 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Exhaled nitric oxide (FENO) is a marker of type-2 inflammation in asthma and is used in its management. However, smokers and ex-smokers have lower FENO values, and the clinical use of FENO values in COPD patients is unclear. Therefore, we investigated if FENO had a relationship to different COPD characteristics in smoking and ex-smoking subjects. Patients with COPD (n = 533, 58% females) were investigated while in stable condition. Measurements of FENO50, blood cell counts, IgE sensitisation and lung function were performed. Medication reconciliation was used to establish medication usage. Smokers (n = 150) had lower FENO50 9 (8, 10) ppb (geometric mean, 95% confidence interval) than ex-smokers did (n = 383) 15 (14, 16) ppb, p < 0.001. FENO50 was not associated with blood eosinophil or neutrophil levels in smokers, but in ex-smokers significant associations were found (r = 0.23, p < 0.001) and (r = –0.18, p = 0.001), respectively. Lower FENO values were associated with lower FEV1% predicted in both smokers (r = 0.17, p = 0.040) and ex-smokers (r = 0.20, p < 0.001). Neither the smokers nor ex-smokers with reported asthma or IgE sensitisation were linked to an increase in FENO50. Ex-smokers treated with inhaled corticosteroids (ICS) had lower FENO50 14 (13, 15) ppb than non-treated ex-smokers 17 (15, 19) ppb, p = 0.024. This was not found in smokers (p = 0.325). FENO is associated with eosinophil inflammation and the use of ICS in ex-smoking COPD subjects, but not in smoking subjects suggesting that the value of FENO as an inflammatory marker is more limited in smoking subjects. The association found between low FENO values and low lung function requires further investigation.
Introduction
Exhaled nitric oxide (FENO) is a marker of type-2 inflammation [Citation1, Citation2] and it has a role in asthma management. It can be used to titrate anti-inflammatory medication, identify individuals at risk for exacerbation [Citation3, Citation4] and study the effects of new biological treatments affecting the IL-13 pathway [Citation5]. A high FENO of more than 50 ppb, measured at an exhalation flow of 50 mL/s (FENO50) [Citation6, Citation7], can be used to indicate the possible presence of type-2 inflammation in symptomatic asthmatic patients and the likely location of the response to inhaled corticosteroids (ICS) [Citation8]. The clinical use of FENO in stable COPD patients is unclear as summarised by a recent scoping review [Citation9]. Finding a relevant FENO cut-off level for determining the use of ICS in COPD is challenging since many studies have not reported on the smoking status, which lowers FENO levels in both the general population and the asthma and COPD population [Citation10–12]. In GOLD 2019, https://goldcopd.org, ICS is recommended to group D patients, or those with an eosinophil blood count ≥300 cells/μL. In COPD, the FENO value during an exacerbation can be elevated and correlates well to the eosinophil count [Citation13]. However, ICS treatment has been reported to reduce FENO in COPD subjects [Citation14], but the effect does not appear to be dose dependent.
Asthma is a relatively common comorbidity in COPD, which is recognised as asthma-COPD overlap (ACO). According to recent publications, both increased FENO values [Citation13, Citation15, Citation16], and non-increased FENO values [Citation17] are reported in ACO. IgE sensitisation is an important determinant of FENO50 both in population-based studies [Citation18] and among subjects with asthma [Citation19], yet it has been sparsely studied in COPD [Citation20].
In a Swedish study, Tools Identifying Exacerbations in COPD (TIE-study), stable COPD subjects were investigated. The present objective was to explore the role of FENO among the smokers and ex-smokers with COPD, and correlate the FENO to COPD characteristics and inflammatory markers.
Material and methods
Study design and subjects
The study subjects were recruited for the multi-disciplinary, prospective TIE-study from the central Swedish regions of Dalarna, Gävleborg and Uppsala [Citation21]. Patients from primary and secondary care settings with a diagnosis of COPD (ICD code J44.0, J44.1, J44.8 and J44.9) were included. The present analysis is based on the data acquired from the inclusion visits made between September 2014 and September 2016. The Regional Review Board in Uppsala, Sweden (Dnr 2013/358) approved the study on 28 April 2014. All subjects gave written informed consent.
Methods
The COPD diagnosis was confirmed with spirometry using the post-bronchodilator (400 µg salbutamol) forced expiratory volume measured in the 1 second (FEV1) divided by the highest vital capacity (VC) value from a slow (SVC) or forced manoeuvre (FVC) with a ratio of <0.70 (SpiroPerfect spirometer, Welch Allyn, Skaneateles Falls, NY, USA or Jaeger Masterscreen PFT, Erich Jaeger GmbH, Würzburg, Germany). FEV1 and FVC are presented as a predicted percent using Swedish reference values [Citation22, Citation23]. Height and weight were taken at the visit to calculate the body mass index (BMI).
Exhaled nitric oxide (NO) at an exhalation flow of 50 mL s−1 (FENO50) was measured at the different study locations with NIOX VERO (Circassia AB, Solna, Sweden), NIOX Flex (Aerocrine AB, Solna, Sweden) or Eco Medicks CLD 88 (Eco Medicks, Duernten, Switzerland). The measurements were performed in accordance with current ATS/ERS 2005 recommendations for NO measurements. Blood cell counts analysing neutrophils (B-Neu) and eosinophils (B-Eos) were performed (Cell-Dyn 4000, Abbott, Abbott Park, IL, USA and Sysmex XN-10, Sysmex America Inc., Lincoinshire, IL, USA).
Questionnaires including the COPD Assessment Test (CAT) and modified Medical Research Council Scale (mMRC) were used together with questions regarding subject demographics, smoking habits, contacts with the healthcare system and medication use. Having ever asthma was self-reported. The research nurse reviewed the answers to the questionnaires with the subjects. Medication reconciliation was used to establish pharmacological treatment. When a subject did not answer the questions regarding smoking habits in the questionnaire (n = 2), measurements of exhaled carbon monoxide were used to define their smoking status (Smoke Check, Intramedic AB, Sollentuna, Sweden).
The presence of IgE sensitisation was assessed with a multi-allergen test against a mix of aeroallergens (birch, timothy, mugwort, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Cladosporium herbarum, cat, dog, and horse) using the ImmunoCAP system (Immunodiagnostics, Thermo Fischer Scientific, Uppsala, Sweden). A subject was considered sensitised if IgE levels to the Phadiatop were ≥0.35 kUA/L.
Analysis
To classify the COPD subjects with regard to severity of the disease, the GOLD risk assessment version 2017 A-D with symptoms (CAT scale) and number of exacerbations/hospitalizations was used. An exacerbation was defined as an unscheduled health care visit, and/or a course of oral corticosteroids and/or a course of antibiotics due to COPD deterioration (questionnaire assessed). Frequent exacerbations were defined as having at least two exacerbations and/or at least one hospital admittance (information retrieved from hospital records) due to a COPD exacerbation during the previous year.
The analyses were performed separately for current smokers and ex-smokers. There are no established cut-off values for elevated FENO50 in COPD, as opposed to asthma [Citation8]. Therefore, to study the clinical value of FENO50 in COPD and the influence of current smoking, the ex-smokers and smokers were divided into three groups or tertiles of about equal numbers and given the labels low, intermediate and high FENO50. Log transformation was performed on data with a skewed distribution. Data in the results are given as n (%), mean ± SD and geometric mean (CI95% lower and upper limit). For the statistical calculations Binominal test, Pearson χ2-test and non-parametric test, i.e. Mann–Whitney U test, Kruskal-Wallis test, Spearman’s rho and a post hoc test Tukey HSD, (SPSS, v. 24 for Windows, SPSS Inc., Chicago, MI, USA) were used. A p-value of <0.05 was considered significant. In the boxplots the horizontal line in each box corresponds to the median value, the upper and lower margins correspond to the 25th and 75th percentiles, and the whiskers correspond to the 10th and 90th percentiles.
Results
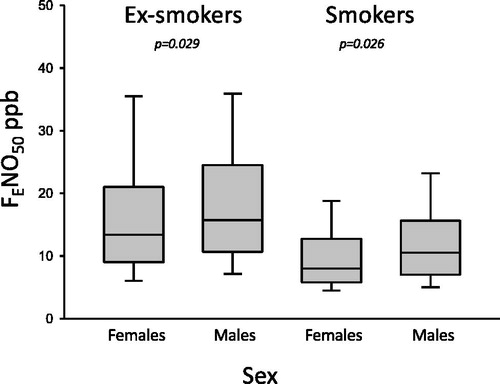
FENO50 was measured in 533 subjects (58% female), 13 (12, 14) ppb. Women had lower FENO50 values 12 (11, 13) ppb than men 15 (13, 16) ppb, p = 0.001. Smokers (n = 150, 65% female) had lower FENO50 values 9 (8, 10) ppb than ex-smokers (n = 383, 55% female) 15 (14, 16) ppb, p < 0.001. Smoking women had lower FENO50 than smoking men. In addition, ex-smoking women had lower FENO50 than ex-smoking men ().
Figure 1. The FENO50 values in ex-smokers and smokers according to sex. Females had lower FENO50 values than men did.
A lower FENO50 was associated with lower lung function. In smokers there were correlations between FENO50 and FEV1% predicted (r = 0.17, p = 0.04) and FVC% predicted (r = 0.22, p = 0.007). In ex-smokers corresponding values were r = 0.20, p < 0.001 and r = 0.17, p = 0.001. The lung function values found in the different FENO50 groups of smokers and ex-smokers are presented in and .
Table 1. Characteristics of the ex-smoking subjects divided into tertiles with regard to FENO50.
Table 2. Characteristics of the smoking subjects divided into tertiles with regard to FENO50.
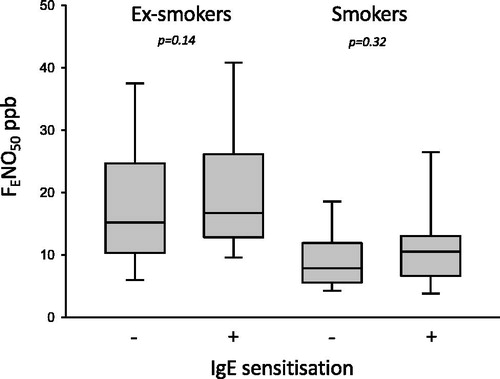
Subjects reported having asthma, smokers 33% and ex-smokers 34%, had no significant difference in FENO50 values from those who did not report having asthma. IgE sensitisation was analysed in subjects (n = 309) from two of the regions. A positive IgE test was found in 21% of the smokers and in 18% in ex-smokers. There were no significant differences in the FENO50 values for the IgE sensitised and non-sensitised subjects within the smoking and ex-smoking groups (; and ). There was a positive association between FENO50 and B-Eos (r = 0.23, p < 0.001) and a negative association between FENO50 and B-Neu (r = –0.18, p = 0.001) in the ex-smokers. In smokers there were no statistically significant associations found. Values of the inflammatory markers are presented for the different FENO50 group in and .
Figure 2. The FENO50 values in ex-smokers and smokers according to positive or negative IgE sensitisation (defined as ≥0.35 kUA/L). No significant difference was found.
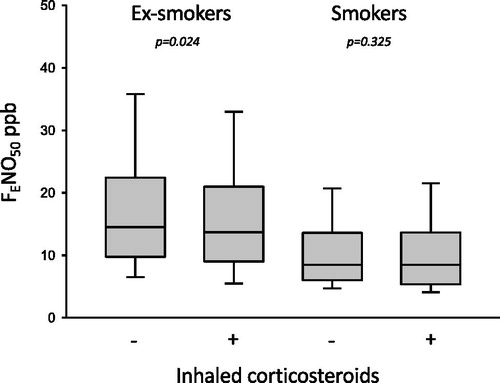
Smokers had a lower usage of ICS (49%) compared to the ex-smokers (62%), p = 0.005. Ex-smokers treated with ICS had lower FENO50 than ex-smokers not treated with ICS (). This FENO50 difference with ICS treatment or non-treatment was not seen in the smokers. There were no differences in ICS usage when the smokers and ex-smokers were divided into the low, intermediate and high FENO groups ( and ).
Figure 3. The FENO50 values in ex-smokers and smokers according to usage of ICS. Ex-smokers had a significantly lower FENO50 value statistically when treated with ICS, while no difference was found in smokers.
A CAT score ≥ 10 was found in 59% of the smokers and 62% of the ex-smokers (p = 0.588). A value of ≥ 2 on the mMRC scale was present in 33% of the smokers and 48% of the ex-smokers (p = 0.001).
Subjects who had four or more exacerbations during the year before inclusion (3 smokers and 20 ex-smokers) had a FENO50 of 13 (4, 43) and 10 (7, 14) ppb, respectively. Those with three exacerbations (8 smokers and 13 ex-smokers) had a FENO50 of 11 (8, 16) and 11 (8, 15) ppb, respectively. Those with one or two exacerbations (49 smokers and 99 ex-smokers) had a FENO50 of 9 (8, 11) and 16 (14, 18) ppb, respectively; and subjects with zero exacerbations (90 smokers and 251 ex-smokers) had a FENO50 of 9 (8,10) and 15 (14, 17) ppb, respectively. Accordingly, in smokers there was no significant difference in FENO50 (p = 0.469), but ex-smokers did show a decrease in FENO50 when the exacerbations increased (p = 0.008).
Subjects who had at least one hospital admission the year before inclusion (11 smokers and 26 ex-smokers) had a FENO50 of 8 (5, 12) and 12 (9, 15) ppb, respectively; and subjects with zero hospital admissions (139 smokers and 357 ex-smokers) had 9 (8, 10) and 15 (14, 16) ppb, respectively. Thus, in smokers there was no statistical difference in FENO50 levels (p = 0.521) nor was there a difference found in the ex-smokers (p = 0.104).
A sensitivity analysis was performed to confirmed our findings by using the 5th percentile of reference population as the lower limit of normality (LLN). Hence, a COPD diagnosis set by FEV1/VC-ratio < LLN was found in 432 subjects, among ex-smokers 305 subjects and smokers 127 subjects. Ex-smokers had a significant lower FENO50 when on ICS (p = 0.01) while no difference was found in smokers (p = 0.30). Regarding IgE sensitisation there was no significant difference in FENO50 in ex-smokers and smokers (p = 0.46 resp. p = 0.64).
Discussion
This multicentre study in COPD has shown that smokers have much lower FENO50 levels than ex-smokers, and the relationship between FENO50 and the inflammatory markers, such as blood eosinophil and blood neutrophil counts, was only found in the ex-smokers. Similarly, the relationship of lower FENO50 and ICS usage was only found in the ex-smokers. A low FENO50 was associated with a lower lung function in both the smokers and ex-smokers. Neither a reported history of asthma nor IgE sensitisation was shown to have a relationship with FENO50.
The interesting finding from this study is that there were no associations found between the exhaled NO and the eosinophil and neutrophil blood counts in the current smokers with COPD. However, there was a positive correlation of FENO50 with the eosinophil blood count and a negative correlation with the neutrophil blood count found in the ex-smokers. FENO is known to correlate with eosinophils in many asthma studies [Citation24–26]. As far as we know, relationships of this kind have not been reported in COPD patients in stable condition, but a positive relationship between FENO50 and blood eosinophil levels has been found in COPD patients experiencing exacerbations [Citation13, Citation27]. Additionally, there was no difference in the FENO values of current smokers with or without ICS treatment. As this study is cross-sectional, we cannot infer that ICS treatment will not reduce FENO levels in our smokers, which is suggested by current data concerning the impaired action of ICS in smokers [Citation28–30].
A low FENO was associated with low lung function in this study. This has been reported in subjects with cystic fibrosis [Citation31] where a negative correlation was also found with neutrophils, which is similar to our findings. A reduction of NO by superoxide released from neutrophils has been presented as an alternative explanation for the low FENO [Citation32]. Cystic fibrosis and primary ciliary dyskinesia (two diseases with low levels of FENO) and COPD share some common features such as chronic bacterial colonisation and repeated inflammatory periods leading to lung function decline [Citation33, Citation34]. NO has many biological functions in the respiratory system. It is bacteriostatic, it halts viral replication and eliminates various pathogens [Citation35]. It has also been suggested that the antiproliferative effects of NO on airway smooth muscle might become an important clue in the quest for future strategies to prevent airway remodelling [Citation36]. In this study, 25% of the subjects had FENO50 values under 8 ppb which can be compared to the lower normal limit for healthy subjects in the same age group [Citation37]. Even though the lower level of FENO50 did not reach statistical significance in smokers, we did note that ex-smokers with frequent exacerbations had significantly lower FENO50, and when they were hospitalised for exacerbations their FENO50 was more likely to be on the low side. Everything considered, a functional level of NO in the airways is a necessity.
One third of our COPD subjects reported having asthma, but only one fifth had atopy. In the reported subjects, there was no significant difference in the FENO50 values of the IgE sensitised and non-sensitised subjects among the smokers or ex-smokers. There are few studies on allergic sensitisation to aeroallergens among COPD subjects. A recent Polish study reports that one third of the investigated COPD subjects had a positive skin prick test to at least one of the investigated aeroallergens [Citation38]. In a recent Japanese study, based on the definition of total IgE above 173 kU/L, one third of the COPD subjects were considered atopic [Citation39]. Kobayashi et al. identified participants with ACO as variable respiratory symptoms and variable expiratory airflow limitation and they found that about half of the ACO participants had a total serum IgE level above 173 kU/L [Citation20]. The differences in prevalence of IgE sensitisation in our study might be due to different methodologies used to assess IgE sensitisation, type of patients included in the study and regional differences. Our study has tried to relate the presence of IgE aeroallergen sensitisation to the levels of exhaled NO. We were unable to find this relationship in COPD, as opposed to studies with healthy [Citation18] and asthmatic subjects [Citation19]. Accordingly, allergic sensitisation appears to be less important in the interpretation of FENO levels in COPD subjects.
A self-reported asthma diagnosis, not physician diagnosed, was found in about one third of the subjects, and this was not related to FENO values in the present material. There are divergent results reported in the literature regarding levels of FENO in subjects with both COPD and asthma, and the definitions of asthma-COPD overlap used in these studies are also heterogeneous.
In this study, women had lower FENO50 values than men did, and this was the case for both smokers and ex-smokers. This differs from what was reported for the same age group in one study of healthy individuals [Citation37], but is in line with results from other studies [Citation40, Citation41]. The FENO50 values in our study are slightly lower than values from healthy individuals, which is a known effect of smoking [Citation10].
The strength of the present study is in the large number of patients investigated. Another strength comes from the inclusion of subjects with different levels of care and disease severity, which leads to a higher external validity of the present findings. However, the study does have limitations that stem from the fact that the subjects already had their diagnoses and had already received treatment. This means that we cannot answer the question regarding the value of FENO in predicting the response to ICS in ICS-naïve subjects. Furthermore, the cross-sectional analysis does not allow us to study the relationship that low FENO has to lung function decline. This study has used both electrochemical sensors and chemiluminescence technique to measure FENO and the difference between them are considered by most users to be clinically acceptable Citation[42].
In conclusion, smoking decreases FENO in COPD subjects and the value of FENO as inflammatory marker appears to be limited in current smokers. Higher levels of FENO in our COPD subjects did not relate to the presence of reported asthma and IgE sensitisation. Ex-smokers that had frequent exacerbations had low FENO when in a stable condition. This is perhaps what we must be observant for and mindful of in clinical practice. In many COPD studies smokers are analysed together with ex-smokers. This study has shown that smokers need to be analysed separately from ex-smokers. Future studies are needed to investigate if the lower FENO values, seen in subjects with poor lung function, can be used to identify individuals at risk for rapid lung function decline.
| Abbreviations | ||
| ACO | = | asthma – COPD overlap |
| B-Eos | = | eosinophil blood count |
| B-Neu | = | neutrophil blood count |
| BMI | = | body mass index |
| CAT | = | COPD assessment test |
| COPD | = | chronic obstructive pulmonary disease |
| FENO50 | = | fraction of exhaled nitric oxide at the flow of 50 mL/s |
| FEV1 | = | forced expiratory volume in 1 second |
| FVC | = | forced vital capacity |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| ICS | = | inhaled corticosteroids |
| mMRC | = | modified British Medical Research Council |
| NO | = | nitric oxide |
| SVC | = | slow vital capacity |
| TIE-study | = | Tools Identifying Exacerbations in COPD |
| VC | = | vital capacity |
Acknowledgments
The authors are grateful to the research nurses/technicians at the three study sites: Dalarna, Gävle and Uppsala. The authors wish to thank Robin Quell for proofreading and editing this manuscript.
Additional information
Funding
Notes on contributors
M. Högman
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
A. Thornadtsson
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
K. Bröms
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
C. Janson
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
K. Lisspers
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
B. Ställberg
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
H. Hedenström
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
A. Malinovschi
MH, BS, KL, KB, CJ and AM participated in the study design and together with AT collected and analysed the data. MH prepared the initial draft. All authors have discussed the draft, and have read and approved the final manuscript.
References
- Munakata M. Exhaled nitric oxide (FeNO) as a non-invasive marker of airway inflammation. Allergol Int. 2012;61:365–372. doi:10.2332/allergolint.12-RAI-0461.
- Fahy JV. Type 2 inflammation in asthma-present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi:10.1038/nri3786.
- Petsky HL, Kew KM, Chang AB. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev. 2016;11:CD011439. doi:10.1002/14651858.CD011439.pub2.
- Normansell R, Cates C. Tailoring asthma therapies using FeNO: can a new objective measure help more people to gain control and reduce over-treatment? Cochrane Database Syst Rev. 2016;9:ED000115.
- Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi:10.1056/NEJMoa1106469.
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912–930. https://www.atsjournals.org/doi/full/10.1164/rccm.200406-710ST?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed
- Horvath I, Barnes PJ, Loukides S, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J. 2017;49:1600965. doi:10.1183/13993003.00965-2016.
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi:10.1164/rccm.9120-11ST.
- Mostafavi-Pour-Manshadi S-M-Y, Naderi N, Barrecheguren M, et al. Investigating fractional exhaled nitric oxide in chronic obstructive pulmonary disease (COPD) and asthma-COPD overlap (ACO): a scoping review. COPD. 2018;15:377–391. doi:10.1080/15412555.2018.1485637.
- Malinovschi A, Janson C, Holmkvist T, et al. Effect of smoking on exhaled nitric oxide and flow-independent nitric oxide exchange parameters. Eur Respir J. 2006;28:339–345. doi:10.1183/09031936.06.00113705.
- Verleden GM, Dupont LJ, Verpeut AC, et al. The effect of cigarette smoking on exhaled nitric oxide in mild steroid-naive asthmatics. Chest. 1999;116:59–64. doi:10.1378/chest.116.1.59.
- Lehouck A, Carremans C, De Bent K, et al. Alveolar and bronchial exhaled nitric oxide in chronic obstructive pulmonary disease. Resp Med. 2010;104:1020–1026. doi:10.1016/j.rmed.2010.01.001.
- Río Ramírez MT, Juretschke Moragues MA, Fernández González R, et al. Value of exhaled nitric oxide (FeNO) and eosinophilia during the exacerbations of chronic obstructive pulmonary disease requiring hospital admission. COPD. 2018;15:369–376. doi:10.1080/15412555.2018.1482532.
- Williamson PA, Menzies D, Clearie KL, et al. Dose-response for inhaled fluticasone on airway and systemic inflammation in COPD. Eur Respir J. 2011;37:206–209. doi:10.1183/09031936.00062210.
- Takayama Y, Ohnishi H, Ogasawara F, et al. Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma-COPD overlap. COPD. 2018;13:2525–2532. doi:10.2147/COPD.S167600.
- Shi F, Qiu C, Yu J, et al. Comparison of fractional exhaled nitric oxide in elderly patients with asthma-chronic obstructive pulmonary disease overlap and other airway inflammatory diseases. Iran J Allergy Asthma Immunol. 2018;17:232–239.
- Colak Y, Afzal S, Nordestgaard BG, et al. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the Copenhagen General Population Study. Eur Respir J. 2018;52:1800616. doi:10.1183/13993003.00616-2018.
- Malinovschi A, Janson C, Holmkvist T, et al. IgE sensitisation in relation to flow-independent nitric oxide exchange parameters. Respir Res. 2006;7:92. doi:10.1186/1465-9921-7-92.
- Al-Shamkhi N, Alving K, Dahlen SE, et al. Important non-disease-related determinants of exhaled nitric oxide levels in mild asthma - results from the Swedish GA(2) LEN study. Clin Exp Allergy. 2016;6:1185–1193. doi:10.1111/cea.12749.
- Kobayashi S, Hanagama M, Yamanda S, et al. Inflammatory biomarkers in asthma-COPD overlap syndrome. COPD. 2016;11: 2117–23. doi:10.2147/COPD.S113647.
- Högman M, Sulku J, Ställberg B, et al. 2017 Global initiative for chronic obstructive lung disease reclassifies half of COPD subjects to lower risk group. COPD. 2018;13:165–173. doi:10.2147/COPD.S151016.
- Hedenström H, Malmberg P, Agarwal K. Reference values for lung function tests in female. Regression equations with smoking variables. Bull Eur Physiopathol Respir. 1985;21:551–557.
- Hedenström H, Malmberg P, Fridriksson HV. Reference values for pulmonary function test in men: regression equations which include tobacco smoking variables. Upsala J Med Sci. 1986;91:299–310. doi:10.3109/03009738609178670.
- Högman M, Lúdvı́ksdóttir D, Anderson SD, et al. Inhaled mannitol shifts exhaled nitric oxide in opposite directions in asthmatics and healthy subjects. Respir Physiol. 2001;124:141–150. doi:10.1016/S0034-5687(00)00195-X.
- Jatakanon A, Lim S, Kharitonov SA, et al. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi:10.1136/thx.53.2.91.
- Berry MA, Shaw DE, Green RH, et al. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy. 2005;35:1175. doi:10.1111/j.1365-2222.2005.02314.x.
- Soter S, Barta I, Antus B. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation. 2013;36:1178–1185. doi:10.1007/s10753-013-9653-8.
- Tomlinson JEM, McMahon AD, Chaudhuri R, et al. Efficacy of low and high dose inhaled corticosteroid in smokers versus non-smokers with mild asthma. Thorax. 2005;60:282–287. doi:10.1136/thx.2004.033688.
- Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J. 2013;41:716–726. doi:10.1183/09031936.00073312.
- Bourbeau J, Christodoulopoulos P, Maltais F, et al. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax. 2007;62:938–943. doi:10.1136/thx.2006.071068.
- Krantz C, Janson C, Hollsing A, et al. Exhaled and nasal nitric oxide in relation to lung function, blood cell counts and disease characteristics in cystic fibrosis. J Breath Res. 2017;11:026001. doi:10.1088/1752-7163/aa61aa.
- Jones KL, Bryan TW, Jinkins PA, et al. Superoxide released from neutrophils causes a reduction in nitric oxide gas. Am J Physiol. 1998;275:L1120–6. doi:10.1152/ajplung.1998.275.6.L1120.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi:10.1056/NEJMra0800353.
- Acosta N, Heirali A, Somayaji R, et al. Sputum microbiota is predictive of long-term clinical outcomes in young adults with cystic fibrosis. Thorax. 2018;73:1016–1025. doi:10.1136/thoraxjnl-2018-211510.
- Ricciardolo F. Multiple roles of nitric oxide in the airways. Thorax. 2003;58:175–182. doi:10.1136/thorax.58.2.175.
- Ricciardolo FLM, Sterk PJ, Gaston B, et al. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi:10.1152/physrev.00034.2003.
- Högman M, Thornadtsson A, Liv P, et al. Effects of growth and aging on the reference values of pulmonary nitric oxide dynamics in healthy subjects. J Breath Res. 2017;11:047103. doi:10.1088/1752-7163/aa7957.
- Bożek A, Rogala B. IgE-dependent sensitization in patients with COPD. Ann Agric Environ Med. 2018;25:417–420. doi:10.26444/aaem/83413.
- Tamada T, Sugiura H, Takahashi T. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015;10:2169–2176.
- Olin A-C, Rosengren A, Thelle DS, et al. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130:1319–1325. doi:10.1378/chest.130.5.1319.
- Jacinto T, Malinovschi A, Janson C, et al. Evolution of exhaled nitric oxide levels throughout development and aging of healthy humans. J Breath Res. 2015;9:036005. doi:10.1088/1752-7155/9/3/036005.
- Antus B, Horvath I, Barta I. Assessment of exhaled nitric oxide by a new hand-held device. Respir Med. 2010;104:1377–1380. doi:10.1016/j.rmed.2010.06.005.
