Abstract
Whether the use of adaptive support ventilation (ASV) during noninvasive ventilation (NIV) is as effective as pressure support ventilation (PSV) remains unknown. In this exploratory study, we compared the delivery of NIV with PSV vs. ASV. We randomized consecutive subjects with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) to receive NIV either with the PSV or the ASV mode. The primary outcome was NIV failure (endotracheal intubation, re-institution of NIV within 48 h of discontinuation or mortality). The secondary outcomes were the duration of mechanical ventilation (invasive and noninvasive), the number of NIV manipulations, the visual analogue score (VAS) for physician’s ease of use and patient’s comfort, and the complications of NIV use. We enrolled 74 subjects (n = 38, PSV; n = 36, ASV; 78.4% males) with a mean (SD) age of 60.5 (9.5) years. The baseline characteristics were similar between the two groups. The overall NIV failure rate was 28.4% and was similar between the two groups (PSV vs. ASV: 34.2% vs. 22.2%, p = 0.31). There was a 9% reduction in the intubation rate with ASV. There were six deaths (PSV vs. ASV: 2 vs 4, p =0.311). There was no difference in the secondary outcomes. The application of NIV using ASV was associated with a similar success rate as PSV in subjects with AECOPD. Due to the small sample size, the results of our study should be confirmed in a larger trial.
Trial registry: ww.clinicaltrials.gov (NCT02877524).
Introduction
Noninvasive ventilation (NIV) is the standard of care in the management of patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) (Citation1–5). The use of NIV during AECOPD not only avoids endotracheal intubation but also reduces the mortality (Citation1, Citation2, Citation6, Citation7). Traditionally, NIV has been delivered using the pressure support ventilation (PSV) mode. Although PSV allows the patient to influence the breathing pattern, the ventilator cycling criteria and the inability of pressure support (PS) to vary with changing lung mechanics are significant limitations of this mode (Citation8).
Adaptive support ventilation (ASV) is a closed loop mode of ventilation that maintains an operator-preset target minute ventilation, independent of the patients’ activity. The ASV mode calculates the patient’s expiratory time constant (RCexp, the product of lung compliance and airway resistance) on a breath-to-breath basis (Citation9). It then uses the Otis’ equation, which calculates the optimal respiratory rate and inspiratory pressure based on the minimal work of breathing concept (Citation10). By matching the patient’s respiratory demand, it can potentially improve the ventilator success rates (Citation10–13). In some of our patients (unpublished observation), we have successfully delivered NIV using the ASV mode. We hypothesized that the use of ASV during NIV in subjects with AECOPD would result in better outcomes compared to the conventional PSV mode. In this exploratory study, we compare the efficacy of NIV delivered using either the PSV or the ASV mode in subjects with AECOPD.
Methods
This was an investigator-initiated, single-centre, randomized controlled trial (RCT) conducted between September 2016 and December 2017 in the respiratory intensive care unit (RICU) of our Institute. The study protocol was approved by the Ethics Review Committee (NK/2609/DM/105), and a written informed consent was obtained from all the subjects (or the next of kin). The trial was registered at www.clinicaltrials.gov (NCT02877524).
Study subjects
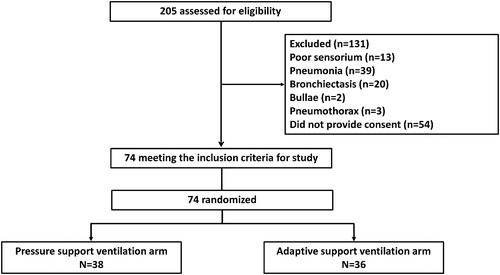
We included consecutive subjects with AECOPD if they met all of the following: (a) an acute (<7 days) sustained worsening of any of the patient’s respiratory symptoms (cough, sputum quantity or character, dyspnoea) beyond the normal day-to-day variation; (b) arterial blood gas analysis showing a PaCO2 >45 mm Hg with either pH between 7.26 and 7.35 or respiratory rate (fR) >30 breaths/min; and, (c) exclusion of other causes of acute breathlessness such as acute heart failure, pulmonary embolism, pneumonia, and pneumothorax. We also excluded subjects with any of the following: (a) acute hypercapneic respiratory failure due to other causes; (b) hypotension (systolic blood pressure <90 mm Hg); (c) severe encephalopathy (Glasgow coma score <8); (d) upper gastrointestinal bleeding; (e) inability to protect the airway and clear respiratory secretions; (f) abnormalities that precluded proper fit of the interface (agitated or uncooperative patient, facial trauma or burns, facial surgery, or facial anatomical abnormality); (g) subjects on domiciliary NIV; (h) pregnancy; and, (i) failure to provide informed consent.
Randomization
Subjects meeting the inclusion criteria were sequentially randomized 1:1 to either the PSV or the ASV group (). The randomization sequence was computer-generated with blocks of four. The assignments were placed in an opaque sealed envelope, which was opened by the ICU physician, not directly involved in the study or the analysis of results. Due to the nature of the study, blinding was not possible.
Study procedure
NIV was administered using an ICU ventilator (Galileo Gold [Hamilton Medical, Bonaduz, Switzerland]). The entire procedure of NIV application was explained to the subjects to improve compliance. NIV was delivered using an oronasal mask (UltraMirage, Resmed, Sydney, Australia) of appropriate size. Subjects were allowed intermittent periods off NIV, for eating or expectoration of secretions, and during this period they were given oxygen through a nasal cannula to maintain SpO2 between 89 and 92%. Subjects in both the study groups received the standard medical care (nebulized bronchodilators, intravenous antibiotics, systemic glucocorticoids) for AECOPD, in addition to NIV (Citation14).
PSV mode
Subjects in the PSV mode were ventilated with initial pressure support (PS) set at 6–8 cm H2O and increased by 2 cm to achieve clinical response defined as relief of dyspnoea, fR <30 breaths/min and tidal volume >6–8 mL/kg ideal body weight (IBW). Positive end-expiratory pressure (PEEP) was commenced at 3–4 cm H2O and increased by 1 cm H2O to achieve SpO2 >92%. The maximum PS and PEEP allowed was 20 and 10 cm H2O, respectively. A minimum difference of pressure of 3 cm H2O between PS and PEEP was always maintained. The expiratory trigger sensitivity was set at 30–35% (Citation15).
ASV mode
In the ASV arm, the ICU physician had to set the percentage minute volume (%MinVol) and PEEP. The %MinVol was calculated as 0.1 L/kg IBW/min. For example, in a subject with an IBW of 60 kg, the 100%-MinVol is 6 L/kg/min. Subjects randomized to the ASV mode were ventilated with an initial setting of 100%-MinVol. Further increments of 10% MinVol (maximum 200%) were made every 15 min to achieve a clinical response similar to the PSV arm. The PEEP was set similar to the PSV mode. The expiratory trigger sensitivity was maintained at 30–35% (Citation15).
Monitoring of the subjects
Subjects were initially monitored every 15 min for fR, heart rate, blood pressure, Glasgow coma score, pulse oximetry, and signs of respiratory fatigue (respiratory alternans, paradoxical breathing), fit of mask, and any peri-mask leak. Arterial blood gas analysis was performed at the initiation of NIV, and at 1, 2, 4, 12 and 24 h after the initiation of NIV, at study endpoint and discharge from ICU. The total duration of NIV applied during each day was also recorded.
Endpoints
The primary outcome was NIV failure defined as the occurrence of any of the following events: subject requiring endotracheal intubation or re-institution of NIV within 48 h of initial discontinuation of NIV or death (in the ICU or hospital). The secondary outcomes were the duration of mechanical ventilation (invasive and noninvasive), duration of NIV, time to intubation, number of NIV manipulations, physician’s ease of the use of the mode on visual analog scale (VAS, on a 100 mm scale [0 being easy to use and 100 being very complicated]), patient’s comfort rated on VAS (0 as comfortable; 100 as extreme discomfort), complications related to NIV, number of weaning failures, and the ICU and the hospital length of stay. The subjects were followed up until hospital discharge or death, whichever was earlier.
The decision to intubate endotracheally was made if any one of the following criteria were met: (a) failure to improve or stabilize gas exchange or dyspnoea in 1-h; (b) bradycardia (heart rate <60 beats/min with altered mental status); (c) hypotension (systolic blood pressure <90 mm Hg); (d) failure to maintain SpO2 >88% despite a FiO2 of 0.6; (e) persistent respiratory acidosis (pH <7.35) despite 1 h of NIV; (f) pooling/inability to handle secretions; (g) inability to tolerate the NIV; and, (h) maximum allowed pressures reached without clinical improvement. We recorded the reason for intubation.
NIV weaning
NIV weaning was commenced if a patient met all of the following criteria: (a) PS ≤12 cm H2O & PEEP ≤6 cm H2O (for ASV, %MinVol <100%); (b) pH >7.35; (c) SpO2 >90% with FiO2 <0.4; (d) fR <30 breaths/min and heart rate <100 beats/min; (e) systolic blood pressure >90 mm Hg; and, f) Glasgow coma scale >13.
Weaning protocol
In the PSV arm, once the patient’s clinical condition had stabilized, PS/PEEP was reduced by 2/1 cm decrements every 2 hourly till a PS of ≤8 cm H2O and PEEP of ≤4 cm H2O was reached. The SpO2 had to be maintained at >90% with FiO2 <30% and an fR <28 breaths/min along with the tidal volume of 6–8 mL/kg IBW. In the ASV group, weaning was performed by reducing the %MV gradually in decrements of 10% per hour to a %MinVol of 60%.
Once comfortable on these settings, the subject was taken off NIV and connected to a Venturi mask with FiO2 sufficient to maintain SpO2 between 89 and 92%. Subjects were monitored closely for any signs of NIV failure (fR ≥35 breaths/min for >15 min, SpO2 <88% with oxygen flow ≥5 L/min for >15 min, pH ≤7.3, an increase of >10 mm Hg in PaCO2, heart rate ≥120 or ≤50 beats/min, systolic blood pressure ≥180 mm Hg or ≤90 mm Hg, diminished consciousness, diaphoresis, clinical signs of respiratory muscle fatigue. If the subject did not tolerate the weaning, the subject was again ventilated with NIV using the previous settings for the next 24 h before assessing for weaning. This was labelled as weaning failure and documented in the case record form.
Sample size and statistical analysis
Assuming a 5% reduction of NIV failure in the ASV arm (from 30% to 25%), 1291 subjects would be required in each arm (power 0.8, alpha, 0.05). As this was logistically not feasible, a convenient sample size of 74 subjects was chosen.
Data were analysed using the statistical package SPSS (version 22.0, IBM Inc., Armonk, NY United States), and expressed as number with percentage or mean with standard deviation (SD) or 95% confidence intervals (CI) or median (interquartile range). The differences between categorical and continuous variables were analysed using the chi-square test (or Fisher’s exact test) and Mann Whitney U test (or student’s t-test), respectively. Trends in clinical (respiratory rate and mean arterial blood pressure), arterial blood gas parameters (pH, PaO2, PaCO2) and NIV parameters (peak inspiratory pressure, PEEP, tidal volume) were analysed using the mixed model technique (autoregressive method) for repeated measures analysis of variance; the within-groups factor was time (baseline, one, two, four and 24 h), and the between-groups factor was NIV success. Survival curves were constructed to study the effect of treatment on time to endotracheal intubation using the Kaplan–Meier analysis, and the group differences were analysed using the log-rank test. Statistical significance was assumed at a p < 0.05.
Results
Of the 205 subjects screened for eligibility, 131 were excluded before randomization (). Seventy-four subjects with AECOPD were randomized to either the PSV arm (n = 38) or the ASV arm (n = 36). The baseline clinical, respiratory, physiological and the ICU parameters are described in and were similar across the two study arms. The mean (SD) duration of NIV applied during day 1, and 2 was 13.4 (5.9) h and 12.9 (5.2) h, respectively, and was similar between the two arms.
Table 1. Baseline demographic, clinical and ICU parameters.
Primary outcomes
The overall NIV failure rate was 28.4% (21/74), and the rate of failure was similar between the two study arms (). There was a 9% reduction in the intubation rates in the ASV group compared to the PSV group. The events leading to NIV failure included endotracheal intubation (n = 12), reuse of NIV within 48 h after weaning (n = 3) and mortality (n = 6).
Table 2. Outcome parameters.
Secondary outcomes
The duration of NIV use and the total duration of ventilation (noninvasive and invasive) was similar in the two groups (). The time to intubation was similar between the two groups (PSV vs. ASV, median [interquartile range]; 3.5 [2–9.5] vs. 12.5 [1.3–26.8] h, p = 0.89; ). The number of NIV manipulations was similar in both the groups. There was no difference in the VAS score of physicians’ ease of use and the subjects’ comfort across the two arms. The use of NIV resulted in improvements in the respiratory rate, heart rate, pH, and PaCO2 at 1 h, 2 h, 4 h and 24 h compared to baseline; however, the trend in improvement was similar between the two groups (). The mean (SD) peak inspiratory pressure and tidal volume were, however significantly higher in the ASV group in comparison to the PSV group. The median %MinVol used during ASV was 100% with a range of 100–150%.
Figure 2. Kaplan–Meier survival curves comparing the cumulative probability of endotracheal intubation in pressure support ventilation (PSV) and adaptive support ventilation (ASV) mode during noninvasive ventilation use during intensive care unit stay
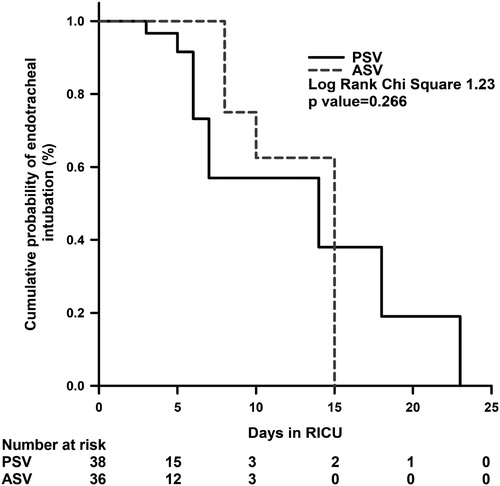
Table 3. Serial clinical and arterial blood gas parameters during the initial 24 h of noninvasive ventilation.
The complications attributed to NIV use were seen in ten subjects and were not different between the two study arms. The common complications were mask intolerance (n = 7), skin ulceration due to mask (n = 2), and gastric distension (n = 1).
There was no difference in the ICU and the hospital length of stay between the two groups. There were six deaths (8.1%) and were similar between the two study arms. Two deaths occurred due to intractable cardiac arrhythmias, one subject suffered from intracranial bleed, and three subjects died because of refractory septic shock. Sixty-eight subjects in the study population were discharged successfully. Twelve subjects were discharged on support that included domiciliary oxygen (n = 3), home NIV support (n = 4) and both oxygen and NIV support (n = 5). The proportion of subjects discharged with support was similar in the two groups.
Discussion
The results of this study suggest that NIV can be successfully delivered using ASV. ASV provides benefits akin to PSV, and both the modes resulted in a similar improvement of the clinical and blood gas parameters. The physician and the patient comfort were also similar with either mode.
Conventionally, during NIV, the PSV mode is used, where the level of ventilatory assistance remains static irrespective of the patient’s ventilatory demand. Theoretically, this could increase the patient’s work of breathing as the patient’s flow requirements are not met, thereby increasing the chances of NIV failure. On the other hand, ASV modulates the respiratory rate and the tidal volume breath-to-breath depending on the patients’ RCexp (Citation10, Citation11, Citation16, Citation17). In an intubated passive (paralysed) patient, using the ASV mode, the ventilator generates pressure-controlled breaths and modulates the inspiratory pressure and the respiratory rate; while in the spontaneously (non-paralysed) breathing intubated patients, the ventilator adjusts the inspiratory pressure; the respiratory rate is changed only if it is less than the target rate. This results in a better patient-ventilator interaction, with a potential of reducing the weaning time and duration of mechanical ventilation.
To our knowledge, the use of ASV during NIV has not been previously reported. Theoretically, the use of ASV mode during NIV may be hindered by some factors. For instance, the ASV mode assesses the RCexp on a breath-to-breath basis. Leaks around the mask may cause erroneous estimation of the RCexp that could cause inappropriate ventilatory support. In the current study, the peak inspiratory pressure and the tidal volume were significantly higher in the ASV arm during the initial 24-h of NIV use. This is similar to the scenario of using ASV during invasive ventilation of COPD patients where the tidal volumes are higher compared to PSV (Citation11). This suggests that the RCexp estimation was probably correct, even with ASV-NIV. Further, we also ensured the use of a mask with proper fit and minimized the leaks around the mask. Elsewhere, proportion assist ventilation (another closed loop ventilation mode) has been successfully used with mask ventilation (Citation18, Citation19); this provides support to our concept that ASV can be delivered with the mask.
What are the clinical implications of the study? The most important conclusion is that ASV can be delivered noninvasively. The only caveat is that proper fit of the mask should be ensured to avoid major leaks. We did not find any difference in the primary outcome across the two groups, which was probably due to the small sample size. We did, however, find a trend towards lower endotracheal intubation rates with ASV. Elsewhere, the use of ASV (compared to PSV) has also been shown to be associated with shorter weaning time and ventilator duration, and shorter hospital stay during invasive mechanical ventilation in subjects with AECOPD (Citation13, Citation20). Thus, this study not only provides the basis for future trials using ASV mode during NIV but also provides a template to calculate the sample size.
Finally, our study is not without limitations. The obvious limitation is the small sample size and conduct at a single centre that limits the generalizability. We also did not measure the asynchrony events in the study population. It is likely that the asynchrony events could be different in the two study arms. Finally, the results of this study are valid only for those with AECOPD and cannot be generalized to other forms of respiratory failure. The strength of the study is the uniform protocol and objective criteria for intubation.
In conclusion, ASV mode performs as well as PSV during NIV of subjects with AECOPD. There was a trend towards lower intubation rates with the use of ASV during NIV. The results of this study require confirmation in a larger multicenteric trial.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Inderpaul Singh Sehgal
ISS conceived the idea, performed statistical analysis, and drafted and revised the manuscript. HK collected patient data, and drafted and revised the manuscript. SD drafted and revised the manuscript. ANA performed statistical analysis, and drafted and revised the manuscript. KTP drafted and revised the manuscript. RA provided intellectual content to the manuscript, and drafted and revised the manuscript.
References
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi: 10.1056/NEJM199509283331301.
- Osadnik CR, Tee VS, Carson-Chahhoud KV, et al. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:CD004104. doi: 10.1002/14651858.CD004104.pub4.
- Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016.
- Sharma S, Agarwal R, Aggarwal AN, et al. A survey of noninvasive ventilation practices in a respiratory ICU of North India. Respir Care. 2012;57(7):1145–1153. doi: 10.4187/respcare.01541.
- Agarwal R, Gupta R, Aggarwal AN, et al. Noninvasive positive pressure ventilation in acute respiratory failure due to COPD vs other causes: effectiveness and predictors of failure in a respiratory ICU in North India. Int J Chron Obstruct Pulmon Dis. 2008;3(4):737–743. doi: 10.2147/COPD.S3454.
- Antonelli M, Conti G. Noninvasive positive pressure ventilation as treatment for acute respiratory failure in critically ill patients. Crit Care. 2000;4(1):15–22. doi: 10.1186/cc645.
- Carlucci A, Richard JC, Wysocki M, et al. Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med. 2001;163(4):874–880. doi: 10.1164/ajrccm.163.4.2006027.
- Vignaux L, Vargas F, Roeseler J, et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 2009;35(5):840–846. doi: 10.1007/s00134-009-1416-5.
- Agarwal R, Srinivasan A, Aggarwal AN, et al. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology. 2013;18(7):1108–1115. doi: 10.1111/resp.12126.
- Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med. 2008;34(1):75–81. doi: 10.1007/s00134-007-0847-0.
- Jaber S, Sebbane M, Verzilli D, et al. Adaptive support and pressure support ventilation behavior in response to increased ventilatory demand. Anesthesiology. 2009;110(3):620–627. doi: 10.1097/ALN.0b013e31819793fb.
- Tassaux D, Dalmas E, Gratadour P, et al. Patient-ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med. 2002;30(4):801–817. doi: 10.1097/00003246-200204000-00014.
- Kirakli C, Naz I, Ediboglu O, et al. A randomized controlled trial comparing the ventilation duration between adaptive support ventilation and pressure assist/control ventilation in medical patients in the ICU. Chest. 2015;147(6):1503–1509. doi: 10.1378/chest.14-2599.
- Gupta D, Agarwal R, Aggarwal AN, et al. Guidelines for diagnosis and management of chronic obstructive pulmonary disease: Joint ICS/NCCP (I) recommendations. Lung India. 2013;30(3):228–267. doi: 10.4103/0970-2113.116248.
- Moerer O, Harnisch LO, Herrmann P, et al. Patient-ventilator interaction during noninvasive ventilation in simulated COPD. Respir Care. 2016;61(1):15–22. doi: 10.4187/respcare.04141.
- Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin N Am. 2001;7(3):425–440, ix.
- Brunner JX, Iotti GA. Adaptive Support Ventilation (ASV). Minerva Anestesiol. 2002;68(5):365–368.
- Wysocki M, Richard JC, Meshaka P. Noninvasive proportional assist ventilation compared with noninvasive pressure support ventilation in hypercapnic acute respiratory failure. Crit Care Med. 2002;30(2):323–329. doi: 10.1097/00003246-200202000-00010.
- Gay PC, Hess DR, Hill NS. Noninvasive proportional assist ventilation for acute respiratory insufficiency. Comparison with pressure support ventilation. Am J Respir Crit Care Med. 2001;164(9):1606-–1611. doi: 10.1164/ajrccm.164.9.2011119.
- Kirakli C, Ozdemir I, Ucar ZZ, et al. Adaptive support ventilation for faster weaning in COPD: a randomised controlled trial. Eur Respir J. 2011;38(4):774–780. doi: 10.1183/09031936.00081510.