Abstract
One in four patients with chronic obstructive pulmonary disease (COPD) has depression. However, the effect of depressive symptoms on self-reported physical function among patients with COPD is not fully defined. We tested the hypothesis that depressive symptoms among patients with COPD are associated with increased self-reporting of physical limitations in a nationally representative sample of adults living in the USA. We sampled 775 adults with obstructive lung disease and history of regular smoking from 2007 to 2012 National Health and Nutrition Examination Survey (NHANES). Multivariable logistic regression was used to estimate odds ratios for the association between depressive symptoms and self-reported difficulty performing three activities. We used a Patient Health Questionnaire (PHQ-9) score ≥ 10 to define depressive symptoms. Covariates in the multivariable analysis were age, gender, percent predicted forced expiratory volume in 1 second, current smoking, low-income status, and number of co-morbidities. In models predicting any difficulty walking a quarter mile, walking up 10 stairs, and walking between rooms on a level surface, adjusted odds ratio estimates (95% CI) for depressive symptoms were 2.03 (1.06, 3.88), 3.40 (1.77, 6.50), and 4.92 (2.29, 10.60), respectively. The presence of depressive symptoms was associated with increased self-reported difficulty with physical activities in our sample. Unexpectedly, the effect of depressive symptoms was greater with easier activities in our sample. These results suggest that depressive symptoms are associated with activity-dependent self-reporting of physical limitations among patients with COPD.
Introduction
Approximately one in four patients with chronic obstructive pulmonary disease (COPD) has depression (Citation1,Citation2). Among patients with COPD, depression has been associated with increased morbidity and decreased quality of life (Citation3). COPD patients with depression are more likely to experience a COPD exacerbation and have higher self-reported dyspnea compared to COPD patients without depression (Citation3–5). Mechanisms behind how depression increases COPD morbidity are complex and include associations with reduced adherence to treatment and negative emotions like low self-esteem and hopelessness (Citation3).
However, the effect of depressive symptoms on self-reported physical function among patients with COPD is not fully defined. In a prospective study of COPD patients living in Korea, depression was associated with decreased 6-minute walking distance and increased BMI, Airflow Obstruction, Dyspnea, and Exercise (BODE) Index (Citation6). Similar results were reported by authors examining roughly 1,700 COPD patients enrolled in the multinational Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort (Citation7). In a US survey-based study of Veterans Affairs patients with COPD, depression accounted for 11–18% of the variation in self-reported difficulty with physical activities (Citation4). Whether these findings are generalizable to the US population at large is not yet known.
Understanding the effect of depressive symptoms on self-reported physical limitations in COPD is critical because treatment of COPD is based on patients’ symptoms. The current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend using a questionnaire such as the COPD Assessment Test (CAT) to assess COPD symptoms (Citation8,Citation9). A patient’s score on these questionnaires represents an aggregate of multiple COPD symptoms including self-reported physical limitations that can be used to categorize the patient into one of four groups (A through D) and guide treatment with inhalers (Citation8,Citation9). Current questionnaires used to assess COPD severity do not address the effect of mood disorders such as depression on COPD symptoms (Citation8,Citation9). Given the high prevalence of depression in COPD, understanding the effect of depressive symptoms on self-reported physical limitations is required to accurately categorize COPD severity and guide COPD management.
In this study, we tested the hypothesis that depressive symptoms among patients with COPD are associated with increased self-reporting of physical limitations across different levels of physical activity in a large, nationally representative sample of adults living in the USA. We used data from the National Health Examination Survey (NHANES) 2007–2012 surveys because these surveys included a diverse patient population and collected spirometry data, smoking history, depression screening, and self-reported difficulty at different levels of physical activity.
Materials and methods
Data source and collection
Data were obtained from the NHANES 2007–2012 surveys (Citation10). NHANES data are nationally representative, cross-sectional samples of non-institutionalized, civilian population in the USA (Citation11,Citation12). NHANES data are publicly available and de-identified, therefore, are exempt from human subject review.
From 2007 to 2012, NHANES collected spirometry data from roughly 20,000 participants. Detailed spirometric testing methods were per American Thoracic Society standards and are outlined in the NHANES manual (Citation12). Subjects excluded from spirometry testing were those with current chest pain, with a physical problem limiting forceful expiration, on supplemental oxygen, and with recent surgery, stroke, heart attack, TB exposure, or hemoptysis (Citation12).
To screen for depression, NHANES asked subjects to complete the Patient Health Questionnaire (PHQ-9), a widely used and validated tool to screen for major depression in the primary care setting (Citation13–15). Details of methods regarding PHQ-9 administration are outlined in the NHANES manual (Citation12). To quantify physical functioning, NHANES asked participants “By {yourself/himself/herself} and without using any special equipment, how much difficulty {do you/does SP} have … walking for a quarter of a mile” (Citation11). Participants were similarly asked about difficulty walking up 10 steps without stopping, and walking between rooms on the same floor (Citation11). Participants were able to respond that they had no difficulty, some difficulty, much difficulty, were unable to perform, do not do this activity, or don’t know. Among the physical functioning questions reported in NHANES data, we chose these three activities based on the relatively well-defined nature of each task and the relatively direct relationship between these tasks and pulmonary function. We also chose these questions because they are similar to questions used in the COPD Assessment Test (Citation8).
Definition of COPD and depressive symptoms
The NHANES 2007–2012 database contains spirometric values, including forced expiratory volume in 1 second (FEV1) and functional vital capacity (FVC), reported in mL. NHANES uses the NHANES III dataset to calculate age, sex, height, and race-/ethnicity-specific percent predicted spirometric values (Citation12,Citation16). NHANES also tested patients with obstructive lung disease, defined as FEV1/FVC ratio < 70% and/or FEV1/FVC < lower limit of normal per NHANES III reference values, for bronchodilator response. In this study, COPD was defined as the combination of obstructive lung disease as defined above, smoking at least 100 cigarettes in lifetime, and history of regular smoking. A positive bronchodilator response was defined as a ≥200 mL absolute increase and ≥12% relative increase in FEV1 after albuterol treatment.
For the purpose of this study, the presence of depressive symptoms is defined as a PHQ-9 score ≥ 10. Prior studies demonstrate that a PHQ-9 score ≥ 10 has a sensitivity of 70–90% and specificity of 90% for the diagnosis of major depression (Citation13,Citation14). Furthermore, the PHQ-9 questionnaire is endorsed by the US Preventative Services Task Force as a validated screening tool for major depression (Citation15).
Data abstraction
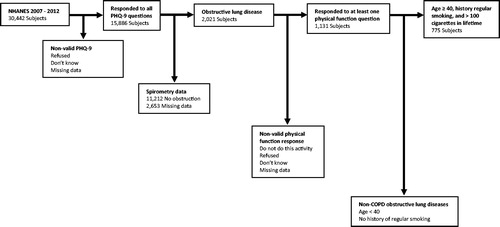
We limited our analysis to subjects age 40–79 who (1) had complete survey responses to all questions on the PHQ-9, (2) had obstructive lung disease as defined above, (3) had survey responses to at least one of the physical function questions of interest, (4) smoked at least 100 cigarettes during lifetime and had a history of regular smoking (). Subjects with incomplete data, including subjects who responded that they didn’t know or refused to answer, were excluded.
Figure 1. Study selection criteria. The 2007–2012 NHANES surveys included 30,442 individuals from across the USA. From this cohort, our study included the 775 subjects age 40–79 who had valid responses to all questions on the PHQ-9, had obstructive lung disease, responded to at least one of the physical function questions, and had history of regular smoking.
Statistical methods
All analysis was performed using SAS Version 9.4 of the SAS System for Windows (copyright 2002–2012 SAS Institute Inc.). For smoking history, we estimated that the number of years smoked was equivalent to the difference between age starting regular smoking and age quitting regular smoking. For spirometry, NHANES uses the NHANES III reference values to define obstructive lung disease. However, we chose to recalculate and report percent predicted spirometry using the GLI macro for SAS (Macro Version 1 – April 7, 2013), which uses data collected by the Global Lung Function Initiative, because of the diversity of our study population (Citation17).
Unadjusted and adjusted odds ratios were estimated via logistic regression for the association between the presence of depressive symptoms and self-reported difficulty (some difficulty, much difficulty, or unable to perform) performing the physical activities defined above. Additional covariates in the adjusted model were age, gender, FEV1% predicted, low-income status (household income < 2 times the national poverty line), smoking status, and number of co-morbidities (including obesity, defined as a BMI ≥ 30, and self-reported diagnosis of any of the following: congestive heart failure, coronary artery disease, liver disease, or anemia). These variables were chosen based on clinical relevance, the demographics of self-reported difficulty with physical activity (Supplemental Tables S1–S3), and univariable logistic regression (see Results). Using obesity or BMI as an independent covariate did not affect the results of our analysis. Given the possibility that some of the studied population had asthma and not COPD, we performed an additional analysis that excluded patients with a positive bronchodilator response. Results were unchanged in this analysis (see Supplemental Material).
All patients in this cohort responded to NHANES survey question about difficulty walking between rooms. However, some patients in this cohort either did not respond to subsequent questions about more challenging physical activity or responded that they didn’t know how to answer. To address this, we performed statistics in two different ways. First, we assumed patients reporting difficulty walking between rooms but missing data for subsequent outcomes would also report difficulty with the comparably more challenging activities. This analysis is presented in the main text. Second, we performed analysis without the above assumption and excluded patients with missing outcome data. This analysis is presented in the Supplemental Material. Excluding patients with missing outcome data did not substantively change our results.
The multivariable regression analysis described above was a complete case analysis. To address missing data, we performed multiple imputations using SAS Proc MI with 20 imputations and recalculated OR estimates for the association between the presence of depressive symptoms and self-reported difficulty with walking a quarter mile, walking up 10 stairs, and walking between rooms.
To address potential selection bias, we used the inverse probability of treatment weighting (IPTW) method to generate a balanced IPTW cohort. First, we used logistic regression to calculate each patient’s probability of having depressive symptoms, given their gender, age, FEV1% predicted, low-income status, smoking status, and number of co-morbidities. Next, using these probabilities we applied IPTW method with weights stabilized by a number of subjects in each group to generate the IPTW cohort. Finally, we estimated adjusted odds ratios using this IPTW cohort (Citation18). Covariates included in this logistic regression were the same as those applied in the original, non-IPTW sample.
While NHANES provides weighting variables for national-level estimates of prevalence, we did not use weighting in this study because our sample is a nonrandom subset of the 2007–2012 NHANES dataset.
Results
Demographics
This study included 775 adult subjects (age 40–79) identified from 2007 to 2012 NHANES database with obstructive lung disease, a history of smoking, and survey responses to PHQ-9 and physical activity questions (). Demographics of the study sample are shown in . The sample was 67% male and ethnically diverse (63% Caucasian, 20% non-Hispanic Black, and 13% Hispanic). The median age (IQR) was 67 (61, 72). 43% of patients reported ongoing smoking at the time of the survey. The median FEV1% predicted (IQR) was 79% (64, 91).
Table 1. Demographics by the presence of depressive symptoms.
Bronchodilator testing was available for 329 patients. Of these, only 42 had a positive bronchodilator response. The median age (IQR) of this group was 68 (61, 74). All of these subjects had smoked regularly in the past and 13 continued to smoke. Among patients not actively smoking, the median number of years smoking (IQR) was 33 (22, 39). The extensive smoking history suggests that patients with bronchodilator response have COPD rather than asthma.
Prevalence of depressive symptoms, defined as a PHQ-9 score ≥ 10, was 10% (n = 75). Subjects with depressive symptoms were younger (median age 55), less likely to be male (45%), more likely to be current smokers (71%), and more likely to have low-income (87%) compared to subjects without depression. In the IPTW sample, differences between patients with depressive symptoms and those without depressive symptoms were reduced for the covariates used in multivariable regression models ().
Table 2. IPTW sample demographics by the presence of depressive symptoms.
Response to physical functioning questions
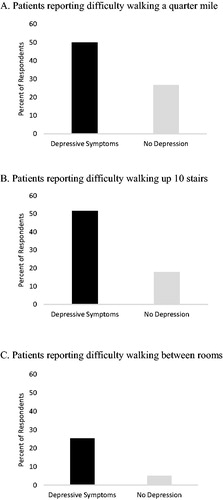
Subjects’ response to three physical functioning questions are depicted in . Among subjects with depressive symptoms, 50%, 52%, and 25% reported difficulty walking a quarter mile, walking up 10 stairs, and walking between rooms on a level surface, respectively. Among subjects without depression, 27%, 18%, and 5% reported difficulty with the same tasks.
Figure 2. Self-report of difficulty with physical activities by the presence of depressive symptoms. COPD patients with depressive symptoms were more likely to report difficulty with the physical activities walking a quarter mile, walking up 10 stairs, and walking between rooms than non-depressed COPD patients.
Covariate assessment
Characteristics of patients with self-reported difficulty with physical activities are summarized in Supplemental Tables S1–S3. Patients reporting difficulty were more likely to be female, current smokers, have low-income, have lower FEV1% predicted, and have more co-morbidities than patients who did not report difficulty with physical activity. In unadjusted logistic regression models, low-income status, female sex, and the number of co-morbidities were significantly associated with self-reported difficulty with all physical activities; age, smoking status, and FEV1% predicted were significantly associated with self-reported difficulty with some but not all physical activities. Ethnicity and insurance status showed no association with self-reported difficulty with physical activities ().
Table 3. Unadjusted odds ratio estimates for the association between demographic variables and self-reported difficulty with physical activity.
Depressive symptoms and physical activity
summarizes results from our models examining the association between depressive symptoms and self-reported difficulty with different physical activities. In the unadjusted models, we observed an association between depressive symptoms and self-reported difficulty with the physical activities walking between rooms on a level surface, walking up 10 stairs, and walking a quarter mile. The odds ratio (OR) estimates (95% CI) were 2.74 (1.62, 4.67), 4.88 (2.85, 8.35), and 6.26 (3.37, 11.62), respectively. After adjustment for age, gender, FEV1% predicted, low-income status, smoking status, and number of co-morbidities, OR estimates (95% CI) for depressive symptoms were 2.03 (1.06, 3.88), 3.40 (1.77, 6.50), and 4.92 (2.29, 10.60). In the IPTW sample, OR (95% CI) estimates for depressive symptoms were 2.82 (1.49, 5.33), 5.01 (2.65, 9.48), and 5.42 (2.55, 11.50). In unadjusted, adjusted, and IPTW models, the effect of depressive symptoms on self-reported difficulty walking between rooms was larger than the effect of depressive symptoms on self-reported difficulty with harder tasks. Excluded respondents with a significant bronchodilator response did not change our findings (Supplemental Table S4).
Table 4. Odds ratio estimates for depressive symptoms as a predictor of self-reported difficulty with physical activity.
For patients with missing outcome data, we assumed patients reporting difficulty walking between rooms would also report difficulty with the more challenging physical activities walking up 10 stairs and walking a quarter mile (see Materials and methods). In adjusted models without this assumption, we observed no association between depressive symptoms and self-reported difficulty walking a quarter mile. The OR estimates for depressive symptoms as a predictor of self-reported difficulty walking up 10 stairs was reduced or lost. The OR estimate for depressive symptoms as a predictor of self-reported difficulty walking between rooms was unchanged (Supplemental Table S5). Overall, the activity-dependent changes noted in were accentuated when patients with missing outcome data were excluded.
The multivariable logistic regression analysis reported in was a complete case analysis. However, 61 subjects (7.8% of total patients) had missing covariate data. The majority of these subjects (n = 59) were missing data related to income. To address these missing data, we used multiple imputations and reran our multivariable logistic regression. The imputed multivariable logistic regression is compared with our main results, the non-imputed multivariable logistic regression analysis, in Supplemental Table S6. While estimates are reduced in the imputed multivariable model, the overall trend in our results and conclusion that depressive symptoms are associated with self-reported difficulty with physical activities that are activity-dependent remains unchanged.
Discussion
In this study, we observed that depressive symptoms, defined as a PHQ-9 ≥ 10, was associated with self-reported difficulty with physical activities in a nationally representative cross-sectional sample of adults with COPD living in the USA. An unexpected finding was that the relationship between depressive symptoms among COPD patients was dependent on the difficulty of the physical activity. For the easiest task in our study, walking between rooms on a level surface, depressive symptoms were associated with 5 times increase in odds of self-reported difficulty. For the hardest task in our study, walking a quarter mile, depressive symptoms were associated with 2.5 times increase in odds of self-reported difficulty.
One possible explanation for the results presented here is that COPD patients with depressive symptoms have a negative response bias such that they report more physical limitations than patients without depressive symptoms despite similar lung function. However, it is also possible that the relationship between functional limitations due to COPD and depressive symptoms is bidirectional. Under this interpretation, patients with depressive symptoms might have reduced 6-minute walk testing compared to COPD patients without depression. This interpretation is supported by recent studies suggesting depressive symptoms are significantly associated with reduced 6-minute walk testing (Citation6,Citation7).
The strong association between depressive symptoms and self-reported physical limitations observed here raises several questions in the management of COPD patients with co-morbid depressive symptoms. For example, how should we interpret self-reported physical limitations in this population? How should we assess response to COPD treatment? Based on our results, physicians treating patients with COPD and co-morbid depressive symptoms likely need to determine whether depressive symptoms, COPD, or both are the primary drivers of patients’ physical limitations. For this reason, further research is needed to guide clinicians on how to assess and manage COPD in patients with co-morbid depressive symptoms.
Our findings have several limitations. Many patients, including all patients requiring supplemental oxygen, were excluded by NHANES from spirometry testing. While the study sample is drawn from a nationally representative cohort, the sample probably excludes most patients with severe COPD. Second, this study is a nonrandom cross-section of patients with COPD and all results presented should be interpreted as associations rather than causal relationships. Third, the PHQ-9 has been validated the primary care setting but has not yet been validated in a population of COPD patients; thus, applying the threshold PHQ-9 score ≥ 10 might not accurately classify some patients with COPD in our sample. Fourth, 6-minute walk testing or similar objective data regarding physical functioning is not available in the NHANES data. We could not examine the relationship between depressive symptoms and objective physical limitations (such as decreased 6-minute walk testing).
In conclusion, our analysis shows that among patients with COPD, the presence of depressive symptoms is associated with increased self-reported physical limitations. Surprisingly, the effect of depressive symptoms on self-reported physical limitations is activity dependent. As patient-reported physical limitations are integral to guideline-based COPD assessment and treatment algorithms (Citation9), the impact of depressive symptoms could have wide implications in the management of COPD and deserves further attention in future research.
Author contributions
J.O. had full access to all of the data in the study and takes responsibility for the integrity of data and accuracy of data analysis. J.O. developed the original research question, collected data, performed initial statistical analysis, and wrote the first draft of the manuscript. G.L. also had full access to all of the data in the study and performed the final statistical analysis. D.H. supervised and directed the project from conception to submission. J.O., G.L., and D.H. all contributed to interpreting data and writing the manuscript.
Declaration of interest
The authors have no conflicts of interest to disclose.
Supplemental Material
Download PDF (396.7 KB)Acknowledgments
The authors would like to acknowledge the faculty of the University of Virginia Department of Public Health Sciences for assistance with data collection and data analysis. The authors are especially grateful to Aaron Pannone, Elizabeth Rogawski, and Min-Woong Sohn.
References
- Matte DL, Pizzichini MMM, Hoepers ATC, et al. Prevalence of depression in COPD: a systematic review and meta-analysis of controlled studies. Respir Med. 2016;117:154–161. doi: 10.1016/j.rmed.2016.06.006.
- Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med. 2012;12(1):26. doi: 10.1186/1471-2466-12-26.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345–349. doi: 10.1183/09059180.00007813.
- Felker B, Katon W, Hedrick SC, et al. The association between depressive symptoms and health status in patients with chronic pulmonary disease. Gen Hosp Psychiat. 2001;23(2):56–61. doi: 10.1016/S0163-8343(01)00127-X.
- Papaioannou AI, Bartziokas K, Tsikrika S, et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823. doi: 10.1183/09031936.00013112.
- Kim UK, Park H, Jung HY, et al. Association of depression with disease severity in patients with chronic obstructive pulmonary disease. Lung. 2014;192:243–249. doi: 10.1007/s00408-013-9547-4.
- Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104(6):849–857. doi: 10.1016/j.rmed.2009.12.007.
- Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP.
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Retrieved February 16, 2019, from https://www.cdc.gov/nchs/nhanes/index.htm.
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics. National Health and Nutrition Examination Survey Questionnaire. Retrieved from https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?BeginYear=2011. Published 2011.
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Examination Protocol. Retrieved from https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2011. Published 2011.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x.
- Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348–353. doi: 10.1370/afm.1139.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380. doi: 10.1001/jama.2015.18392.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312.
- Thoemmes F, Ong AD. A primer on inverse probability of treatment weighting and marginal structural models. Emerg Adulthood. 2016;4(1):40–59. doi: 10.1177/2167696815621645.
