Abstract
This study examined sociodemographic and clinical characteristics, treatment patterns, and health resource utilization among Medicare beneficiaries with chronic obstructive pulmonary disease (COPD) to identify predictors of nebulized arformoterol treatment. Using Medicare administrative data from 2010 to 2014, beneficiaries with ≥2 COPD outpatient visits ≥30 d apart or ≥1 COPD-related hospitalization(s) (ICD-9-CM 491.xx, 492.xx, and 496) were identified. Inclusion criteria required ≥1 COPD medication claim(s) and continuous enrollment in Parts A, B, and D. Four cohorts were identified: (a) 11,887 arformoterol users, (b) a subsample of arformoterol users (n = 1,778) who were hospitalized and discharged 30 d before initiating arformoterol, (c) 450,178 controls who had not received arformoterol, and (d) a subsample of controls (n = 21,910) who had hospitalizations. Logistic regression analysis was used to evaluate predictors of arformoterol treatment. The majority of beneficiaries were older than 70 years of age, female, Caucasian, and 47% were dual-eligible. The strongest predictors of arformoterol treatment were oxygen therapy, systemic corticosteroid or methylxanthine use, an exacerbation, a COPD-related hospitalization, and receiving care from a pulmonologist (all p < .001). Dual-eligibility, being a racial/ethnic minority, and having severe psychiatric comorbidity or immunodeficiency lowered the odds of receiving nebulized arformoterol (all p < .001). Among beneficiaries with recent hospitalizations, exacerbations and COPD-related admissions increased the odds of receiving arformoterol (p < .001). Nebulized arformoterol treatment was more likely to be initiated in sicker patients with COPD. Ensuring access to nebulized maintenance therapy is important and particularly warranted for COPD populations with greater medical needs.
Introduction
Chronic obstructive pulmonary disease (COPD) affects an estimated 16 million people in the United States (US) (Citation1, Citation2). The condition accounts for more than $32.1 billion in annual medical costs, an economic burden that is projected to increase to $49 billion by 2020 (Citation3). The use of pharmacotherapy in COPD aims to provide symptom relief and avoid costly exacerbations that frequently lead to hospitalizations to (Citation4–7). To this end, therapeutic strategies for COPD recommend the use of maintenance therapy with long-acting bronchodilators (LABDs), (Citation8, Citation9). However, past studies have shown that patients with COPD are often undertreated with LABDs despite exacerbations and repeated hospitalizations (Citation7, Citation10–13).
The largest segment of the US population affected by COPD is adults over 65 years of age (Citation14). Among this older adult population, the Medicare program serves as the primary payer for health coverage through its various components (Citation14). Medicare Part A (hospital insurance) covers inpatients hospital stays, care in a skilled nursing facility, hospice care, and some home health care. Medicare Part B (medical insurance) covers two types of services: medically necessary services and preventive care including doctors’ office visits, outpatient care, mental health care (inpatient, outpatient, and partial hospitalization), ambulance service, clinical research, medical supplies, and durable medical equipment (DME) (e.g., nebulizer devices and nebulizer medications). Medicare Part D covers prescription drugs. In addition, Medicare Advantage (also known as Medicare Part C) is an “all in one” alternative to Medicare Parts A, B, and D that bundles all three components as inclusive coverage.
Approximately one in nine Medicare beneficiaries is diagnosed with COPD, of whom 17% are dual-eligible individuals (i.e., receive Medicare and Medicaid benefits) (Citation12). Dual-eligible individuals are enrolled in Medicare Part A and/or Part B and receive full Medicaid benefits and/or assistance with Medicare premiums or cost sharing through various Medicare Savings Programs (MSPs) (Citation15). Since Medicaid is managed by individual states rather than by the US government, dual-eligible benefits vary by state. Through federal statutes, the US government defines income and resource standards for full Medicaid coverage and MSPs, but states have discretion to effectively raise those limits above the federal floor. Some states offer Medicaid through managed care plans, some provide fee-for-service coverage, while others provide comprehensive plans.
Studies have shown that 40% to 71% of Medicare beneficiaries receive no maintenance treatment for COPD (Citation13, Citation16–19). Although a recent longitudinal study on Medicare populations reported that use of maintenance medications has been improving (Citation20), in general, it is recognized that Medicare beneficiaries with COPD remain undertreated (Citation16, Citation20, Citation21).
COPD inhalation treatments are administered using several types of devices including pressurized metered-dose inhalers, dry powder inhalers, soft-mist inhalers, and nebulizers (Citation22). Factors that limit the successful administration of pharmacotherapy in patients with COPD include complex inhaler regimens that require hand-breath coordination steps and mismatching inhaler devices to patients’ needs (Citation23–26). Inhalation technique errors with handheld devices are common (Citation27–29), and can result in incomplete medication dose delivery to the lungs (Citation30). For some patients, COPD management may be improved with nebulizer use since it requires little to no hand-breath coordination or breath-holding (Citation30, Citation31).
There is limited research on the factors associated with prescribing a nebulized therapy for COPD maintenance treatment. Moreover, no study has focused on predictors of nebulized treatment in the Medicare population. The purpose of our study was to examine the sociodemographic and clinical characteristics, treatment patterns, and health resource utilization (HRU) trends among Medicare beneficiaries with COPD to identify predictors of nebulized therapy using Brovana® (arformoterol tartrate) Inhalation Solution, one of two commercially available nebulized long-acting beta2 agonists (LABA) in the US.
Methods
Data sources
This study used a 100% sample of Medicare administrative data from the Chronic Condition Warehouse at the Centers for Medicare and Medicaid Services (CMS). Demographic data were obtained from Master Beneficiary Summary Files. Information on hospitalizations was extracted from MedPar files, outpatient services from carrier and outpatient files, and home healthcare utilization from home health files. Information on medication utilization was obtained from Part D and DME files. Our study was approved by the Human Research Protections Program at the University of California San Diego. In addition, our study was subject to a data use agreement with CMS.
Sample selection
As illustrated in , our primary study population was 10,371,035 Medicare beneficiaries who had a COPD-related claim (i.e., International Classification of Diseases, 9th Revision, Clinical Modification codes 490.xx, 492.xx, 494.xx, 496.xx) between 2010 and 2014 and a COPD diagnosis before 1st January 2013. Our study sample was further refined to 3,642,497 beneficiaries who met the inclusion criteria of having continuous coverage under Medicare Parts A, B, and D. Among these beneficiaries, 106,442 used nebulized LABA therapy during 2010 and 2014 and 36,061 of these were new users during 2012 and 2103; 3,536,055 beneficiaries did not use nebulized LABA therapy during this time period.
Figure 1. Flow chart depicting sample selection. a: Full coverage and COPD diagnosis <1 year prior to first nebulized LABA claim. Excluded deaths before first nebulized LABA claims. COPD: chronic obstructive pulmonary disease; LABA: long-acting beta2 agonist; SNF: skilled nursing facility; ESRD: end stage renal disease.
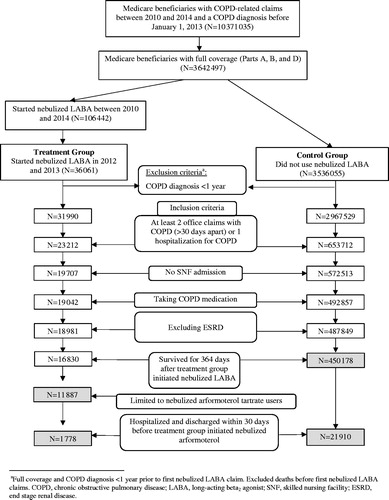
To support the analyses described below, a start date was randomly assigned to control group beneficiaries consistent with the distribution of initiation dates for nebulized LABA therapy in the study group. Both samples of new users and controls were limited to beneficiaries with: a diagnosis of COPD for at least 1 year; at least two office claims with COPD 30 d apart or one hospitalization for COPD in the prior year; without an admission to a skilled nursing facility; using COPD medication; without End Stage Renal Disease, who survived for at least 364 d following initiation of nebulized LABA therapy or their assigned start date. We further limited the sample of users of nebulized LABA therapy to those using only arformoterol.
As a final restriction, we limited both samples to beneficiaries who were hospitalized and discharged within 30 d prior to initiation of nebulized LABA therapy or their assigned start date. After applying additional exclusion and inclusion criteria, four cohorts were identified: (a) 11,887 nebulized arformoterol users, (b) a subsample of nebulized arformoterol users (n = 1,778) who had been discharged within 30 d before initiating arformoterol, (c) 450,178 controls who had received no nebulized arformoterol treatment, and (d) a subsample of controls (n = 21,910) who had been discharged within 30 d prior to the assigned start date.
Measures
The following predictor variables were examined for the year prior to initiating nebulized LABA or the assigned start date: (a) sociodemographic characteristics including age, sex, race/ethnicity, and US region in which care was delivered; (b) dual-eligibility status; (c) comorbidity as measured by the Chronic Illness and Disability Payment System (CDPS) diagnostic classification (Citation32) and the Charlson Comorbidity Index (Citation33); (d) COPD medications based on drug refill rates for short-acting bronchodilators (SABDs) which included short-acting muscarinic antagonists (SAMAs) and short-acting beta2 agonists (SABAs), LABAs, long-acting muscarinic antagonists (LAMAs), inhaled corticosteroids (ICS), systemic corticosteroids (CS), methylxanthines, phosphodiesterase (PDE)-4 inhibitors, non-specific PDE inhibitors, mucolytics, and antibiotics; (e) oxygen therapy; (f) exacerbation frequency, defined by an emergency department (ED) visit or a hospitalization for acute exacerbation of COPD (ICD-9 491.21 or 491.22) or a physician office visit followed by prescription for a systemic CS and/or a respiratory antibiotic; and (g) HRU including DME use, hospitalizations (all cause and COPD-related), intensive care unit stays, length of stay (LOS) during hospitalizations; pulmonary specialist visits (inpatient and outpatient), ED visits, and home health visits.
Statistical analysis
Descriptive statistics including means, frequencies, and proportions were used to compare sociodemographic characteristics, COPD treatment patterns, exacerbation frequency, and HRU between nebulized arformoterol users and controls. Logistic regression analysis was used to evaluate predictors of nebulized arformoterol treatment. Odds ratios (OR), 95% confidence intervals (CI), and p values were computed. p < .05 denoted statistical significance. All analyses were performed using SAS Enterprise Guide 7.1.
Results
Sociodemographic characteristics
All sociodemographic characteristics were comparable between nebulized arformoterol users and controls, except for a higher proportion of controls being dual-eligible compared to those treated with arfomoterol (47.3% vs. 36.5%, respectively) (). Across both groups, the majority of beneficiaries were >70-years-old, female, and Non-Hispanic White. The largest proportion of beneficiaries received care in the Southern or Midwest regions of the US.
Table 1. Sociodemographic characteristics of nebulized arformoterol users and controls.
COPD treatment patterns
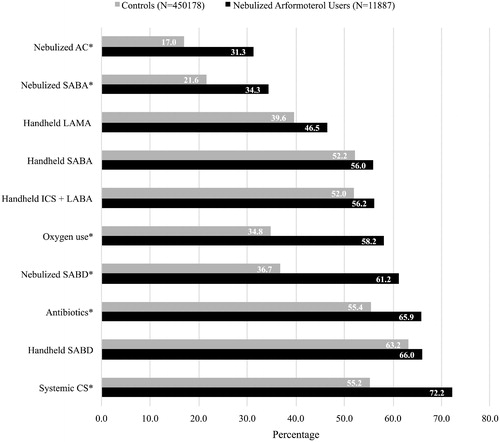
The top 10 most common COPD treatments received by nebulized arformoterol users and controls are shown in . Combination therapy with multiple medications was highly prevalent across all beneficiaries, irrespective of hospitalization history. A significantly higher proportion of nebulized arformoterol users (n = 11,887) compared to controls (n = 450,178) were receiving systemic CS (72.2% vs. 55.2%, respectively; p < .05), antibiotics (65.9% vs. 55.4%, respectively; p < .05), nebulized SABDs (61.2% vs. 36.7%, respectively; p < .05), oxygen therapy (58.2% vs. 34.8%, respectively; p < .05), and nebulized anticholinergics (AC) (31.3% vs. 17.0%, respectively; p < .05).
Figure 2. Top 10 most common COPD treatments among nebulized arformoterol users and controls in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls). COPD: chronic obstructive pulmonary disease; CS: corticosteroids; SABD: short-acting bronchodilator; ICS: inhaled corticosteroids; LABA: long-acting beta2 agonist; SABA: short-acting beta2 agonists; LAMA: long-acting muscarinic antagonist; AC: anticholinergics; *p < .05.
Exacerbations and HRU
Unadjusted results revealed that, compared to controls, a significantly higher proportion of nebulized arformoterol users had at least one exacerbation (63.5% vs. 76.4%, respectively; p < .05), an all-cause (35.2% vs. 46.2%, respectively; p < .05) or a COPD-related (12.3% vs. 21.1%, respectively; p < .05) hospitalization, and received inpatient (12.3% vs. 20.6%, respectively; p < .05) or outpatient care (42.3% vs. 57.6%, respectively; p < .05) from a pulmonologist within the previous 12 months ().
Figure 3. a: Exacerbations and health resource utilization patterns in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls). ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; ED: emergency department; * p < .05. b: Exacerbations and health resource utilization patterns in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls) among Medicare beneficiaries with recent hospitalizations. ED: emergency department; ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; * p < .05.
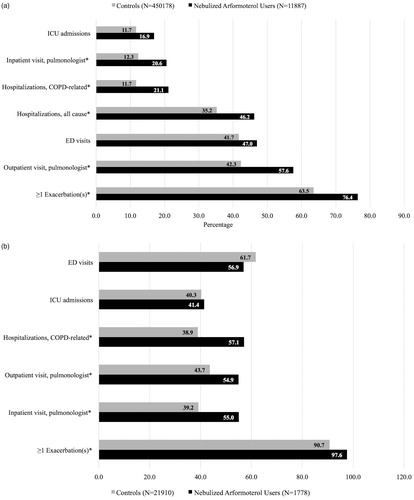
Among beneficiaries with recent hospitalizations (), nearly all nebulized arformoterol users and controls had at least one exacerbation within the previous 12 months (97.6% vs. 90.7%, respectively; p < .05). In addition, prior COPD-related hospitalization was significantly higher among nebulized arformoterol users than controls (57.1% vs. 38.9%, respectively; p < .05).
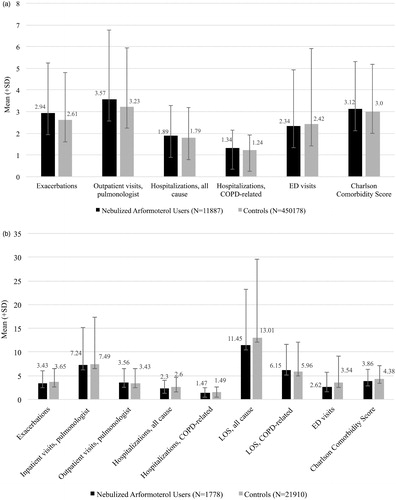
The average number of exacerbations, outpatient visits, all-cause and COPD-related hospitalizations, and ED visits within the previous 12-months were similar between nebulized arformoterol users and controls (). Charlson comorbidity scores were also similar between the two groups. Beneficiaries who had hospitalizations had frequent exacerbations and their HRU was high (). Although not statistically different from one another, within the previous 12 months, beneficiaries treated with nebulized arformoterol and controls had an average LOS that exceeded 11 d for all-cause admissions (11.45 ± 11.8 vs. 13.01 ± 16.5, respectively) and 6 d for COPD-related admissions (6.15 ± 6.1 vs. 5.96 ± 5.4, respectively). Moreover, these beneficiaries had a high number of comorbidity (3.86 ± 2.5 for nebulized arformoterol group vs. 4.38 ± 2.7 for controls).
Figure 4. a. Average number of exacerbations, health resources used, and comorbidity in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls). COPD: chronic obstructive pulmonary disease; ED: emergency department. b. Average number of exacerbations, health resources used, and co-morbidities in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls) among Medicare beneficiaries with recent hospitalizations. COPD: chronic obstructive pulmonary disease; LOS: length of stay; ED: emergency department.
Predictors of nebulized arformoterol treatment
After adjusting for potential confounders such as age and sex, several factors including sociodemographic and clinical characteristics, COPD treatments, and HRU patterns were found to be associated with the initiation of nebulized arformoterol therapy (). The strongest predictors of nebulized arformoterol treatment were being on oxygen therapy (OR: 2.01, 95% CI: 1.93, 2.09; p < .001); using a systemic CS (OR: 1.50, 95% CI: 1.43, 1.57; p < .001) or methylxanthine (OR: 1.37, 95% CI: 1.28, 1.47; p < .001); having an exacerbation (OR: 1.33, 95% CI: 1.26, 1.41; p < .001) or a COPD-related hospitalization (OR: 1.31, 95% CI: 1.24, 1.39; p < .001); and receiving outpatient care from a pulmonologist (OR: 1.40, 95% CI: 1.34, 1.46; p < .001) or a respiratory therapist (OR: 1.23, 95% CI: 1.11, 1.36; p < .001).
Figure 5. Predictors of nebulized arformoterol treatment among Medicare beneficiaries with COPD. COPD: chronic obstructive pulmonary disease; OR: odds ratio; CI: confidence interval; US: United States; AIDS: acquired immune deficiency syndrome; GI: gastrointestinal; CNS: central nervous system; CS: corticosteroids; LAMA: long-acting muscarinic antagonist; SABD: short-acting bronchodilator; HH: home healthcare; ED: emergency department; OP: outpatient; IP: inpatient; * p < .05; ** p < .01; *** p < .001.
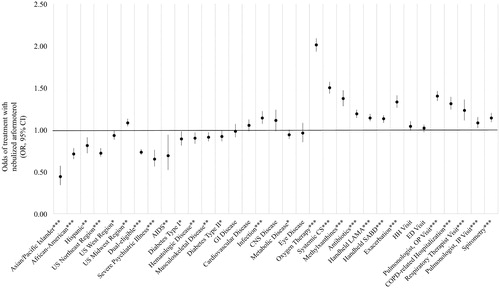
By contrast, the odds of being treated with nebulized arformoterol were significantly lower for Asians/Pacific Islanders (OR: 0.44, 95% CI: 0.34, 0.57); p < .001), African-Americans (OR: 0.71, 95% CI: 0.65, 0.78; p < .001), or and Hispanics (OR: 0.81, 95% CI: 0.72, 0.91; p < .01); the dual-eligible (OR: 0.73, 95% CI: 0.70, 0.77; p < .001); and beneficiaries with a severe psychiatric comorbidity (OR: 0.65, 95% CI: 0.56, 0.76; p < .001) or acquired immune deficiency syndrome (AIDS) (OR: 0.69, 95% CI: 0.52, 0.94; p < .01).
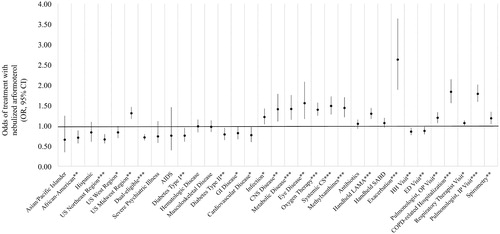
Among beneficiaries with recent hospitalizations (), the strongest predictors of receiving nebulized arformoterol were having an exacerbation (OR: 2.62, 95% CI: 1.88, 3.63; p < .001) or a recent COPD-related hospitalization (OR: 1.83, 95% CI: 1.55, 2.14; p < .001); and receiving inpatient care from a pulmonologist (OR: 1.78, 95% CI: 1.58, 2.01; p < .001). Other significant predictors followed a similar trend to those observed in the larger cohort of beneficiaries (), with the exception of three additional positive predictors: having a metabolic disease (OR: 1.41, 95% CI: 1.14, 1.75; p < .001), a central nervous system (CNS disease) (OR: 1.40, 95% CI: 1.10, 1.78; p < .01), or eye disease (OR: 1.55, 95% CI: 1.16, 2.08; p < .01); and one negative predictor: receiving care in the northeast region of the US (OR: 0.66, 95% CI: 0.56, 0.79; p < .001).
Figure 6. Predictors of nebulized arformoterol treatment among Medicare beneficiaries with recent hospitalizations. COPD: chronic obstructive pulmonary disease; OR: odds ratio; CI: confidence interval; US: United States; AIDS: acquired immune deficiency syndrome; GI: gastrointestinal; CNS: central nervous system; CS: corticosteroids; LAMA: long-acting muscarinic antagonist; SABD: short-acting bronchodilator; HH: home healthcare; ED: emergency department; OP: outpatient; IP: inpatient; *p<.05; **p<.01; ***p<.001.
Discussion
In this case–control study between Medicare beneficiaries who initiated nebulized arformoterol and control beneficiaries who did not, the distribution of nearly all of the sociodemographic characteristics were as anticipated except for the higher proportion of women than men in both cohorts. Since reports have shown that in the Medicare population the prevalence of COPD is higher in men than women (Citation34), our results initially appeared to be somewhat counterintuitive. However, further examination of our data revealed that 56% of beneficiaries 65 years and older were female. Since most Medicare beneficiaries with COPD are older than 70 years of age, the higher percentage of women than men in our two cohorts can be explained by the age distributions that ensued as a result of applying our various inclusion and exclusion criteria. Moreover, our findings with regards to gender distribution are consistent with previous studies that have applied similar sampling criteria to ours to explore factors associated with maintenance treatment patterns among Medicare beneficiaries with COPD (Citation20).
Our study revealed that the most important factors associated with the initiation of nebulized arformoterol were oxygen therapy, use of systemic CS or methylxanthine, and a previous exacerbation or COPD-related hospitalization. In contrast, beneficiaries who were dual-eligible, had severe psychiatric comorbidity or acquired immune deficiency syndrome (AIDS) were less likely to be treated with nebulized arformoterol. These results reveal several insights that can inform clinical practice.
From a clinical management perspective, we found several important predictors that collectively suggest sicker beneficiaries were more likely to be treated with nebulized arformoterol. The odds of receiving nebulized arformoterol treatment were increased if a beneficiary was receiving oxygen therapy, had an exacerbation, or a COPD-related hospitalization. While similar studies have not been conducted on Medicare populations and limited to nebulized therapy, there is some evidence from an international study that concurs with our findings that having a previous exacerbation is a positive predictor of initiating LABA treatment with handheld devices among patients with COPD (Citation35). Among Medicare beneficiaries, the use of any maintenance medication substantially reduces the risk of hospitalizations (Citation19). Unfortunately, past research conducted on large commercial and Medicare databases has found that hospitalizations do not necessarily impact LABD initiation, and that most patients with COPD continue to be undertreated even after a hospitalization episode (Citation36). Our findings suggest a more positive outlook in that clinicians seem to be incorporating knowledge from empirical studies (Citation37–39) into their patient treatment plans. Further support for our interpretation comes from the other positive predictors we found to be associated with initiating nebulized arformoterol therapy, namely receiving care from a pulmonologist or respiratory therapist. This latter finding is supported by past research showing that non-pulmonary clinicians tend to have more limited awareness and knowledge of current COPD treatment guidelines, a factor that has been posited to contribute to the under treatment of COPD (Citation21, Citation40, Citation41).
We also found that certain pharmacotherapies were significant predictors of nebulized arformoterol treatment including systemic CS, methylxanthines, antibiotics, and handheld LAMAs and SABDs (all p < .001). Our study did not evaluate whether nebulized arformoterol was used in combination or as a replacement therapy. However, past studies have shown that patients with COPD who receive nebulized therapies generally have more advanced disease compared to those who receive other forms of pharmacotherapy (Citation30, Citation42). Thus, it is reasonable to assume that nebulized arformoterol was likely added as a combination therapy rather than as a monotherapy (Citation11, Citation43).
Our results further revealed that certain predictors were associated with lower odds of receiving nebulized arformoterol. Beneficiaries with African-American, Hispanic, and Asian/Pacific Islander race/ethnicity as well as the dual-eligible were less likely than non-Hispanic Whites and non-dual-eligibles to receive nebulized maintenance therapy. These results are concerning in light of past research that has demonstrated many negative health and economic outcomes associated with disparities in access to treatment among these groups of beneficiaries (Citation44–49). For example, dual-eligible status is associated with a higher rate of COPD exacerbations and hospitalizations, a greater number of co-morbidities, and higher HRU (Citation48–50). Although recent studies in Medicare populations have found that dual-eligible beneficiaries were more likely to receive maintenance medications compared to non-dual eligible beneficiaries, the rates of access to nebulized therapies have remained low (Citation20). Past research has shown that treatment with nebulized arformoterol can substantially improve the health status of COPD patients through better disease management, leading to fewer exacerbations and lower COPD-related costs (primarily related to hospitalizations) (Citation37–39, Citation51). Thus, increasing access to nebulized maintenance therapy is likely to benefit dual-eligible beneficiaries, many (>40%) of whom despite being much younger than 65 years old, have substantial unmet medical needs (Citation8, Citation52, Citation53). Future research employing well-controlled study designs that quantify this potential benefit would be insightful.
Another important finding of the current study was that certain co-morbidities including severe psychiatric disorders, AIDS, diabetes, musculoskeletal disease and cardiovascular conditions significantly lower the odds of receiving nebulized arformoterol treatment. Although not specific to Medicare populations, past studies have shown cardiovascular diseases, diabetes, musculoskeletal disease, and psychiatric disorders to be negatively associated with initiation of LABA treatment, a finding that resonates with our results (Citation32, Citation54, Citation55). Among these predictors, the most disconcerting result was that beneficiaries with severe psychiatric illness were less likely to be prescribed nebulized maintenance therapy. In Medicare populations, patients with concurrent COPD and psychiatric conditions experience more exacerbations, have higher HRU, and subsequent healthcare costs (Citation55, Citation56). In addition, poor adherence to inhaled COPD maintenance medications is common among beneficiaries with psychiatric illness (Citation13, Citation16, Citation57). Since nebulizers require more passive participation from users to administer medications than handheld inhalers, increasing reliance on nebulized maintenance therapy may improve adherence to prescribed COPD treatment regimens (Citation13, Citation27, Citation40).
There are several limitations to our study. As with any retrospective claims database study, there is the potential for coding inaccuracy, missing data, and other reliability and validity indicators related to data quality. Specifically, the diagnosis of COPD is not based on spirometry but rather on the appropriate Medicare coding; therefore, the diagnosis was likely based on clinical interpretation in many of the patients included in our study. Our analysis was limited to fee-for-service Medicare beneficiaries with Parts A, B, and D. Thus, the findings may not be generalizable to managed Medicare or commercially insured populations. Information was not available on factors that may have influenced treatment management such as patient’s cognitive impairments, limited hand-breath coordination abilities, poor dexterity, inhalation device preferences, or inadequate adherence (Citation29, Citation58–62). We had no direct measures of COPD severity, such as spirometry values and dyspnea scores. However, our inclusion criteria required a COPD diagnosis of ≥1 year and past research has provided ample evidence that by the time a COPD diagnosis is given, most patients have progressed beyond mild disease (Citation1, Citation2, Citation63–65). In addition, medication use was determined based on prescription fills. Thus, we could not account for medications that may have been prescribed but never filled or filled but not used as prescribed. Reliance on ICD-9-CM codes to identify beneficiaries with psychiatric disorders may not have accurately captured the true prevalence of these conditions (Citation66–68). Lastly, the role of formulary placement may have influenced the decision to initiate nebulized arformoterol therapy, an unobservable heterogeneous treatment effect that was not considered in the current analysis (Citation69).
Conclusions
Nebulized arformoterol was more likely to be initiated in sicker patients with COPD. Given the results of this study, increasing access to nebulized maintenance therapy is warranted for select populations with COPD including racial/ethnic minorities, the dual-eligible, and those with certain co-morbidities, such as psychiatric disorders. Future studies are needed to explore the optimal time to initiate nebulized maintenance therapy, and the potential differential impact of early versus late initiation on patient outcomes.
Disclosure statement
T.P.G. and Z.X. are employees of the University of California San Diego and received institutional research funding from Advance Health Solutions to conduct this study. B.R.C. received consultation remuneration as a member of the Medical Advisory Board at Advance Health Solutions. He has also been a consultant for Glaxo Smith Kline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. S.C. and M.N. are employed by Advance Health Solutions, LLC. C.D. is an employee of Sunovion Pharmaceuticals Inc. This project was funded by Sunovion Pharmaceuticals Inc.
Additional information
Funding
References
- Chronic Obstructive Pulmonary Disease [Internet]. Atlanta (GA): Centers for Disease Control and Prevention. What is COPD? 2018 June 6 [cited 2018 Nov 1]; [about 1 screen]. Available from: https://www.cdc.gov/copd/index.html
- Murphy SL, Xu JQ, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017:66(6):1–75.
- Ford ES, Murphy LB, Khaviou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi:10.1378/chest.14-0972.
- Guarascio AJ, Ray SM, Finch CK, et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi:10.2147/CEOR.S34321.
- Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benefits. 2014;7(2):98–106.
- Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473–526. doi:10.1016/S2213-2600(16)00094-1.
- Petite SE. Characterization of chronic obstructive pulmonary disease prescribing patterns in the United States. Pulm Pharmacol Ther. 2018;49:119–122. doi:10.1016/j.pupt.2018.02.003.
- Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD 2018 report [Internet]. GOLD; 2018. 142 p. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi:10.7326/0003-4819-155-3-201108020-00008.
- Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi:10.1016/j.amjmed.2008.09.042.
- Ford ES, Mannino DM, Giles WH, et al. Prescription practices for chronic obstructive pulmonary disease: findings from the national ambulatory medical care survey 1999-2010. COPD. 2014;11(3):247–255.
- Centers for Medicare and Medicaid Services (CMS). Chronic conditions among medicare beneficiaries, Chartbook, 2012 edition [Internet]. Baltimore (MD): CMS; 2012. p. 30. Available from: https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/chronic-conditions/downloads/2012chartbook.pdf
- Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. doi:10.2147/COPD.S25805.
- Henry J. Kaiser Family Foundation. An overview of medicare. Issue Brief, February 2019. Available from: https://www.kff.org/medicare/issue-brief/an-overview-of-medicare/.
- Centers for Medicare and Medicaid Services. Dual eligible beneficiaries under medicare and medicaid. 2018. Available from: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Medicare_Beneficiaries_Dual_Eligibles_At_a_Glance.pdf
- Celli BR, Navaie M, Xu Z, et al. Medication management patterns among Medicare beneficiaries with chronic obstructive pulmonary disease who initiate nebulized arformoterol treatment. Int J Chron Obstruct Pulmon Dis. 2019;14:1019-1031. doi:10.2147/COPD.S199251.
- Bishwakarma R, Zhang W, Kuo YF, et al. Long-acting bronchodilators with or without inhaled corticosteroids and 30-day readmission in patients hospitalized for COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:477–486. doi:10.2147/COPD.S122354.
- Qian J, Simoni-Wastila L, Rattinger GB, et al. Association between depression and maintenance medication adherence among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2014;29(1):49–57. doi:10.1002/gps.3968.
- Stuart BC, Simoni-Wastilla L, Zuckerman IH, et al. Impact of maintenance therapy on hospitalization and expenditures for Medicare beneficiaries with chronic obstructive pulmonary disease. Am J Geriatr Pharmacother. 2010;8(5):441–453. doi:10.1016/j.amjopharm.2010.10.002.
- Nishi SPE, Maslonka M, Zhang W, et al. Pattern and adherence to maintenance medication use in Medicare beneficiaries with chronic obstructive pulmonary disease: 2008–2013. Chronic Obstr Pulm Dis. 2018;5(1):16–26. doi:10.15326/jcopdf.5.1.2017.0153.
- Make B, Dutro MP, Paulose-Ram R, et al. Undertreatment of COPD: a retrospective analysis of us managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:201–209.
- Carlin BW, Schuldheisz SK, Noth I, et al. Individualizing the selection of long-acting bronchodilator therapy for patients with COPD: considerations in primary care. Postgrad Med. 2017;129(7):725–733. doi:10.1080/00325481.2017.1353885.
- Koehorst-ter HK, Movig K, van der Valk P, et al. The influence of type of inhalation device on adherence of COPD patients to inhaled medication. Expert Opin Drug Deliv. 2016;13(4):469–475. doi:10.1517/17425247.2016.1130695.
- Darbà J, Ramírez G, Sicras A, et al. The importance of inhaler devices: the choice of inhaler device may lead to suboptimal adherence in COPD patients. Int Chronic Obstruct Pulmon Dis. 2015;10:2335–2345.
- Braido F, Chrystyn H, Baiardini I, et al; Respiratory Effectiveness Group. "Trying, but failing" - the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract. 2016;4(5):823–832. doi:10.1016/j.jaip.2016.03.002.
- Barrons R, Wheeler J, Woods JA. Opportunities for inhaler device selection in elderly patients with asthma or COPD. Patient Intell. 2015;7:53–65.
- Melzer AC, Ghassemieh BJ, Gillespie SE, et al. Patient characteristics associated with poor inhaler technique among a cohort of patients with COPD. Respir Med. 2017;123:124–130. doi:10.1016/j.rmed.2016.12.011.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22. doi:10.1038/s41533-017-0016-z.
- Cho-Reyes S, Celli BR, Dembek C, et al. Inhalation technique errors with metered-dose inhalers among patients with obstructive lung diseases: a systematic review and meta-analysis of US studies. Chronic Obstr Pulm Dis. 2019;6(3): In press.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49:1601794. doi:10.1183/13993003.01794-2016.
- Patel M, Steinberg K, Suarez-Barcelo M, et al. Chronic obstructive pulmonary disease in post-acute/long-term care settings: seizing opportunities to individualize teatment and device selection. J Am Med Dir Assoc. 2017;18(6):553.e17–553.e22. doi:10.1016/j.jamda.2017.03.020.
- Kronick R, Gilmer TP, Dreyfus T, et al. CDPS-Medicare: the chronic illness and disability payment system modified to predict expenditures for Medicare beneficiaries [Internet]. Final Report to CMS; 2002. 147 p. Available from: http://cdps.ucsd.edu/CDPS_Medicare.pdf
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Centers for Medicare and Medicaid Services. Medicare chronic conditions prevalence state level: all beneficiaries by sex and age, 2007–2017 [Internet]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/CC_Main.html
- Drivenes E, Ostrem A, Melbye H. Predictors of ICS/LABA prescribing in COPD patients: a study from general practice. BMC Fam Pract. 2014;15:42. doi:10.1186/1471-2296-15-42.
- Baker CL, Zou KH, Su J. Long-acting bronchodilator use after hospitalization for COPD: an observational study of health insurance claims data. Int J Chron Obstruct Pulmon Dis. 2014;9:431–439.
- Baumgartner RA, Hanania NA, Calhoun WJ, et al. Nebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trial. Clin Ther. 2007;29(2):261–278. doi:10.1016/j.clinthera.2007.02.009.
- Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5(1):25–34. doi:10.1080/15412550701816187.
- Chen YJ, Makin C, Bollu VK, et al. Exacerbations, health services utilization, and costs in commercially-insured COPD patients treated with nebulized long-acting β2-agonists. J Med Econ. 2016;19(1):11–20. doi:10.3111/13696998.2015.1079530.
- Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9(3):24.
- Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis. 2008;3(2):311–318. doi:10.2147/COPD.S2486.
- Wise RA, Acevedo RA, Anzueto AR, et al. Guiding principles for the use of nebulized long-acting beta2-agonists in patients with COPD: an expert panel consensus. Chronic Obstr Pul Dis. 2017;4(1):7–20. doi:10.15326/jcopdf.4.1.2016.0141.
- Lane DC, Stemkowski S, Stanford RH, et al. Initiation of triple therapy with multiple inhalers in chronic obstructive pulmonary disease: an analysis of treatment patterns from a U.S. Retrospective database study. J Manag Care Spec Pharm. 2018;24(11):1165–1172. doi:10.18553/jmcp.2018.24.11.1165.
- Pleasants RA, Riley IL, Mannino DM. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2475–2496. doi:10.2147/COPD.S79077.
- Centers for Medicare and Medicaid Services (CMS) Office of Minority Health and RAND Corporation. Racial and ethnic disparities by gender in health care in Medicare advantage [Internet]. Baltimore (MD): CMS; 2017. 39 p. Available from: https://www.cms.gov/About-CMS/Agency-Information/OMH/Downloads/Health-Disparities-Racial-and-Ethnic-Disparities-by-Gender-National-Report.pdf
- Gandhi K, Lim E, Davis J, et al. Racial disparities in health service utilization among Medicare fee-for-service beneficiaries adjusting for multiple chronic conditions. J Aging Health. 2017;30(8):1224–1243. doi:10.1177/0898264317714143.
- Siegel M ,Krishnan JA ,Lamson-Sullivan J, et al. Health disparities in chronic obstructive pulmonary disease. In: Gerald LB, Berry CE, editors. Health disparities in respiratory medicine. New York (NY): Springer; 2016. p. 189–206.
- D’Souza AO, Shah M, Dhamane AM, et al. Clinical and economic burden of COPD in a Medicaid population. COPD. 2014;11(2):212–220. doi:10.3109/15412555.2013.836168.
- Congressional Budget Office (CBO). Dual-eligible beneficiaries of Medicare and Medicaid: characteristics, health care spending, and evolving policies [Internet]. Washington (DC): CBO; 2013. 45 p. Available from: https://www.cbo.gov/sites/default/files/113th-congress-2013-2014/reports/44308dualeligibles2.pdf
- Jiang HJ, Wier LM, Potter DEB, et al. Potentially preventable hospitalizations among Medicare-Medicaid dual eligibles, 2008: statistical brief #96 [Internet]. Rockville (MD): Agency for Healthcare Quality and Research; 2010. 11 p. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb96.pdf
- Donohue JF, Ganapathy V, Bollu V, et al. Health status of patients with moderate to severe COPD after treatment with nebulized arformoterol tartrate or placebo for 1 year. Clin Ther. 2017;39(1):66–74. doi:10.1016/j.clinthera.2016.11.021.
- The Medicare Payment Advisory Commission (MedPac). A data book: Health care spending and the Medicare program [Internet]. Washington (DC): MedPac; 2016. Section 4, Dual-eligible beneficiaries; p. 35–40. Available from: http://www.medpac.gov/docs/default-source/data-book/june-2016-data-book-section-4-dual-eligible-beneficiaries.pdf?sfvrsn=0
- Terasaki J, Nishi SPE, Ameredes BT, et al. Arformoterol: rationale for use in chronic obstructive pulmonary disease. Clin Invest. 2014;4(5):429–439. doi:10.4155/cli.14.32.
- Di Martino M, Agabiti N, Bauleo L, et al. Use patterns of long-acting bronchodilators in routine COPD care: the OUTPUL study. COPD. 2014;11(4):414–423. doi:10.3109/15412555.2013.839646.
- Singh G, Zhang W, Kuo YF, et al. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905–915. doi:10.1378/chest.15-0449.
- Albrecht JS, Huang TY, Park Y, et al. New episodes of depression among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2016;31(5):441–449. doi:10.1002/gps.4348.
- Albrecht JS, Park Y, Hur P, et al. Adherence to maintenance medications among older adults with chronic obstructive pulmonary disease. The role of depression. Ann Am Thorac Soc. 2016;13(9):1497–1504. doi:10.1513/AnnalsATS.201602-136OC.
- Sharafkhaneh A, Wolf RA, Goodnight S, et al. Perceptions and attitudes toward the use of nebulized therapy for COPD: patient and caregiver perspectives. COPD. 2013;10(4):482–492. doi:10.3109/15412555.2013.773302.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221–1232. doi:10.2146/ajhp100452.
- Baird C, Lovell J, Johnson M, et al. The impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic review. Respir Med. 2017;129:130–139. doi:10.1016/j.rmed.2017.06.006.
- Ding B, Small M, Scheffel G, et al. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma-COPD overlap syndrome consulting for routine care. Int J Chron Obstruct Pulmon Dis. 2018;13:927–936. doi:10.2147/COPD.S154525.
- Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28(3):219–228. doi:10.1089/jamp.2014.1142.
- Martinez FJ, Han MK, Allinson JP, et al. At the Root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(12):1540–1551. doi:10.1164/rccm.201710-2028PP.
- Rossi A, Butorac-Petanjek B, Chilosi M, et al. Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research - a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis. 2017;12:2593–2610. doi:10.2147/COPD.S132236.
- Agustí A, Celli B. Natural history of COPD: gaps and opportunities. ERJ Open Res. 2017;3(4):00117-2017. doi:10.1183/23120541.00117-2017.
- Grossberg GT, Beck D, Zaidi SNY. Assessment in rapid depression geriatric patients. Clin Geriatr Med. 2017;33(3):383–391. doi:10.1016/j.cger.2017.03.007.
- Small GW. Differential diagnoses and assessment of depression in elderly patients. J Clin Psychiatry. 2009;70(12):e47. doi:10.4088/JCP.8001tx20c.
- Wells KB, Hays RD, Burnam MA, et al. Detection of depressive disorder for patients receiving prepaid or fee-for-service care. Results from the medical outcomes study. JAMA. 1989;262(23):3298–3302.
- Shenolikar R, Bruno AS, Eaddy M, et al. Sensitivity of medication use to formulary controls in Medicare beneficiaries: a review of the literature. Am Health Drug Benefit. 2011;4(7):465–474.
