Abstract
Detailed treatment regimens for patients with chronic obstructive pulmonary disease (COPD) were developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Every few years the method of classification of COPD severity and the treatment recommendations are significantly revised. The aim of this study was to determine the clinical implications of changing GOLD reports (2007–2011–2017) and the impact that these changes would have on pharmacological treatment regimens of patients with COPD. A group of 500 randomly chosen primary care physicians in Poland each provided information on 10 consecutive patients diagnosed with COPD. This data was used to simulate the therapeutic consequences of the update of the GOLD 2007 report to GOLD 2011 and GOLD 2017. Pharmacological treatment algorithms from the GOLD 2007 report prefer the use of inhaled corticosteroids (ICS) and short-acting bronchodilators (60.2% and 50%, respectively). Compared to the GOLD 2007 report, there would be an almost eightfold reduction in the frequency of short-acting bronchodilator using the GOLD 2011 report and over fourfold decrease using the GOLD 2017 report. With each subsequent update of the GOLD report, the frequency of use of ICS would be significantly (p < 0.001) reduced. Pharmacological treatment by the GOLD 2011 and 2017 reports would be dominated by the use of long-acting bronchodilators from the group β2-agonists and muscarinic antagonist groups. Updates from the GOLD 2007 COPD report to GOLD 2011 and 2017 would have a significant impact on everyday clinical practice. Changes would result in a reduction of treatment intensity.
| Abbreviations | ||
| COPD | = | chronic obstructive pulmonary disease |
| FEV1 | = | forced expiratory volume in 1 second |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| GPs | = | general practitioners |
| ICS | = | inhaled corticosteroids |
| LAMA | = | long-acting muscarinic antagonist |
| LABA | = | long-acting β2-agonists |
| p-value | = | probability value |
| SAMA | = | short-acting muscarinic antagonist |
| SABA | = | short-acting β2-agonists |
Introduction
Chronic obstructive pulmonary disease (COPD) is commonly occurring, progressive chronic respiratory disorder (Citation1). The goal of treatment is to improve the quality and comfort of the patient’s life as well as to alleviate the disease symptoms (Citation1, Citation2). The main clinical treatment includes pharmacotherapy but can also include oxygen therapy, surgical treatment, respiratory rehabilitation, noninvasive mechanical ventilation, and a number of activities in the field of patient education (Citation2). European Respiratory Society (ERS) has estimated that annual costs of healthcare and lost productivity due to COPD in 28 EU countries can reach even 48.4 billion euros (Citation3). The total direct cost of COPD management was estimated as 23.3 billion euros, wherein almost one-third of the quota was the drug costs (approximately 7.1 billion euros) (Citation3).
Detailed treatment regimens for patients with COPD are defined in international reports developed by Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation4–6). The clinical management and pharmacotherapy scheme is determined by assignment of the patient to the specific severity stage of the disease according to the GOLD recommendation. While GOLD reports are adjusted regularly, every few years substantial revisions are made to update the method of classification of COPD severity and the treatment recommendations. These major revisions of the GOLD report were made in 2007, 2011, and 2017 (Citation4–Citation6).
The aim of this study was to determine the clinical implications of changing GOLD reports (2007–2011–2017) and their impact on pharmacological treatment regimen of patients diagnosed with COPD. We expected that with the implementation of each subsequent update of the GOLD report (2007–2011–2017), the pharmacological treatment regimen would be changed.
Methods
This study is a simulation based on the results from the cross-sectional study. To complete this study and meet our objective, we used data from a cross-sectional survey performed among 500 randomly chosen primary care physicians in Poland who individually provided information on 10 consecutive COPD patients along with the 2007–2011–2017 GOLD recommendations to simulate the effects of the implementation of each report. The questionnaire addressed: history of the disease, current clinical status, disease control, treatment, and pharmacotherapy. We applied the recommendations from the three sets of reports to each of the patients’ data to predict the COPD severity stage for each subject using three classification schemes provided by GOLD 2007, 2011, and 2017 reports, as previously described (Citation7). Recommended COPD pharmacotherapy was then determined, also based on the three sets of GOLD reports (Supplementary Tables 1–3) (Citation4–6).
COPD severity definitions
Based on the individual questionnaire-derived data (including spirometric status, results of Medical Research Council dyspnea scale (mMRC), frequency of COPD exacerbations, and number of hospitalization and ambulance intervention during last 12 months), subjects were assigned to the specific therapeutic groups according to the GOLD 2007, 2011, and 2017 reports (Citation4–7).
COPD pharmacotherapy treatment regimen
As criteria of correct COPD treatment, we have used the sets of drugs recommended in particular GOLD editions (2007–2011–2017) dedicated to the specific severity stage of COPD (Citation4–Citation6). Pharmacological treatment algorithms according to the GOLD 2007, 2011, and 2017 reports are shown in Supplementary Tables 1–3.
For the purpose of the analysis, we have assumed the ideal situation that all patients were treated according to the GOLD criteria (100% adherence). Due to the fact that the GOLD reports indicate groups of drugs, which can be used in a particular COPD severity stage, (without favoring any group of drugs), it was also assumed that within a given COPD severity stage, each group of drugs included in a GOLD report can be used with equal frequency. For example: if a patient with mild COPD according to GOLD 2007 should be treated with short-acting β2-agonists (SABA) or short-acting muscarinic antagonist (SAMA), we assumed that half of the patients in this category (mild COPD) will be treated with SABA and half will be treated with SAMA.
GOLD reports include two treatment regimens (recommended/preferred treatment and other defined as alternative choice or other possible treatments), so the simulations of the therapeutic consequences of the GOLD report updates implementation were conducted in two ways. The first method assumed that all patients were treated with the first-line treatment according to the GOLD treatment regimens. Based on the data obtained from the cross-sectional study (the number of hospitalizations, ambulance intervention, exacerbations, and the mMRC scale result), we estimated that 40% of patients will not respond to first-line (preferred) treatment and will be required to include additional (second-line) treatment. The second method of analysis included using both first- (60% of patients) and second-line (40% of patients) treatment scenario.
The assessment of pharmacotherapy also includes the use of methylxanthines (theophylline), as a treatment of the second choice, by patients with severe and very severe COPD, which results from the GOLD 2007 and 2011 reports. Based on the frequency of application of theophylline obtained from the cross-sectional study, the percentage of patients using theophylline was estimated within individual groups, amounting to 65% of patients among those with severe disease according to GOLD 2007 or in category C according to GOLD 2011, and 75% of patients among those with a very severe form of the disease according to GOLD 2007 or in category D according to GOLD 2011. In the case of GOLD 2017, theophylline was not included in analysis, because the GOLD 2017 report did not recommend the use of methyloxantynes as a potential pharmacotherapy in patients with COPD.
Assumptions, details of the presented analyses, and data related to changes in the pharmacological treatment of COPD for each particular disease severity category are presented in Supplementary materials.
This study was carried out in accordance with the principles expressed in the Declaration of Helsinki. Individual patients’ data were coded in an anonymous manner that would prevent the identification of the patient and the physician by any member of the research team at any stage of the study. According to the Ethical Review Board at the Medical University of Silesia, Poland, scientific simulations based on anonymous, questionnaire-based cross-sectional study do not require separate consent.
Statistical analysis
Data analysis was performed using the procedures available in the Statistica 12 package. Descriptive statistics were used to present the distributions of the analyzed quantitative (arithmetic mean, standard deviation,) and qualitative (frequency and proportion with 95% confidence intervals) variables. The normality of the distributions of quantitative variables was assessed using the Shapiro–Wilk test. Statistical significance of differences between groups of qualitative variables was assessed using the McNemar’s test. The criterion of statistical significance was p < 0.05.
Results
Fully completed questionnaires were obtained from 298 general practitioners (GPs), with a response rate of 59.6%. In the final analysis, data from 2597 patients (women 35.3%) aged 29–96 years (mean age 61.6 ± 11.1 years) were included. According to the GOLD 2007 report, 13.1% of patients fulfilled the criteria for mild disease, 26.7% for moderate disease, 56.7% for severe disease, and 3.4% for very severe disease stages of COPD. Stages A, B, C, D according to GOLD 2011 presented 14.8%; 10.4%; 39.7%; 35.1%, and according to GOLD 2017 46.8%; 23.8%; 7.7%; 21.6% of patients, respectively. Detailed reclassification of patients between severity stages following application of criteria in GOLD 2011 and 2017 is described in detail in another article (Citation7).
COPD pharmacotherapy – therapeutic consequences of updating the GOLD reports
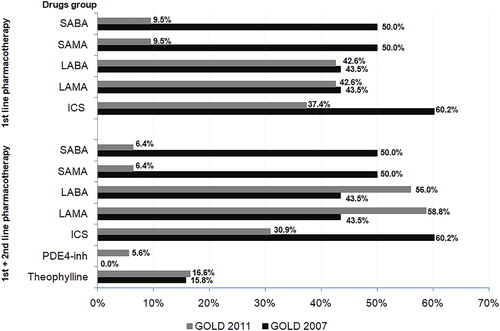
Pharmacological treatment algorithms by the GOLD 2007 report prefers the use of inhaled corticosteroids (ICS) and short-acting bronchodilators, which would be used in 60.2% and 50% of COPD patients, respectively. As a result of the implementation of the GOLD 2011 report, significant changes (p < 0.001) would occur in the pharmacotherapy of patients previously treated according to GOLD 2007. A significant decrease (p < 0.001) in the number of patients treated with SABA or SAMA as first or first- and second-line therapy [from 50% to I: 6.4; or I + II: 9.5%)] and ICS (from 60.2% to 30.9–37.4%) would be observed. Taking into account the pharmacotherapy scheme of first and second choice according to GOLD 2011, in comparison to GOLD 2007, the frequency of long-acting β2-agonists (LABA) and long-acting muscarinic antagonist (LAMA) use, would increase by 12.5% and 14.5%, respectively. Details of this simulation are presented in and Supplementary Table 4.
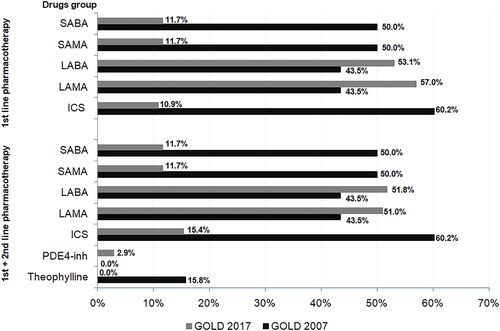
The application of the GOLD 2017 report would also significantly (p < 0.001) modify the pharmacotherapy treatment regimen used according to the GOLD 2007 report for a number of categories. There would be a decrease (p < 0.001) in the number of patients treated with short-acting drugs from the β2-agonists and muscarinic antagonist group (from 50% to 11.7%) and ICS (from 60.2% to 10.9–15.4%). The percentage of patients using theophylline would decrease almost fourfold and in 2.9% of patients, the phospodiesterase-4 inhibitor would be included in the treatment scheme. and Supplementary Table 5 shows the details of this simulation.
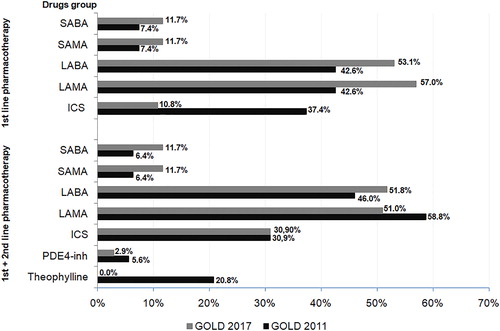
Pharmacological treatment algorithms by the GOLD 2011 report would prefer the use of long-acting bronchodilators (LABA or LAMA; 42.6–56.0% and 42.6–58.8%, respectively) and inhaled corticosteroids (30.9–37.4% of subjects). Implementation of the GOLD 2017 report would significantly (p < 0.001) modify treatment algorithm. In comparison to the pharmacotherapy regimen recommended by GOLD 2011, the percentage of patients treated with short-acting β2-agonists and muscarinic antagonist would be significantly (p < 0.001) increased. Furthermore, pharmacotherapy by the GOLD 2017 report would significantly (p < 0.001) reduce using long-acting drugs from LABA and LAMA groups, phospodiesterase-4 inhibitor. The frequency of ICS application would be comparable (p = 0.9) regardless of the used GOLD criteria. Details of this simulation are presented in and Supplementary Table 6.
Discussion
To the author’s best knowledge, this is the first available study comparing pharmacological treatment of the patients diagnosed with COPD, according to the GOLD 2007, 2011, and 2017 reports. Our study showed that updates from the GOLD 2007 COPD report to GOLD 2011 and 2017 would have a significant impact on everyday clinical practice. According to the GOLD 2007 report, the most frequently used drug classes would be short-acting bronchodilators from the group β2-agonists and muscarinic antagonist groups. In comparison to pharmacotherapy recommended by GOLD 2007 report, the classification of patients based on the GOLD 2011 report would be associated with an almost eightfold reduction in the frequency of use of short-acting bronchodilators and over fourfold decrease after implementation of the GOLD 2017 report. With each subsequent update of the GOLD reports, the frequency of use of inhaled corticosteroids would be also significantly reduced. Pharmacological treatment by the GOLD 2011 and 2017 reports would be dominated by the use of long-acting bronchodilators from the group β2-agonists and muscarinic antagonist groups. Wherein, the highest frequency of use of long-acting bronchodilators would be observed after implementation of treatment regimen recommended by the GOLD 2011 report. Significant differences in the pharmacological treatment of patients diagnosed with COPD would also be observed after an update from GOLD 2011 to 2017. However, the number of patients requiring change of treatment after the update from GOLD 2011 to 2017 would be lower, compared to the number of patients requiring change of treatment regimen after update from GOLD 2007 to GOLD 2011.
Updates of GOLD 2007–2011–2017 reports would also affect the frequency of drug interactions and the presence of adverse effects of used pharmacotherapy. Compared to the GOLD 2007 report, implementation of GOLD 2011 and 2017 COPD reports would result in a reduction of the intensity of treatment. It can be assumed that the significant decrease in the number of patients treated with inhaled corticosteroids after implementation of GOLD 2011 and 2017 reports would reduce the risk of pneumonia associated with ICS among COPD patients (Citation8). Update of the GOLD reports (2011 and 2017) would also reduce the frequency of the use of theophylline. This drug, after introduction to the treatment regimen, significantly increases the risk of drug interactions, so the pharmacotherapy according to the GOLD 2011 and 2017 seems to be safer, compared to the GOLD 2007.
Currently available data assessing clinical implications of an update of the GOLD report are very limited. Marçôa et al. showed that after update of documents from GOLD 2011 to GOLD 2017, most of the patients previously assigned to group D (GOLD 2011) had migrated toward the lighter (group B according to the GOLD 2017) form of the disease (Citation9). Despite that, the overall frequency of ICS use in a group of 200 patients with COPD had increased after reclassification of the patients from GOLD 2011 to GOLD 2017 (9). Our results are in contrast to Marçôa et al. (Citation9). After implementation of the GOLD 2017 report, the frequency of ICS use in first-line pharmacotherapy would decrease more than three times, compared to pharmacological treatment according to the GOLD 2011. Taking into account the pharmacological treatment of first and second choice, the frequency of ICS use in a group of 2597 patients with COPD would be almost identical, regardless of using the GOLD 2011 or 2017 reports. Differences in the results between our study and by Marçôa et al. study can be caused by differences in the study protocols and population: follow-up of 200 COPD patients from outpatient pulmonology clinic, while our study is a simulation based on the results from cross-sectional study of 2597 COPD patients, being under the care of GPs.
In this simulation, we have assumed that each patient was treated according to the GOLD report. Nevertheless, the real life application of the GOLD reports for COPD is not 100% implemented and adherence to the GOLD recommendation among physicians differs worldwide (Citation10–12). Excessive use of inhaled corticosteroids was well described in the literature (Citation8, Citation12, Citation13). Analysis of the pharmacological treatment regimen of patients with COPD in the United States and Europe showed that about 70% of patients with COPD used ICS, wherein half of newly diagnosed has used ICS in combination with LABA (Citation13). GOLD 2017 report, which is currently in force, significantly limits the use of inhaled corticosteroids in patients with COPD (Citation6). Maio et al. had compared the COPD management by general practitioners in Italy, according to either the GOLD 2007 or the GOLD 2011 (Citation10). Among 526 GPs, only 35.6% of the physicians had treated their patients in accordance with the GOLD 2007 report (Citation10). After the introduction of the GOLD 2011 report, the correctness of the prescribed treatment in Italy increased to 61.4% (Citation10). The correctness of treatment increased with an increase of the severity stage of the disease (Citation10). The results of the “European COPD audit” assessing the pharmacological treatment of patients with exacerbations of COPD in 422 hospitals from all over Europe also indicate on the growing educational needs (Citation14).
Moreira et al. evaluated the distribution of 830 patients with COPD to particular therapeutic groups depending on the used GOLD 2001–2011–2017 report (Citation15). After introduction of newer GOLD reports, a significant migration toward groups of lighter COPD severity was observed (Citation15). This observation is similar to that presented in our previous paper (Citation7). In the study by Mathioudakis et al., a significant reduction in the cost of COPD pharmacotherapy would be observed after applying the GOLD 2017 recommendations in UK primary care (Citation16).
Results of our study show the magnitude of changes and the impact of GOLD report update on everyday clinical practice. Within ten years, the treatment regimen of 2597 COPD patients primarily treated according to the GOLD 2007 report would significantly change. Implementation of GOLD update (2011 and 2017) would significantly change the pharmacological treatment in regards to individual patients. Consequently, every substantial update of the GOLD report should be widely promoted in the medical environment, especially among GPs, who daily manage the pharmacotherapy of patients with stable stage of COPD.
We have calculated that the number of potential treatment changes after the implementation of the GOLD 2007–2011–2017 updates covers almost 3000 combinations. The GOLD report also distinguishes two treatment regimens: recommended/preferred treatment and other defined as alternative choice or other possible treatments. Due to this fact our simulation was based on two scenarios. Because the populations of patients with COPD differ between countries, as well as between regions within one country (Citation7, Citation9, Citation10, Citation14), we decided, in case of any doubts, to refer to the cross-sectional study, which was the source of data for simulation. Taking into account the results of the Medical Research Council dyspnea scale (mMRC), frequency of COPD exacerbations, and number of hospitalization and ambulance intervention during the last 12 months, we have calculated that 40% of patients with COPD, due to the severity of disease symptoms, will require treatment modification/inclusion of new drugs in the pharmacotherapy regimen. Based on the frequency of application of theophylline obtained from the cross-sectional study, the percentage of patients using theophylline was estimated. The assumption of a different treatment intensity in the group of patients with the most severe form of COPD could affect the pharmacotherapy scheme.
The GOLD reports do not distinguish the COPD–asthma overlap syndrome (ACOS) (Citation17, Citation18). A review of national guidelines for management of COPD in 11 European countries revealed that ACOS phenotype was recognized in recommendations from the five countries (Citation18). Polish COPD guidelines also did not include the presence of ACOS (Citation18). The diagnosis of ACOS could change the preferred treatment regimen, including the use of ICS or ICS/LABA. To better reflect “real-life scenario”, the presence of ACOS should be included in the future research.
Our study has several limitations. First of all, our simulation was based on a population of the patients with COPD attending primary care physicians in Poland and does not correspond to the complete population of the patients diagnosed with COPD in Europe. However, it is one of the largest cross-sectional studies carried out among patients with COPD attending GPs in Poland.
Moreover, in Poland, the majority of COPD patients remain under the care of a primary care physician and primary care physicians actively manage their COPD pharmacotherapy. Second, we have assumed that each group of drugs included in the GOLD report can be used with equal frequency. In clinical practice, the frequency of the use of each group of drugs as well as adherence to GOLD recommendations may differ depending on the prescribing preferences of physicians. The assumption of dividing patients equally to each group of drugs (especially 50%–50% to SABA/SAMA or LABA/LAMA) may affect the interpretations of the results, especially in case of the frequency of use of these pharmacological choices. Third, we have analyzed the frequency of use of each group of drugs separately only, without considering the double or triple therapy. Nevertheless, our simulations of the therapeutic consequences of the GOLD reports updates implementation were conducted in detail, including first-line (preferred) treatment and second-line treatment.
There is a need for further studies assessing clinical implications of updates GOLD documents. Studies conducted in “real-life scenario” are necessary to fully understand the consequences of GOLD up-to-date both in individual and population dimension.
Conclusions
Updates from the GOLD 2007 COPD report to GOLD 2011 and 2017 may have a significant impact on the pharmacological treatment regimen of patients with COPD. During the last decade, GOLD recommendations on the treatment for the same clinical COPD status have significantly changed three times.
Implementation of GOLD 2011 and 2017 compared to GOLD 2007 reports would result in a reduction of the intensity of treatment, which could lead to fewer drug interactions and adverse events. Moreover, it would significantly decrease the frequency of using short-acting bronchodilators from the group β2-agonists and muscarinic antagonist groups. Use of inhaled corticosteroids would also be significantly reduced after implementation of updates of the GOLD reports. The pharmacotherapy of COPD patients would be mainly based on long-acting bronchodilatorators (β2-agonists or muscarinic antagonist), additionally adding a phosphodiesterase-4 inhibitor to the treatment algorithm.
Every substantial update of the GOLD report should be widely promoted in the medical environment, especially among GPs, who commonly manage the pharmacotherapy of patients with stable stage of COPD.
Disclosure statement
The authors report no conflict of interest.
References
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304.
- Fromer L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int J Gen Med. 2011;4:729–739. doi: 10.2147/IJGM.S21387.
- European Respiratory Society. European Lung White Book. [cited 2017 July 4]. Available from: https://www.erswhitebook.org/chapters/the-economic-burden-of-lung-disease/.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2006 revision [cited 2017 July 4]. Available from: http://www.who.int/respiratory/copd/GOLD_WR_06.pdf.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2011 revision [cited 2017 July 4]. Available from: http://www.goldcopd.org/.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2017 revision [cited 2017 July 4]. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/.
- Nowak M, Brozek GM, Zejda JE, Jankowski M, Pierzchała W. Impact of changing GOLD guidelines (2007–2011–2017) on assignment of a COPD patient to disease severity category. Adv Dermatol Allergol. 2018 [cited 2017 July 4]; [1–8]. doi: 10.5114/ada.2018.79143.
- Iannella H, Luna C, Waterer G. Inhaled corticosteroids and the increased risk of pneumonia: what’s new? A 2015 updated review. Ther Adv Resp Dis. 2016;10(3):235–255. doi: 10.1177/1753465816630208.
- Marçôa R, Rodrigues DM, Dias M, et al. Classification of chronic obstructive pulmonary disease (COPD) according to the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: comparison with GOLD 2011. COPD. 2018;15(1):21–26. doi: 10.1080/15412555.2017.1394285.
- Maio S, Baldacci S, Martini F, et al. COPD management according to old and new GOLD guidelines: an observational study with Italian general practitioners. Curr Med Res Opin. 2014;30(6):1033–1042. doi: 10.1185/03007995.2014.884492.
- Nowak M, Jankowski M, Brożek GM, et al. Profile of adults suffering from COPD in Poland. Wiad Lek. 2017;70(1):9–15.
- Glaab T, Banik N, Rutschmann OT, Wencker M. National survey of guideline-compliant COPD management among pneumologists and primary care physicians. COPD. 2006;3(3):141–148. doi: 10.1080/15412550600829299.
- Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J. 2009;34(1):13–16. doi: 10.1183/09031936.00190908.
- Roberts CM, Lopez-Campos JL, Hartl S. The European COPD Audit: brothers in arms. Breathe. 2012;8:267–270. doi: 10.1183/20734735.012812.
- Moreira S, Oliveira C, Escaleira D, et al. The impact of the three GOLD severity classifications of COPD (2001, 2011 and 2017) in the same population of the patients. Paper presented at: ERS International Congress 2017; Milan, Italy; 2017 Sept 9–13.
- Mathioudakis A, Vestbo J, Gayle A, et al. Estimating potential cost savings associated with managing COPD according to GOLD 2017 recommendations in UK primary care: a CPRD study. Paper presented at: ERS International Congress 2018; 2018 Sept 15–19; Paris, France.
- Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48(3):664–673. doi: 10.1183/13993003.00436-2016.
- Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016;47(2):625–637. doi: 10.1183/13993003.01170-2015.