Abstract
Exposure to organic dusts is an independent causative factor of chronic obstructive pulmonary disease (COPD). Unhealthy dietary patterns have been associated with poor lung function in smokers. This study investigated whether dietary patterns were associated with post-bronchodilator airway obstruction, a hallmark of COPD, in dairy farmers exposed to organic dusts.
All subjects were identified by screening programs and patients with airflow obstruction were matched with subjects with normal spirometry. Six groups were compared, defined by their exposures (non-smoking dairy farmers, smokers ≥ 10 pack-years with no occupational exposure, and smoking dairy farmers) and the presence or absence of post-bronchodilator airflow obstruction, resulting in 321 study subjects. The Alternative Healthy Eating Index (AHEI) score was calculated based on an adapted food frequency questionnaire.
Mean total AHEI scores were similar in all groups. Comparison between smokers with post-bronchodilator airway obstruction and subjects with post-bronchodilator airway obstruction related to occupational exposure found minimal differences in dietary patterns: dairy farmers had lower scores for the ratio of white to red meat and higher scores for cereal fiber consumption. As in previous studies, smokers with post-bronchodilator airway obstruction exhibited higher lipid intakes and lower carbohydrate intakes than their counterparts with normal spirometry.
No evidence of any meaningful difference in dietary patterns was found between subjects with post-bronchodilator airway obstruction detected by screening and healthy controls, either in dairy farmers or in smokers with no occupational exposure.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by the association of post-bronchodilator airway obstruction with chronic respiratory symptoms (dyspnea and/or cough and/or sputum production) (Citation1). COPD is one of the leading causes of morbidity and mortality worldwide and results in an increasing economic and social burden (Citation2). The most important etiological factor for COPD is tobacco smoking (Citation3), but many other factors can trigger lung inflammation, leading to COPD. Occupational exposure to biological dusts ranks high among these factors (Citation4), especially in dairy farmers (Citation5, Citation6). Nevertheless, COPD develops only in a subset of subjects exposed to these noxious particles (Citation7), indicating that other factors leading to disease development are involved in these patients. There is increasing evidence to suggest that these factors include not only genetic factors but also many modifiable conditions (Citation8).
Diet counts among these modifiable conditions. Using the Alternative Healthy Eating Index (AHEI), a score based on the results of food frequency questionnaires that quantify diet quality (Citation9), dietary patterns have been associated with the risk of COPD development, with a lower risk being associated with high intake of antioxidants (Citation10–12) and a greater risk with high intake of processed meats (Citation13–15). Nevertheless, most studies have essentially focused on smoking-related COPD, and very little is known regarding the effects of diet on the development of COPD in nonsmokers exposed to noxious particles.
The main objective of the present study was to investigate whether dietary patterns differed between subjects with post-bronchodilator airway obstruction exposed to tobacco smoking and those exposed to organic dusts. We also aimed to study body composition in these subjects. For this purpose, we compared AHEI scores and body composition between six groups of patients: three groups with mild-to-moderate post-bronchodilator airway obstruction (exposed either to tobacco smoking, to organic dusts or to both) and three groups of matched counterparts with normal spirometry.
Methods
Screening programs
Data for this study were collected as part of the BALISTIC project (COPD in dairy farmers: screening, characterization and constitution of a cohort; ClinicalTrials.gov Identifier: NCT02540408) which was conducted from 2011 to 2015 at the University Hospital of Besançon in collaboration with the French national social security system for agricultural workers (Mutualité Sociale Agricole, MSA) and the federation of community health practices of Franche-Comté (Fédération des Maisons de Santé Comtoises, FeMaSaC). This study was set up primarily to assess the prevalence and some specific characteristics associated with post-bronchodilator airway obstruction in dairy farmers (subjects exposed to organic dusts) as compared with subjects with smoking-related post-bronchodilator airway obstruction without occupational exposure and matched controls without airway obstruction (Citation16).
Subjects with post-bronchodilator airway obstruction and matched controls were recruited through two parallel screening programs (the screening phase of the BALISTIC study). Inclusion criteria in the screening programs were as follows: men or women aged 40–74 years, with no previous diagnosis of asthma or hypersensitivity pneumonitis and who were either dairy farmers (“dairy farmers” subgroups) or who were unexposed to any occupational hazard associated with COPD (“non-farmers” subgroups). One of these two screening programs was conducted during free health checkups organized by the MSA for their affiliated members, where COPD screening was proposed to all subjects who attended the health checkup and fulfilled the inclusion criteria. In parallel, general practitioners of the FeMaSaC conducted a second COPD screening program targeting all their patients who met the same inclusion criteria.
For COPD screening, both programs used the same material, that is, a pneumotachograph (MedGraphics; MSE Medical, Strasbourg, France) calibrated at least once daily using a 3-L syringe, and the same protocol. Spirometry was performed according to the ATS/ERS recommendations by nurses or technicians who had followed the same training course in the same pulmonary function laboratory, as previously described (Citation17). Spirometry outcomes included forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). A bronchodilation test was applied when the FEV1/FVC ratio was less than 0.70. Predicted values were based on the Global Lung Function Initiative (GLI) equations (Citation18).
Study groups
A diagnosis of post-bronchodilator airway obstruction was retained when the post-bronchodilator FEV1/FVC ratio was less than the lower limit of normal (LLN) (Citation18). Subjects with post-bronchodilator airway obstruction were rated either as stage 1 (FEV1 > 80% of the predicted value) or stage 2+ (FEV1 ≤ 80% of the predicted value). Spirometry was considered normal when the FEV1/FVC ratio was > LLN and FEV1 was > 80% of the predicted value before bronchodilator administration.
During the study period, all subjects with post-bronchodilator airway obstruction detected during the screening phase of the BALISTIC study were invited to participate in the characterization phase of the study. Those who accepted were matched 1:1 with subjects with normal spirometry (as defined above) in terms of age, body mass index (BMI), tobacco smoking (in pack-years) and sex.
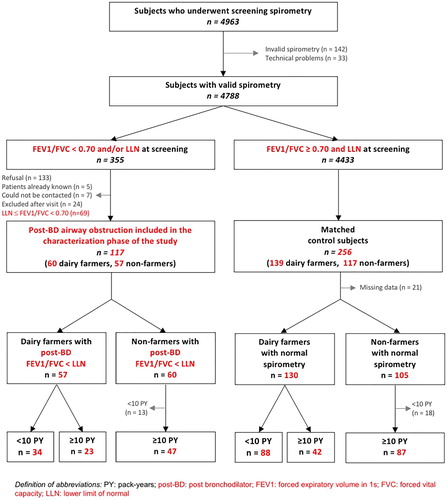
All subjects who participated in the characterization phase attended a visit at the University Hospital of Besançon, during which examinations were performed and questionnaires were administered as described in detail elsewhere (Citation16). The distribution of the study population is shown in .
Table 1. Main characteristics and pulmonary function of the study participants according to exposure to dairy-farming, number of smoked pack-years and presence of post-bronchodilator airway obstruction (defined by FEV1/FVC).
Ethical approval was received from the local Ethics Committee (CPP Est; 11/617), and written consent was obtained from all subjects.
Questionnaires
A medical questionnaire (completed by the physician) collected data on demographic characteristics, medical history, treatments, symptoms and smoking history. Dyspnea was assessed using the modified Medical Research Council (mMRC) scale. Smoking status was recorded and the number of pack-years was calculated. Subjects who smoked on average less than one cigarette (cigarette, cigar or pipe) a day for a year were categorized as never-smokers. If the amount smoked was higher than this value, patients were considered as smokers, former smokers being those who had abstained from smoking for the last 30 days before the characterization visit.
The dietary questionnaire was an adaptation of a French Food Frequency Questionnaire (FFQ) that includes 24 items regarding food and drinks (Citation19). Its objective was to assess retrospectively the usual intake during the past year for foods or food groups. The questionnaire was self-administered in a single interview. The questionnaire included semi-quantitative questions about the frequency of consumption (with a “never” option as well as options ranging from very infrequent to several times a day), and portion size information (with portion size images to enhance reporting accuracy).
Alternate Healthy Eating Index (AHEI)
An adapted Alternate Healthy Eating Index (AHEI) (Citation9) was calculated for each completed FFQ by converting each food item to a daily intake. The original components of the index include vegetables, fruit, nuts and soy, the ratio of white (seafood and poultry) to red meat, cereal fiber, trans fat, the ratio of polyunsaturated fatty acids to saturated fatty acids, long-term multivitamin use (< 5 or ≥ 5 years), and alcohol consumption. Because multivitamin use was not available in our nutrient data set, we adapted the score using the eight other components, with a potential contribution of 0–10 points to the total score for each. Intermediate intakes were scored proportionately between 0 and 10, with scoring decisions made a priori, based on food nutrient composition. All the component scores were summed to obtain a total AHEI score, which ranged from 0 to 80, with higher scores corresponding to healthier diets.
Bioelectrical impedance analysis
A nurse measured height and weight. BMI was calculated as weight/height2 (kg/m2). Body composition was assessed by multiple frequency bioelectrical impedance analysis (BIA) (Z-Metrix, Bioparhom, France) under standardized conditions, i.e., ambient temperature between 23 °C and 25 °C, fasting during at least 3 hours, empty bladder, clean skin surface. For body composition assessment, participants were asked to remain in the supine position for at least 10 minutes before starting the measurement, with legs and arms slightly abducted at 30° so there was no contact between the extremities and the trunk.
A standard tetrapolar technique was used, with the measuring electrodes placed on the anterior surface of the wrist and ankle, and the injecting electrodes placed on the dorsal surface of the hand and the foot. Impedance (Z) was determined at two frequencies (5 and 250 kHz) with an imperceptible electrical current of 800 mA. Z at 5 kHz and Z at 250 kHz were considered as inversely related to extracellular water (ECW) and total body water (TBW), respectively, TBW being the sum of ECW and intracellular water (ICW). Fat-free mass (Citation20) and FFM index (fat-free mass index [FFMI] equal to FFM/height2) were calculated by estimating that FFM is composed of 73% of water, and thus FFM = TBW/0.73 (Citation21).
Statistical analysis
Data are presented as number (percent) and mean ± standard deviation for qualitative and quantitative variables, respectively. Quantitative variables were compared with the Student t test or the Wilcoxon test, as appropriate. Qualitative variables were compared with the chi-square (χ2) test.
Comparisons were made between six groups defined by their exposures (non-smoking dairy farmers, smokers > 10 pack-years with no occupational exposure and smoking dairy farmers) and the presence or absence of post-bronchodilator airflow obstruction. To identify factors associated with post-bronchodilator airway obstruction among dairy farmers and non-farmers, bivariate logistic regressions for all candidate factors were performed.
A p-value < 0.05 was considered statistically significant. Analyses were performed using SAS software (version 9.3; SAS Institute, Inc., Cary, NC, USA).
Results
Population characteristics
The flowchart of subjects is shown in . A total of 117 subjects with post-bronchodilator airway obstruction and 256 controls with normal spirometry were included in the characterization phase of the study. Thirty patients were excluded after their visit, mainly owing to a history of asthma or occupational exposure other than dairy farming. In addition, 21 control subjects (12 dairy farmers and 9 non-farmers) were not included in the current analysis because of missing data for the dietary evaluation. Finally, 31 non-farmers (13 with post-bronchodilator airway obstruction and 18 with normal spirometry) who had smoked less than 10 pack-years were not analyzed because group sizes were too small.
The main characteristics of the population analyzed are displayed in and Figure 1 in Supplementary Material. The number of current smokers and former smokers was much lower among farmers than among non-farmers. The proportion of men was higher among farmers than among non-farmers. A large majority of subjects with post-bronchodilator airway obstruction had preserved FEV1. Comorbidities and treatments were similar in all subgroups (Table 1 in Supplementary Material).
Comparisons made between subjects recruited through the MSA and those recruited through the FeMaSaC demonstrated that the main characteristics of the recruited subjects were similar in both conditions of recruitment (Table 2 in Supplementary Material).
Dietary patterns
Total AHEI score and each dietary component of the score are shown in . The mean total AHEI score was very similar in all groups. Each available component of the AHEI score was compared between groups. The only significant difference was for the ratio of white to red meat and for cereal fiber between dairy farmers with post-bronchodilator airway obstruction and the other groups of subjects with post-bronchodilator airway obstruction (). In addition, there was no relationship between FEV1 and AHEI in patients with post-BD airway obstruction, the coefficient of correlation (r) being equal to [95% confidence interval] 0.13 [–0.30;0.52], p = 0.55; –0.05 [–0.38;0.30], p = 0.80; and –0.02 [–0.31;0.26], p = 0.87 in dairy farmers ≥ 10 pack-years, dairy farmers < 10 pack-years and nondairy farmers ≥ 10 pack-years, respectively.
Table 2. Adapted Alternate Healthy Eating Index scores and subscores and nutritional intake ratios with the results of food-frequency questionnaires according to exposure to dairy farming, number of pack-years smoked and presence of post-bronchodilator airway obstruction (defined by FEV1/FVC).
also lists the participants’ nutritional intake ratios of lipids, carbohydrates and proteins according to the FFQ, showing significantly higher intakes of lipids and lower intakes of carbohydrates in nondairy farmers smokers with post-bronchodilator airway obstruction than in their counterparts with normal spirometry.
Bioelectrical impedance analysis
In the entire population, there was a positive correlation between AHEI score and fat mass expressed as a percentage of body weight (p = 0.04). Fat mass (% body weight) was also positively correlated with the ratio of white to red meat (p < 0.001) and negatively correlated with cereal fiber (p = 0.002).
The body composition in each group is shown in . Patients were overweight in all groups, with mean BMI ranging from 25.6 to 27.3 kg.m−2. FFMI was preserved in all groups, with mean values between 19.8 and 21.4 kg.m−2. There was no significant difference in terms of body composition between groups.
Table 3. Morphometric characteristics and body composition evaluated by bioelectrical impedance analysis according to exposure to dairy-farming, number of smoked pack-years and presence of post-bronchodilator airway obstruction (defined by FEV1/FVC).
Discussion
In this study performed in dairy farmers and in subjects with no occupational exposure in the Franche-Comté region of France, we were unable to find any difference in terms of dietary patterns or body composition between patients with post-bronchodilator airway obstruction and their healthy counterparts. In addition, comparisons between smokers with post-bronchodilator airway obstruction and subjects with post-bronchodilator airway obstruction related to occupational exposure revealed only minimal differences in terms of dietary patterns. Conversely, smokers with post-bronchodilator airway obstruction exhibited slightly higher intakes of lipids and lower intakes of carbohydrates than their counterparts with normal spirometry.
Several associations between dietary patterns on the one hand and COPD prevalence and severity on the other have been previously reported. In a large prospective cohort of US men followed up for 12 years, the risk of newly diagnosed COPD increased with a greater consumption of cured meats (Citation15). Consumption of processed meats has also been significantly associated with lower FEV1 and lower FEV1/FVC (Citation14). In addition, the negative association between higher processed meat consumption and poorer lung function has been shown to be greater in subjects who also have lower fruit and vegetable consumption and low dietary total antioxidant capacity (Citation22). In line with these findings, it has been reported that higher current cured meat consumption increases the risk of readmission to hospital in COPD patients (Citation23). Other studies have reported a negative association between fish intake and COPD-related outcomes (Citation24–27), potentially linked to the beneficial effects of omega-3 polyunsaturated fatty acid (PUFA) on the production of anti-inflammatory and pro-resolving mediators (Citation28). Fish and omega-3 PUFA are an important component of the prudent dietary pattern recommended by the AHEI-2010. A prospective cohort analysis of more than 120,000 US women and men has demonstrated that a higher AHEI-2010 diet score, reflecting high intakes of whole grains, polyunsaturated fatty acids, nuts, and long-chain omega-3 fats and low intakes of red and processed meats, refined grains, and sugar sweetened drinks, was associated with a lower risk of newly diagnosed COPD (Citation29). Some specific nutrients such as black tea have been shown to be protective against developing COPD in male smokers, possibly through an increase in the level of cellular antioxidant defenses (Citation30). Here, with the AHEI score adapted to our FFQ data, we found similar global AHEI scores in subjects with post-bronchodilator airway obstruction and in healthy controls, even after stratification for occupational exposure and tobacco smoking. Detailed items of the AHEI score showed no difference in consumption of each food category between subjects with post-bronchodilator airway obstruction and controls. We only found that farmers with post-bronchodilator airway obstruction who had smoked ≥ 10 pack-years had a lower ratio of white to red meat consumption and a higher consumption of cereal fiber than non-farming smokers with post-bronchodilator airway obstruction. This could be related to better access of dairy farmers to these food categories (cereal fiber and red meat). Of note, dairy farmers with and without post-bronchodilator airway obstruction had strictly similar dietary patterns. In accordance with previous findings, the proportion of total energy from carbohydrates was low in comparison with recommendations while lipid proportions were high (Citation31).
Body weight and body composition are important discriminants in classifying disease heterogeneity. Body weight loss in patients with COPD is at least in part related to disturbance of hormonal appetite stimulation and activation of inflammation by hypoxia (Citation32,Citation33). Fat-free mass index (FFMI) depletion, calculated by bioelectrical impedance analysis (BIA), has been shown to be a better predictor of mortality than BMI in COPD patients (Citation34), and could be a predictor of COPD severity as it is strongly correlated with exercise capacity, dyspnea, respiratory muscle function and FEV1 (Citation35). Lung function has an impact on nutritional status, as both BMI and FFMI values are poorer in the more severe COPD stages than in patients with mild disease (Citation36). Analysis of dietary intakes in patients with moderate to severe COPD showed that patients with low FFMI had lower mean energy intakes than patients with normal FFMI, related to lower daily consumption of dairy products and red meat (Citation37). In this study, patients with post-bronchodilator airway obstruction (a hallmark of COPD) had few symptoms, suggesting a low impact on overall nutritional status, as confirmed by BIA and FFMI comparisons between subjects.
Limitations of the study
Our study has some limitations. Firstly, there were a small number of patients in each group, leading to some lack of power. Statistical power had been calculated for the primary endpoint of the BalistiC study (Citation16). For the study presented here, 52 subjects had to be excluded (therefore, only 321 subjects of the 373 included in the “original” BalistiC study were analyzed). The initial power calculation was therefore no longer appropriate for the current study, resulting in a lower effective statistical power that could explain the lack of significance of our results. Secondly, the FFQ used in our study was not as effective as that used by others (Citation12). It seems difficult to characterize a diet with a single score based on a 24-item FFQ, leading to more approximate data in the AHEI-2010 scoring. This could explain at least in part the lack of significant difference between the groups. Nevertheless, the FFQ used in this study was validated to cover all lipid, carbohydrate and protein intakes, and this dietary score had been validated as a referential score adapted from the Healthy Eating Index (HEI) developed by the US Department of Agriculture. The HEI measures adherence to the Dietary Guidelines for Americans (Citation38) and is designed to target food choices and macronutrient sources associated with reduced chronic disease risk (Citation9). Thirdly, physical activity, which may differ between farmers and non-farmers, is a confounding factor in terms of body composition that was not taken into account in our study. Fourthly, there is a time-dependent confounding factor, as the diet questioning was cross-sectional, with no longitudinal evaluation. It has now been demonstrated that standard methods of analysis may be biased in the presence of such a confounder (Citation39). Fifthly, only one half of subjects detected with post-bronchodilator airway obstruction agreed to attend the hospital for characterization. It has been previously reported that among all subjects who are invited to health checkups organized by the French agricultural health insurance (Mutualité Sociale Agricole), those who attend such checkups and those who do not have different health characteristics (Citation40). Our study population may therefore not be representative of the whole population of dairy farmers.
Conclusion
In conclusion, no evidence of any meaningful difference in dietary patterns was found between subjects with post-bronchodilator airway obstruction detected by screening in the Franche-Comté region and healthy controls, whether in dairy farmers or in smokers with no occupational exposure. Further studies, including more patients and with analysis of micronutrient intakes, would improve our understanding of diet as a risk factor for the onset of COPD, especially in nonsmokers exposed to organic dusts.
Declaration Of Interest
Dr Saussereau reports no conflict of interests.
Dr Guillien reports no conflict of interests.
Dr Soumagne reports no conflict of interests.
Dr Laplante reports no conflict of interests.
Dr Laurent reports no conflict of interests.
Dr. Bouhaddi reports no conflict of interests.
Dr. Annesi-Maesano reports no conflict of interests.
Dr Roche reports grants and personal fees from Boehringer Ingelheim, Pfizer and Novartis, and personal fees from Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, Sanofi, Sandoz, 3M and Zambon, all outside the submitted work.
Dr. Dalphin reports grants from Novartis Pharma, personal fees from Novartis Pharma, Chiesi, Intermune GSK, AstraZeneca, Boehringer Ingelheim, and non-financial support from Novartis, GSK, AstraZeneca, Intermune, Chiesi, Boehringer Ingelheim and Stallergenes, all outside the submitted work.
Dr Degano reports no conflict of interests.
Acknowledgments
The authors would like to express their appreciation to all those who participated in the study. The authors also thank the clinical staff who contributed to the measurements. We are indebted to Raphaëlle Varraso for assistance in analyzing the AHEI score. We would also like to thank the staff of the MSA and the FeMaSaC who participated in the collection of data. The authors thank Nina Crowte for editorial assistance.
Additional information
Funding
References
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82. doi: 10.1164/rccm.201701-0218PP.
- Hoong JM, Ferguson M, Hukins C, Collins PF. Economic and operational burden associated with malnutrition in chronic obstructive pulmonary disease. Clin Nutr. 2017;36(4):1105–9. doi: 10.1016/j.clnu.2016.07.008.
- Liu Y, Pleasants RA, Croft JB, Wheaton AG, Heidari K, Malarcher AM, Ohar JA, Kraft M, Mannino DM, Strange C. Smoking duration, respiratory symptoms, and COPD in adults aged >/=45 years with a smoking history. Int J Chron Obstruct Pulmon Dis. 2015;10:1409–16.
- Sadhra S, Kurmi OP, Sadhra SS, Lam KB, Ayres JG. Occupational COPD and job exposure matrices: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:725–34. doi: 10.2147/COPD.S125980.
- Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136(3):716–25. doi: 10.1378/chest.08-2192.
- Ryu JY, Sunwoo YE, Lee SY, Lee CK, Kim JH, Lee JT, Kim DH. Chronic Obstructive Pulmonary Disease (COPD) and Vapors, Gases, Dusts, or Fumes (VGDF): A Meta-analysis. Copd. 2015;12(4):374–80. doi: 10.3109/15412555.2014.949000.
- Marescaux A, Degano B, Soumagne T, Thaon I, Laplante JJ, Dalphin JC. Impact of farm modernity on the prevalence of chronic obstructive pulmonary disease in dairy farmers. Occup Environ Med. 2016;73(2):127–33. doi: 10.1136/oemed-2014-102697.
- Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor P, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–22. doi: 10.1056/NEJMoa1411532.
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–71. doi: 10.1093/ajcn/76.6.1261.
- Berthon BS, Wood LG. Nutrition and respiratory health-feature review. Nutrients. 2015;7(3):1618–43. doi: 10.3390/nu7031618.
- Butland BK, Fehily AM, Elwood PC. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax. 2000;55(2):102–8. doi: 10.1136/thorax.55.2.102.
- Miedema I, Feskens EJ, Heederik D, Kromhout D. Dietary determinants of long-term incidence of chronic nonspecific lung diseases. The Zutphen Study. Am J Epidemiol. 1993;138(1):37–45. doi: 10.1093/oxfordjournals.aje.a116775.
- Jiang R, Camargo CA, Jr., Varraso R, Paik DC, Willett WC, Barr RG. Consumption of cured meats and prospective risk of chronic obstructive pulmonary disease in women. Am J Clin Nutr. 2008;87(4):1002–8. doi: 10.1093/ajcn/87.4.1002.
- Jiang R, Paik DC, Hankinson JL, Barr RG. Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults. Am J Respir Crit Care Med. 2007;175(8):798–804. doi: 10.1164/rccm.200607-969OC.
- Varraso R, Jiang R, Barr RG, Willett WC, Camargo CA, Jr. Prospective study of cured meats consumption and risk of chronic obstructive pulmonary disease in men. Am J Epidemiol. 2007;166(12):1438–45. doi: 10.1093/aje/kwm235.
- Degano B, Bouhaddi M, Laplante JJ, Botebol M, Annesi-Maesano I, Marescaux A, Roux P, Thaon I, Wolf JP, Regnard J, et al. [COPD in dairy farmers: screening, characterization and constitution of a cohort. The BALISTIC study]. Rev Mal Respir. 2012;29(9):1149–56. doi: 10.1016/j.rmr.2012.08.007.
- Guillien A, Puyraveau M, Soumagne T, Guillot S, Rannou F, Marquette D, Berger P, Jouneau S, Monnet E, Mauny F, et al. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J. 2016;47:95–103.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312.
- Giovannelli J, Dallongeville J, Wagner A, Bongard V, Laillet B, Marecaux N, Ruidavets JB, Haas B, Ferrieres J, Arveiler D, et al. Validation of a short, qualitative food frequency questionnaire in French adults participating in the MONA LISA-NUT study 2005-2007. J Acad Nutr Diet. 2014;114(4):552–61. doi: 10.1016/j.jand.2013.07.002.
- Adam M, Schikowski T, Carsin AE, Cai Y, Jacquemin B, Sanchez M, Vierkotter A, Marcon A, Keidel D, Sugiri D, et al. Adult lung function and long-term air pollution exposure. ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J. 2015;45(1):38–50. doi: 10.1183/09031936.00130014.
- Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151–6. doi: 10.1164/ajrccm/147.5.1151.
- Okubo H, Shaheen SO, Ntani G, Jameson KA, Syddall HE, Sayer AA, Dennison EM, Cooper C, Robinson SM. Processed meat consumption and lung function: modification by antioxidants and smoking. Eur Respir J. 2014;43(4):972–82. doi: 10.1183/09031936.00109513.
- de Batlle J, Mendez M, Romieu I, Balcells E, Benet M, Donaire-Gonzalez D, Ferrer JJ, Orozco-Levi M, Anto JM, Garcia-Aymerich J. Cured meat consumption increases risk of readmission in COPD patients. Eur Respir J. 2012;40(3):555–60. doi: 10.1183/09031936.00116911.
- Schwartz J, Weiss ST. The relationship of dietary fish intake to level of pulmonary function in the first National Health and Nutrition Survey (NHANES I). Eur Respir J. 1994;7(10):1821–4. doi: 10.1183/09031936.94.07101821.
- Shahar E, Folsom AR, Melnick SL, Tockman MS, Comstock GW, Gennaro V, Higgins MW, Sorlie PD, Ko WJ, Szklo M. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. Atherosclerosis risk in communities study investigators. N Engl J Med. 1994;331(4):228–33. doi: 10.1056/NEJM199407283310403.
- Sharp DS, Rodriguez BL, Shahar E, Hwang LJ, Burchfiel CM. Fish consumption may limit the damage of smoking on the lung. Am J Respir Crit Care Med. 1994;150(4):983–7. doi: 10.1164/ajrccm.150.4.7921474.
- Varraso R, Barr RG, Willett WC, Speizer FE, Camargo CA, Jr. Fish intake and risk of chronic obstructive pulmonary disease in 2 large US cohorts. Am J Clin Nutr. 2015;101(2):354–61. doi: 10.3945/ajcn.114.094516.
- Giudetti AM, Cagnazzo R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012;99(3–4):57–67. doi: 10.1016/j.prostaglandins.2012.09.006.
- Varraso R, Chiuve SE, Fung TT, Barr RG, Hu FB, Willett WC, Camargo CA. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. Bmj. 2015;350:h286. doi: 10.1136/bmj.h286.
- Celik F, Topcu F. Nutritional risk factors for the development of chronic obstructive pulmonary disease (COPD) in male smokers. Clin Nutr. 2006;25(6):955–61. doi: 10.1016/j.clnu.2006.04.006.
- de Batlle J, Romieu I, Anto JM, Mendez M, Rodriguez E, Balcells E, Ferrer A, Gea J, Rodriguez-Roisin R, Garcia-Aymerich J. Dietary habits of firstly admitted Spanish COPD patients. Respir Med. 2009;103(12):1904–10. doi: 10.1016/j.rmed.2009.06.001.
- Koehler F, Doehner W, Hoernig S, Witt C, Anker SD, John M. Anorexia in chronic obstructive pulmonary disease-association to cachexia and hormonal derangement. Int J Cardiol. 2007;119(1):83–9. doi: 10.1016/j.ijcard.2006.07.088.
- Raguso CA, Luthy C. Nutritional status in chronic obstructive pulmonary disease: role of hypoxia. Nutrition. 2011;27(2):138–43. doi: 10.1016/j.nut.2010.07.009.
- Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sorensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83. doi: 10.1164/rccm.200506-969OC.
- Luo Y, Zhou L, Li Y, Guo S, Li X, Zheng J, Zhu Z, Chen Y, Huang Y, Chen R, et al. Fat-free mass index for evaluating the nutritional status and disease severity in COPD. Respir Care. 2016;61(5):680–8. doi: 10.4187/respcare.04358.
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest. 2007;132(1):164–9. doi: 10.1378/chest.06-2789.
- Yilmaz D, Capan N, Canbakan S, Besler HT. Dietary intake of patients with moderate to severe COPD in relation to fat-free mass index: a cross-sectional study. Nutr J. 2015 Apr 10;14:35. doi: 10.1186/s12937-015-0020-5.
- Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95(10):1103–8. doi: 10.1016/S0002-8223(95)00300-2.
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011.
- Pelc AD, V.; Gosselin, S. Les Invités aux Instants Sante en 2011: Profil de Consommation de Soins des Participants et des Non Participants. Available from: http://www.msa.fr/lfy/documents/98830/9488297/Les+invit%C3%A9s+aux+Instants+Sant%C3%A9en+2011.pdf.