascular risk, over 3-years also found that withdrawal from ICS had no effect on the risk of exacerbations in all groups compared (Citation26). Similarly, a meta-analysis of various clinical trials exploring the effects of ICS withdrawal among COPD patients without a history of asthma, found that overall the withdrawal of ICS did not significantly affect the risk of moderate-to-severe exacerbations, although they found that the withdrawal of ICS significantly impaired both forced expiratory volume in 1 s (FEV1) and St George Respiratory Questionnaire (SGRQ) score (Citation27).
Only few studies have evaluated the withdrawal of ICS and the risk of exacerbations stratified by absolute or relative blood eosinophil counts (Citation7, Citation18). In the post-hoc analysis of data from the WISDOM trial, Watz et al. (Citation7) reported that blood eosinophil count ≥4% or ≥300 cells/µL might help identify patients with greater risk of exacerbations following ICS withdrawal. Nevertheless, they found no increased risk of exacerbations following withdrawal of ICS at eosinophil counts >6.0% consistent with our findings(Citation7). Furthermore, it is important to note that they only enrolled patients with severe to very severe COPD, a history of exacerbations and over 70% of patients used ICS before study entry (Citation7). COPD patients in CPRD are derived from primary practice, a population not enriched for exacerbation risk in which use of bronchodilators and ICS use are lower compared to the clinical trials. A recent UK population-based cohort study reported that ICS-LABA therapy was more effective than LAMA in patients with blood eosinophil counts >4% or >300 cells/µL (Citation28). In our previous work using COPD patients from the same data source, we found similar results for moderate-to-severe and severe exacerbations. However, an important difference is the fact that in our previous study only patients with prior history of ICS use were included (Citation29). Analyses of data from the FLAME trial reported that LABA/LAMA combination was superior to LABA/ICS combination in exacerbation reduction for relative or absolute blood eosinophil threshold (2%, ≥2%, 3%, <5% and <150 cells/ml subgroups), and at no threshold was LABA/ICS superior to LABA/LAMA (Citation8). However, the SUNSET trial found that ICS withdrawal led to an increased risk of exacerbations among COPD patients with ≥300 cells/µL (Citation18). It is important to note, that unlike our study all patients included in the SUNSET trial were on long-term ICS regimens and were non-frequent exacerbators (i.e. patients with 0 to 1 exacerbation in the previous year). With approximately 29% of COPD patients reported to have two or more exacerbations 1 year prior in UK general practice (Citation30), it raises questions on the generalisability of their findings in a general practice setting. Furthermore, the researchers were not convinced that their study could be referred to as a “pure ICS” withdrawal study given that patients withdrew to a LABA/LAMA combination, which may not be applicable with other therapies (Citation18).
All-cause mortality is an important end-point, which is seldom assessed among COPD patients partly due to short duration of patient follow-up in most studies. We did not find an increased risk of all-cause mortality among patients who withdrew from ICS treatment with elevated absolute or relative blood eosinophil counts in our study. There is conflicting evidence on ICS treatments and reduced mortality in COPD. Two large trials among patients exposed to combination of a long-acting β-agonist (LABA) and an ICS the reduction in mortality was not statistical significant (Citation3, Citation31). However, the INSPIRE trial which included severe and very severe COPD patients receiving LABA/ICS experienced fewer deaths than those receiving LAMA (Citation32). Furthermore, Vestbo et al. (Citation33) performed a stratified pooled analysis of all fatal adverse events comparing ICS-containing versus ICS-free treatments in three recent 52-week studies among patients with severe to very severe COPD at increased risk for exacerbations. They found no statistically significant reduction in the risk of developing a fatal event. Furthermore, they found a reduced risk of non-respiratory fatal events suggesting that therapy containing an ICS may have a direct or indirect effect on chronic diseases that is almost consistently associated with mostly severe symptomatic COPD This might be due to cardiovascular events, being less likely if the underlying COPD is more stable (33).
A major strength of this study was the inclusion of patients from one of the world’s largest primary care databases, thereby providing a large population-based cohort of COPD patients with eosinophil measurements followed over time. Second, in our study we used validated definitions for moderate and/or severe exacerbations of COPD, using read codes reported to have a 96% positive predictive value of identifying an acute exacerbation within the CPRD (Citation16). Nevertheless, we may have missed considerable numbers of exacerbations, which may be miscoded e.g. as respiratory tract infections such as pneumonia. Third, time-varying classification of exposure to ICS and covariates allowed us to conduct an “on treatment analysis”, which results in less non-differential misclassification of exposure than in an `intention to treat analysis` which ignores ICS exposure during follow-up as in other studies. Lastly, data on confounding factors such as smoking status, BMI, comorbidities and drugs prescribed were available and as such these covariates were adjusted for in our models.
Despite numerous strengths, this study also had limitations. In addition to those already mentioned, there is a potential for residual confounding as we lacked information on the disease severity and exacerbation history. While we excluded asthma patients, it was impossible to rule out the inclusion of patients with reversible airflow limitations. Eosinophil counts are not routinely collected as part of diagnosis of COPD patients, they are most likely requested by the GP for a purpose and might have introduced an information bias in our study. We expect this bias to be non-differential among COPD patients who withdrew from ICS and those who continued ICS therapy leading to bias estimate towards the null. While this might have masked the true risk of severe exacerbations among patients with blood eosinophil counts, we found significant associations irrespective of the absolute or relative blood eosinophil counts for moderate and/or severe exacerbations and mortality, suggesting that our results could not have been affected by this bias. Also there have been concerns on stability of blood eosinophil counts overtime, however blood eosinophil counts among COPD patients have been shown to be relatively stable over time (Citation34, Citation35). Confounding by indication is a major concern in observational studies(Citation23), because the reason for withdrawal or continuation of ICS is often associated with the outcome of interest. While the hospital episode statistics (HES) is known to capture most hospitalisations in the UK, a low positive predictive value of identifying COPD-related hospitalisation using read codes for hospitalisation within the CPRD linked to hospital episode statistics (HES) was observed (Citation36). Linkage to HES will further limit our overall sample size and subsequently our ability to detect any risk this is because only 58% of practices have consented to participating in the CPRD linkage scheme (14).
In conclusion, this study did not show an increased risk of moderate and/or severe COPD exacerbations or all-cause mortality among patients with blood eosinophilia who withdrew from ICS therapy.
Supplemental Material
Download PDF (347.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP.
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8.
- Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789.
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD010115.
- Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in COPD: the Copenhagen general population study. Am J Respir Crit Care Med. 2015;193:965–974.
- Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts as markers of response to inhaled corticosteroids in COPD? - authors’ reply. Lancet Respir Med. 2015;3:e27. doi: 10.1016/S2213-2600(15)00259-3.
- Watz H, Tetzlaff K, Wouters EFM, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–398. doi: 10.1016/S2213-2600(16)00100-4.
- Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance COPD treatment: data from the FLAME trial. Am J Respir Crit Care Med. 2017;195:1189–1197.
- Calverley PMA, Tetzlaff K, Vogelmeier C, et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:1219–1221.
- Price D, Rigazio A, Postma D, et al. Blood eosinophilia and the number of exacerbations in COPD patients. Eur Respir J. 2014;44:1697–1700.
- GOLD. Global strategy for the prevention, diagnosis and treatment of chronic obstructive pulmonary disease (2019 Report). Global Initiative for Chronic Obstructive Lung Disease Inc. 2018 [cited 2018 Nov 15]. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
- Vogelmeier C, Worth H, Buhl R, et al. “Real-life” inhaled corticosteroid withdrawal in COPD: a subgroup analysis of DACCORD. Int J Chron Obstruct Pulmon Dis. 2017;12:487–494.
- Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open 2014;4:e005540.
- Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098.
- Taggar JS, Coleman T, Lewis S, et al. The impact of the Quality and Outcomes Framework (QOF) on the recording of smoking targets in primary care medical records: cross-sectional analyses from The Health Improvement Network (THIN) database. BMC Public Health 2012;12:1–11.
- Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11:e0151357.
- Padmanabhan S. CPRD GOLD Data Specification. 2015 [cited 2018 May 15]. Medicines & Healthcare Products Regulatory Agency. Available from: https://www.ed.ac.uk/files/atoms/files/cprd_gold_full_data_specification.pdf.
- Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198:329–339. doi: 10.1164/rccm.201803-0405OC.
- Chatila WM, Thomashow BM, Minai OA, et al. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:549–555. doi: 10.1513/pats.200709-148ET.
- Dal Negro RW, Bonadiman L, Turco P. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med. 2015;10:24.
- Franssen FME, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev. 2014;23:131–141. doi: 10.1183/09059180.00007613.
- Divo M, Cote C, De Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC.
- de Melo MN, Ernst P, Suissa S. Inhaled corticosteroids and the risk of a first exacerbation in COPD patients. Eur Respir J. 2004;23:692–697. doi: 10.1183/09031936.04.00049604.
- Ernst P, Gonzalez A V, Brassard P, et al. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176:162–166. doi: 10.1164/rccm.200611-1630OC.
- Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371:1285–1294. doi: 10.1056/NEJMoa1407154.
- Vestbo J, Anderson JA, Brook RD, et al. Effect of treatment withdrawal on outcomes in the SUMMIT study. C41. Long acting bronchodilator therapy in COPD II. Am J Respir Crit Care Med. 2017:195:A5483–A5483.
- Calzetta L, Matera MG, Braido F, et al. Withdrawal of inhaled corticosteroids in COPD: a meta-analysis. Pulm Pharmacol Ther. 2017;45:148–158. doi: 10.1016/j.pupt.2017.06.002.
- Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;15:415–417.
- Oshagbemi OA, Franssen FME, Braeken DCW, et al. Blood eosinophilia, use of inhaled corticosteroids, and risk of COPD exacerbations and mortality. Pharmacoepidemiol Drug Saf. 2018;27:1191–1199.
- Merinopoulou E, Raluy-Callado M, Ramagopalan S, et al. COPD exacerbations by disease severity in England. Int J Chron Obstruct Pulmon Dis. 2016;11:697–709. doi: 10.2147/COPD.S100250.
- Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817–1826.
- Wedzicha JA, Calverley PMA, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26.
- Vestbo J, Fabbri L, Papi A, et al. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018;52:1801230.
- Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195:1402–1404.
- Landis SH, Suruki R, Hilton E, et al. Stability of blood eosinophil count in patients with COPD in the UK Clinical Practice Research Datalink. COPD J Chronic Obstr Pulm Dis. 2017;14:382–388.
- Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8:771–782.