Abstract
Bronchodilator reversibility occurs in patients with COPD. Pooled analysis of two 12-week, placebo-controlled randomised studies (FLIGHT1 [NCT01727141]; FLIGHT2 [NCT01712516]) assessed the effect of bronchodilator reversibility on lung function, patient-reported outcomes, and safety in 2,043 patients with moderate-to-severe COPD treated with indacaterol/glycopyrrolate (IND/GLY) 27.5/15.6 µg twice daily. Reversibility was defined as post-bronchodilator increase in forced expiratory volume in one second (FEV1) of ≥12% and ≥0.200 L. Overall, mean reversibility (mean post-bronchodilator FEV1 increase) was 22.8%, and 54.5% of patients met reversibility criteria. IND/GLY resulted in significant (p < 0.05) placebo-adjusted improvements from baseline at Week 12 in reversible and non-reversible patients in FEV1 area under the curve from 0 to 12 hours (0.308 L and 0.170 L, respectively), trough FEV1 (0.260 L and 0.174 L), St. George’s Respiratory Questionnaire total score (−6.3 and –3.5), COPD Assessment Test total score (−2.3 and –1.2), daily rescue medication use (−1.52 and −0.79), and daily total symptom score (−0.86 and –0.63); Transition Dyspnoea Index focal score also showed improvements (1.93 and 1.29) at Week 12, irrespective of reversibility status. Improvements in lung function and rescue medication use were significantly (p < 0.05) greater in IND/GLY patients in the reversible subgroup compared with the non-reversible subgroup. The safety profile was similar across treatment groups and reversibility subgroups. Overall, treatment with IND/GLY led to significant improvements in lung function and PROs in patients with moderate-to-severe COPD, regardless of reversibility status, with greater improvements in the reversible subgroup. Safety profile was not affected by reversibility status.
Introduction
Bronchodilator reversibility is defined as a significant change in lung function following bronchodilator treatment with an improvement from baseline in forced expiratory volume in 1 second (FEV1) of ≥12% and ≥0.200 L (Citation1). Although chronic obstructive pulmonary disease (COPD) is usually considered irreversible, several reports show that reversibility frequently occurs in these patients (Citation2–7). Reversibility status at baseline does not appear to predict the long-term response to bronchodilator treatment (Citation4,Citation5). For example, treatment with the long-acting muscarinic antagonist (LAMA) tiotropium improved lung function, dyspnoea, and health status, and reduced rescue medication requirements, independent of short-term reversibility status (Citation5,Citation6).
A combination of a fixed dose of 27.5 µg indacaterol (IND), a long-acting β2-agonist (LABA), and 15.6 µg glycopyrrolate (GLY), a LAMA, is approved in the USA for the long-term maintenance treatment of airflow obstruction in patients with COPD (IND/GLY; UTIBRON® NEOHALER®, Sunovion Pharmaceuticals Inc.). Two pivotal trials (FLIGHT1 [NCT01727141]; FLIGHT2 [NCT01712516]) demonstrated the efficacy and safety of IND/GLY, which were compared with its mono-components and placebo (Citation8). However, the impact of patient bronchodilator reversibility status on the efficacy and safety of IND/GLY has not been assessed. Here, we report a post-hoc analysis of pooled data from the FLIGHT1 and FLIGHT2 studies evaluating the effect of IND/GLY treatment on lung function and patient-reported outcomes (PROs) in patients with moderate-to-severe COPD, categorised by baseline reversibility status. Use of baseline patient characteristics to identify patients with COPD who may achieve a greater benefit with IND/GLY could be useful in defining treatment strategies and improving outcomes.
Methods
Study design and treatment
Details of the FLIGHT1 and FLIGHT2 studies have been described previously (Citation8). These studies were replicate, multicenter, randomised, parallel-group, placebo- and active-controlled studies. Following a 14-day run-in period, patients were randomised 1:1:1:1 to IND/GLY 27.5/15.6 μg, IND 27.5 µg, GLY 15.6 µg, or placebo for 12 weeks, with a 30-day safety follow-up period. All treatments were delivered twice daily via the Neohaler device.
Throughout the treatment period, inhaled corticosteroid use was permitted if treatment was at a stable dose for 30 days before screening, and albuterol was allowed as rescue medication.
Patients
Key eligibility criteria (Citation8) included current or ex-smokers ≥40 years of age, with a ≥10 pack-year smoking history, a modified Medical Research Council grade of ≥2, moderate-to-severe COPD as defined by the Global initiative for chronic Obstructive Lung Disease (GOLD) 2011 Report (Citation9), and qualifying post-bronchodilator (1 hour after sequential inhalation of ipratropium bromide 84 µg, and salbutamol 400 µg [or albuterol 360 µg]) spirometry (FEV1 ≥30% and <80% of predicted normal and FEV1/forced vital capacity [FVC] ratio <0.70). Patients with a history of asthma were excluded from the studies.
FLIGHT1 and FLIGHT2 studies were approved by institutional review boards or ethics committees at participating centres, and were conducted in accordance with International Council for Harmonisation Good Clinical Practice guidelines, with local regulations applied, and the Declaration of Helsinki. All patients provided written informed consent.
Analyses
The replicate nature of FLIGHT1 and FLIGHT2 enabled data from both studies to be pooled for analysis. FEV1 reversibility was calculated as a percentage increase of FEV1 after sequential inhalation of short-acting (anticholinergic [ipratropium bromide, 84 µg] and β2-agonist [salbutamol 400 µg or albuterol 360 µg]) bronchodilators. Reversibility was defined as a post-bronchodilator increase of ≥12% and ≥0.200 L in FEV1. Patient subgroups were categorised based on reversibility status at baseline.
The primary endpoint was change from baseline in FEV1 area under the curve from 0 to 12 hours (FEV1 AUC0–12h; L) at Week 12. Secondary endpoints included change from baseline in trough FEV1 at Week 12; change from baseline in health status at Week 12, measured via the St George’s Respiratory Questionnaire (SGRQ) total score and the COPD Assessment Test (CAT) total score; breathlessness at Week 12, using the Transition Dyspnoea Index (TDI) focal score; and relative effect on safety (including adverse events [AEs]) during 12 weeks of treatment. Patient diary data were used to evaluate changes from baseline in symptom burden and rescue medication use.
Statistical analysis
Efficacy endpoints were analysed using the full analysis set (FAS), which included all randomised patients who received at least one dose of study treatment. Changes from baseline in FEV1 AUC0−12h and trough FEV1 at Week 12 were analysed using a mixed-model for repeated measures, which included terms of treatment, baseline FEV1, smoking status at baseline, baseline inhaled corticosteroid (ICS) use, region, visit, study, FEV1 reversibility, baseline FEV1 by visit interaction, and FEV1 reversibility by treatment and visit, all two- and three-way interactions. Changes from baseline in SGRQ total score, CAT total score, rescue medication use, and symptom scores, as well as overall change in TDI focal score, were analysed using a similar linear mixed model (using the appropriate baseline score, and centre nested within region, nested within study as a random effect). The proportions of patients with a reduction in SGRQ total score of ≥4 (Citation10) or an increase in TDI focal score of ≥1 (Citation11) (defined as minimum clinically important differences [MCIDs]) were determined using logistic regression models; proportions of patients with a reduction in CAT total score of ≥2 were also assessed (Citation12). No multiplicity adjustments were made for the post-hoc multiple comparisons.
The safety set included all patients who received at least one dose of study treatment, and was used for analysis of all safety variables. Safety data were analysed using descriptive statistics. AEs were coded according to the Medical Dictionary for Regulatory Activities Version 17.0 and summarised by treatment, system organ class, and preferred term. Major adverse cardiovascular events (MACE) were defined as non-fatal myocardial infarction (MI), non-fatal unstable angina, non-fatal stroke, heart failure requiring hospitalisation, and coronary revascularization. All potential MACE were reviewed by an independent adjudication committee. Non-MACE serious cardiovascular or cerebrovascular (CCV) AEs were also adjudicated.
All statistical procedures were performed using SAS® Version 9.2 or higher (SAS Institute Inc., Cary, NC).
Results
Patient demographics and baseline characteristics
The pooled analysis included data from 2,043 randomised patients (FLIGHT1: n = 1,042; FLIGHT2: n = 1,001). A total of 1,001 patients randomised to IND/GLY (n = 501) and placebo (n = 500) were categorised as reversible or non-reversible.
Across the two studies, the overall mean reversibility (mean post-bronchodilator increase in FEV1) was 22.8% (standard deviation: 17.2%). Slightly over half (54.5%) of the patients met reversibility criteria, of whom 279 were treated with IND/GLY and 272 with placebo.
Pooled patient demographics and baseline characteristics are displayed in . The majority of patients in all treatment groups and reversibility subgroups were male (61.9%), and Caucasian (92.4%). Notably, across treatment groups, a lower proportion (28.3–31.3%) of patients had severe airflow limitation in the reversible subgroup compared with the non-reversible subgroup (49.1–49.5%). Post-bronchodilator FEV1 at baseline across treatments was 1.618−1.620 L in the reversible subgroup compared with 1.354–1.394 L in the non-reversible subgroup. The pre-bronchodilator FEV1% predicted was lower in the reversible subgroup (43.6−44.6%) than non-reversible subgroup (46.9−47.4%), whereas post-bronchodilator FEV1% predicted was higher in the reversible subgroup (56.5–57.6%) than the non-reversible subgroup (51.5–51.9%).
Table 1. Pooled demographics and baseline characteristics from the FLIGHT1 and FLIGHT2 studies by bronchodilator reversibility (FAS).
Efficacy
Reversibility subgroup results at 12 weeks were generally consistent with pooled overall primary and secondary efficacy results published previously (Citation8).
Changes from baseline in lung function
At Week 12, IND/GLY produced significant improvements from baseline in placebo-adjusted FEV1 AUC0–12h in reversible (0.308 L) and non-reversible (0.170 L) subgroups (both p < 0.001; ). The least squares mean (LSM) change from baseline in FEV1 AUC0–12h at Week 12 with IND/GLY was significantly greater in the reversible versus the non-reversible subgroup (0.291 L vs. 0.137 L, respectively; difference: 0.154 L; p < 0.001).
Figure 1. LSM change from baseline in (A) FEV1 AUC0–12h and (B) trough FEV1 at Week 12 by bronchodilator reversibility (FAS). ***p < 0.001 versus placebo; †††p < 0.001 versus non-reversible. AUC: area under the curve; FAS: full analysis set; FEV1: forced expiratory volume in 1 second; IND/GLY: indacaterol/glycopyrrolate 27.5/15.6 µg; LSM: least squares mean; SE: standard error.
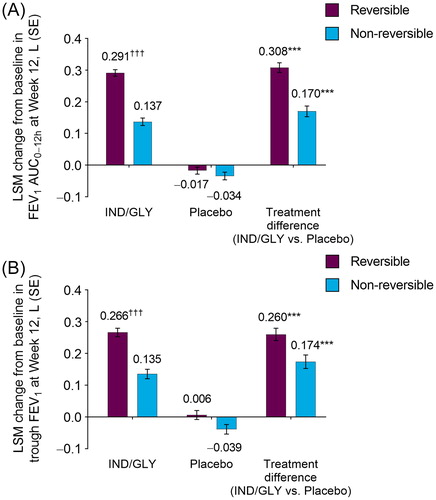
In alignment with Week 12 data, significant improvements from baseline in placebo-adjusted FEV1 AUC0–12h were observed as early as Day 1 for patients treated with IND/GLY in both reversible (0.210 L; p < 0.001) and non-reversible (0.121 L; p < 0.001) subgroups. The difference between change from baseline in FEV1 AUC0–12h for IND/GLY patients in the reversible subgroup and non-reversible subgroup was significant (0.221 L vs. 0.123 L, respectively; difference: 0.098 L; p < 0.001).
Treatment with IND/GLY led to significant improvements (p < 0.001) at Week 12 in placebo-adjusted change from baseline trough FEV1 for patients who were reversible (0.260 L) and who were not reversible (0.174 L; ). The least squares mean change from baseline in trough FEV1 with IND/GLY treatment was significantly greater in patients who were reversible compared with patients who were not reversible (0.266 L vs. 0.135 L, respectively; difference: 0.131 L; p < 0.001).
As with FEV1 AUC0–12h, placebo-adjusted trough FEV1 improvements were consistent across the entire 12 weeks of the studies, and were observed as early as Day 2 in both reversible (0.225 L; p < 0.001) and non-reversible (0.133 L; p < 0.001) subgroups. At Day 2, for patients treated with IND/GLY, the difference between change from baseline in trough FEV1 in the reversible subgroup and non-reversible subgroup was significant (0.250 L vs. 0.132 L, respectively; difference: 0.118 L; p < 0.001).
Additionally, changes from baseline in trough FEV1 at Week 12 among reversible and non-reversible patients were significantly greater (p < 0.001) in those treated with IND/GLY compared with placebo, regardless of background ICS use (Supplementary Information, Table S1). Differences in trough FEV1 between reversible and non-reversible subgroups were significant in patients using background ICS (treatment difference: 0.135 L; p < 0.001) and in patients not using background ICS (treatment difference: 0.127 L; p < 0.001).
SGRQ total score, CAT total score, and TDI focal score
At Week 12, IND/GLY produced significant improvements from baseline in placebo-adjusted SGRQ total score in patients who were reversible (−6.3; p < 0.001) and who were not reversible (−3.5; p < 0.01; ). Improvements in SGRQ total score in the IND/GLY treatment group were greater among patients in the reversible subgroup (−7.7) than those in the non-reversible subgroup (−5.9). The difference between reversible and non-reversible subgroups was not significant (difference: –1.9; p = 0.076).
Figure 2. LSM change from baseline in (A) SGRQ total score, (B) CAT total score, and (C) TDI focal score at Week 12 by bronchodilator reversibility (FAS). *p < 0.05, **p < 0.01, ***p < 0.001 versus placebo. CAT: COPD (chronic obstructive pulmonary disease) Assessment Test; FAS: full analysis set; IND/GLY: indacaterol/glycopyrrolate 27.5/15.6 µg; LSM: least squares mean; SE: standard error; SGRQ: St George’s Respiratory Questionnaire; TDI: transition dyspnoea index.
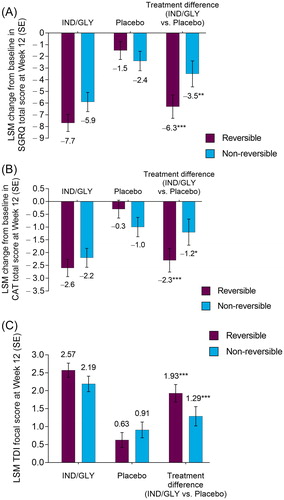
Treatment with IND/GLY led to a significant increase in the odds of being an SGRQ responder (patients meeting MCID of ≥4-unit reduction) versus placebo, with higher odds of patients being a responder in the reversible subgroup (IND/GLY 62.5%; placebo 38.4%; odds ratio [OR]: 2.91 [95% confidence interval (CI): 2.01, 4.22]; p < 0.001) than in the non-reversible (IND/GLY 52.6%; placebo 34.3%; OR: 2.12 [95% CI: 1.41, 3.21]; p < 0.001) subgroup. The odds of being a responder in the IND/GLY treatment group were significantly higher for patients in the reversible subgroup compared with the non-reversible subgroup (OR: 1.63 [95% CI: 1.11, 2.40]; p < 0.05).
At Week 12, versus placebo, IND/GLY produced significant improvements from baseline in CAT total score in patients who were reversible (−2.3; p < 0.001) and those who were not (−1.2; p < 0.05; ). Treatment with IND/GLY led to clinically significant improvements (a reduction from baseline of ≥2.0 units) in CAT total score in reversible and non-reversible subgroups (−2.6 and −2.2, respectively). There was no significant difference between reversibility subgroups in the IND/GLY treatment group (difference: –0.4; p = 0.403). A numerically higher proportion of patients treated with IND/GLY reported a clinically significant improvement in CAT total score compared with placebo in both the reversible (IND/GLY: 56.6%; placebo: 42.4%) and non-reversible (IND/GLY: 51.2%; placebo: 41.9%) subgroups.
At Week 12, IND/GLY treatment led to significant overall change in placebo-adjusted TDI focal score in patients who were reversible (1.93; p < 0.001) and those who were not (1.29; p < 0.001; ). Improvements in TDI score for patients treated with IND/GLY were similar among patients regardless of reversibility status, and were greater than the MCID of 1.0 unit in both subgroups (reversible: 2.57; non-reversible: 2.19; difference: 0.38; p = 0.142).
The odds of being a TDI responder (patients meeting MCID of ≥1-unit increase) were significantly greater with IND/GLY treatment compared with placebo in reversible (IND/GLY 64.9%; placebo 40.7%; OR: 2.87 [95% CI: 1.97, 4.17]; p < 0.001) and non-reversible (IND/GLY 64.6%; placebo 43.3%; OR: 2.74 [95% CI: 1.81, 4.17]; p < 0.001) patients. There were no significant differences in the proportion of TDI responders between reversible and non-reversible patients receiving IND/GLY (p = 0.490).
Rescue medication use and daily total symptom score
Over 12 weeks, patients treated with IND/GLY experienced significant reductions from baseline in placebo-adjusted mean daily puffs of rescue medication use in patients who were reversible (−1.52; p < 0.001) and those who were not (−0.79; p < 0.001; ). Changes from baseline in mean daily puffs of rescue medication use were significantly different between reversible and non-reversible patients receiving IND/GLY (−2.40 vs. –2.00, respectively; difference: –0.40; p < 0.05).
Figure 3. LSM change from baseline in (A) mean number of puffs of rescue medication per day and (B) mean daily total symptom score over 12 weeks by bronchodilator reversibility (FAS). ***p < 0.001 versus placebo. †p < 0.05 versus non-reversible. FAS: full analysis set; IND/GLY: indacaterol/glycopyrrolate 27.5/15.6 µg; LSM: least squares mean; SE: standard error.
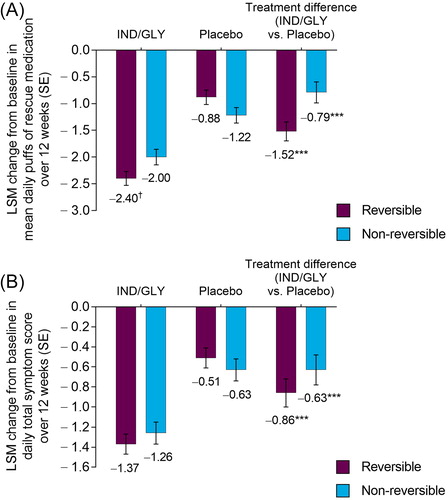
Daytime and nighttime rescue medication use over 12 weeks were also significantly lower (p < 0.001) in patients treated with IND/GLY compared with placebo, regardless of reversibility status (Supplementary Information, Table S2).
Treatment with IND/GLY produced statistically significant placebo-adjusted improvements from baseline in mean daily total symptom score over 12 weeks in patients who were reversible (−0.86; p < 0.001) and those who were not (−0.63; p < 0.001; ). The difference between change from baseline in daily total symptom score for IND/GLY patients in the reversible and non-reversible subgroups was not significant (−1.37 vs. −1.26, respectively; difference: –0.11; p = 0.426).
Other PROs over 12 weeks of treatment
In reversible and non-reversible subgroups, IND/GLY treatment resulted in significant (p < 0.05) improvements versus placebo in percentage of nights with no nighttime awakenings (reversible 8.8%; non-reversible 6.5%) and days able to perform usual daily activities (reversible 9.0%; non-reversible 4.9%). There were also significant (p < 0.05) improvements versus placebo in both reversibility subgroups in daytime and nighttime symptom scores, as well as cough, sputum, and breathlessness scores. Patients treated with IND/GLY who were reversible demonstrated a significant (p < 0.05) improvement versus placebo in percentage of days with no daytime symptoms (3.5%), whereas patients who were not reversible showed improvement (2.4%) that was not significant. Improvements were greater in patients who were reversible compared with those who were not, but there was no substantial difference between reversibility subgroups for any of these PROs (Supplementary Information, Table S2).
Safety
AEs and serious AEs
The overall incidences of AEs and serious AEs were similar across treatment groups and reversibility subgroups (). Worsening of COPD was the most common AE; the incidence was similar between patients treated with IND/GLY and placebo in the non-reversible subgroup (17.9% vs. 16.2%, respectively), but was substantially numerically lower in the reversible subgroup IND/GLY patients compared with placebo (13.0% vs. 23.5%).
Table 2. Incidence of AEs, serious AEs, and the most common AEsTable Footnotea from the FLIGHT1 and FLIGHT2 studies by bronchodilator reversibility (safety population).
MACE and serious CCV AEs
There were two MACE in IND/GLY patients in the reversible subgroup (one non-fatal MI and one non-fatal stroke), and one in the placebo treatment group (non-fatal MI). In the non-reversible subgroup, one patient treated with IND/GLY reported a MACE (non-fatal stroke), with no MACE in the placebo group.
In the reversible subgroup, there was also one non-MACE CCV AE in the IND/GLY treatment group, while in the non-reversible subgroup, there was one non-MACE CCV AE in each of the IND/GLY and placebo treatment groups.
Discussion
The assessment of bronchodilator reversibility continues to be recommended for the diagnosis and severity of COPD (Citation13). In this analysis, just over half of all patients (54.5%) met reversibility criteria, which is comparable to the prevalence reported in similar studies (Citation6,Citation14). The results demonstrate that treatment with IND/GLY resulted in improved lung function and PROs compared with placebo, independent of reversibility status, albeit changes in reversible patients were of greater magnitude than those in non-reversible patients. Importantly, the safety and tolerability of IND/GLY were not impacted by reversibility status.
Baseline pre- and post-bronchodilator lung function parameters showed similar trends to previously published data (Citation6,Citation7,Citation14). The overall mean baseline reversibility in this study was 22.8%, which was comparable to 23.4% baseline reversibility in the 4-year UPLIFT trial investigating once daily tiotropium 18 µg treatment (Citation14). Similar to published data, non-reversible patients generally had more severe airflow limitation (by proportion of patients assessed as GOLD stage 3 (Citation9,Citation13) at baseline), and lower baseline post-bronchodilator FEV1 at baseline compared with reversible patients (Citation7,Citation14).
Treatment with IND/GLY demonstrated significant improvements from baseline, at 12 weeks, with respect to lung function, health status, symptoms, and rescue medication use in reversible and non-reversible subgroups, with PROs aligning with lung function improvements. Notably, improvements in lung function and daily rescue medication use were significantly greater for the reversible subgroup compared with the non-reversible subgroup. The greater lung function improvements observed in the reversible subgroup compared with the non-reversible subgroup consistent with reported results from a LABA/LAMA combination lung function analysis in reversible and non-reversible patients (Citation15), and may be explained by the differences in severity of disease and FEV1 at baseline. Other outcomes did not demonstrate any significant difference across reversibility subgroups.
The responses to IND/GLY treatment at 12 weeks are similar to those demonstrated with once-daily tiotropium 18 µg treatment at 12 months: significant improvements over placebo for both reversible and non-reversible subgroups were observed for lung function, SGRQ total score, TDI focal score, and rescue medication use, and improvements were greater in the reversible subgroup in all cases (Citation6). In the tiotropium studies, TDI focal score was the only PRO to demonstrate significant improvement between subgroups (Citation6). In contrast, the results from our analysis demonstrated significant improvements between reversible and non-reversible subgroups in daily rescue medication use, but did not show significant differences between reversibility subgroups in TDI focal score or other PROs.
There were no differences in the safety profile of IND/GLY in reversible versus non-reversible patients, even though non-reversible patients had more severe baseline disease characteristics. The overall incidences of AEs (including serious, cardiovascular, and cerebrovascular AEs) across reversibility subgroups were similar to results in the overall pooled population (Citation8). These results further support the tolerability of IND/GLY in patients with moderate-to-severe COPD, independent of their baseline reversibility status.
The possibility that a patient’s reversibility status can change between assessments suggests that reversibility is an unreliable predictor of treatment outcome (Citation7). Additional studies would be useful to assess the longer-term effects of IND/GLY treatment on PROs in patients meeting and not meeting standard reversibility criteria.
Conclusions
In this pooled analysis of data from the FLIGHT1 and FLIGHT2 studies, treatment with IND/GLY produced significant improvements in lung function, SGRQ total score, TDI focal score, daily rescue medication use, CAT total score, and daily total symptom score compared with placebo, regardless of reversibility status. Improvements in lung function, health status, symptoms, and daily rescue medication use were numerically greater in reversible patients compared with non-reversible patients, with FEV1 AUC0–12h, trough FEV1, and daily rescue medication use being significantly greater in the reversible subgroup compared with the non-reversible subgroup. Clinically important differences in TDI focal score were observed in reversible and non-reversible patients. The overall and cardiovascular safety of IND/GLY was comparable to other treatment groups, and was not affected by the reversibility status of the patient at baseline. These data support the use of IND/GLY among patients with moderate-to-severe COPD independent of reversibility status.
Declaration of interest
JAO has served on advisory boards for Sunovion Pharmaceuticals Inc., AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Mylan, and Theravance.
S Sharma, TG, AB, BP, AO-G, and S Sanjar are employees of Sunovion Pharmaceuticals Inc.
Acknowledgments
Medical writing support in the preparation of this article was provided by Linda Townsend, PhD, of FireKite, an Ashfield company, part of UDG Healthcare plc, and was funded by Sunovion Pharmaceuticals Inc.
Data sharing statement
Sunovion Pharmaceuticals Inc. is part of a clinical trial data sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability please visit https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on ‘Sunovion’.
Additional information
Funding
References
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. doi: 10.1183/09031936.05.00035205.
- Hanania NA, Celli BR, Donohue JF, Martin, UJ. Bronchodilator reversibility in COPD. Chest. 2011;140(4):1055–63. doi: 10.1378/chest.10-2974.
- Albert P, Agusti A, Edwards L, Tal-Singer R, Yates J, Bakke P, Celli BR, Coxson HO, Crim C, Lomas DA, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–8. doi: 10.1136/thoraxjnl-2011-201458.
- Rossi A, Khirani S, Cazzola M. Long-acting β(2)-agonists (LABA) in chronic obstructive pulmonary disease: efficacy and safety. Int J Chron Obstruct Pulmon Dis. 2008;3(4):521–9. doi: 10.2147/COPD.S1353.
- Hanania NA, Sharafkhaneh A, Celli B, Decramer M, Lystig T, Kesten S, Tashkin D. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res. 2011;12:6.
- Tashkin D, Kesten S. Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest. 2003;123(5):1441–9.
- Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58(8):659–64.
- Mahler DA, Kerwin E, Ayers T, FowlerTaylor A, Maitra S, Thach C, Lloyd M, Patalano F, Banerji D. FLIGHT1 and FLIGHT2: Efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(9):1068–79. doi: 10.1164/rccm.201505-1048OC.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP.
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–9.
- Mahler DA, Witek TJ, Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103.
- Kon SS, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, Haselden BM, Polkey MI, Man WD. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3.
- Global Initiative for Chronic Obstructive Lung Disease Inc. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD; 2019. Available from: http://goldcopd.org/.
- Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31(4):742–50. doi: 10.1183/09031936.00129607.
- Tashkin DP, Ferguson GT, Clerisme-Beaty E, Vob F, Buhl R. Lung-function response to tiotropium + olodaterol in patients with COPD with β-agonist reversibility. Am J Respir Crit Care Med. 2016;193:A6808.
