Abstract
Early diagnosis of COPD exacerbations is vital. Exacerbations are characterised by an increase in dyspnoea that could be affect physical capacity. Changes in the physical capacity of patients with COPD during pulmonary rehabilitation could provide a predictive indication regarding the occurrence of exacerbation. This was a retrospective study of forty for patients with COPD who participated in a pulmonary rehabilitation programme between January 2015 and October 2018. Patients to have experienced at least one exacerbation during their pulmonary rehabilitation programme are included. The performance variable and dyspnoea on the cycle ergometer and the treadmill were collected during the five sessions prior to the exacerbation and the three sessions following the exacerbation. Seventy exacerbations were analysed. We found a significant decrease in the performance on the cycle ergometer during the last session before exacerbation compared with previous sessions (mean difference: 74.5% (95%CI 12.6–136.5); p < 0.01). The optimal threshold value was a 17% decrease in performance compared to the previous training session. Sensitivity was 0.46 (95%CI 0.34–0.59), specificity was 0.83 (95%CI 0.72–0.91) and the area under the curve was 0.65 (95%CI 0.56–0.74) (p < 0.01). The analysis of performance data from cycle ergometer is a potentially useful method to predict the occurrence of exacerbation.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by a relative fixed limitation in airflow. It is usually progressive and is associated with an increase in the inflammatory response within the respiratory tract [Citation1]. In 2015, 3.17 million deaths were caused by COPD (5% of all worldwide deaths) [Citation2]. Exacerbations are characteristic of COPD and cause both short-term worsening of respiratory symptoms as well as disease progression [Citation3]. In English cohort study, the exacerbation rate was 1.43 per year in 2016. Costs relating to respiratory diseases represent 6% of the total health budget in the European Union. COPD represents 56% of these costs, €38.6 million of which is spent on treating exacerbations [Citation4, Citation5]. Moreover, exacerbations that require hospitalisation have a negative impact on quality of life and are associated with increased mortality [Citation6–8]. Early diagnosis of exacerbations and initiation of appropriate therapy is therefore vital [Citation9, Citation10].
Pulmonary rehabilitation and physical exercise are essential components of the management of COPD. The aim of pulmonary rehabilitation is to improve exercise capacity and reduce dyspnoea [Citation11]. It should be carried out over a period of several months, two to three times per week, either in a hospital setting, a specialised centre or the patient’s own home environment. As well as improving the patient’s overall condition, pulmonary rehabilitation provides a means to monitor the patient regularly [Citation12]. During physical exercise, the increased activity of the muscle system increases cardiac and respiratory output. If the patient’s respiratory status deteriorates, as occurs, for example, before the onset of an exacerbation, exercise capacity could be reasonably expected to reduce [Citation13–17]. We hypothesised, therefore, that changes in the physical capacity of patients with COPD during a rehabilitation session could provide a predictive indication regarding the risk of occurrence of exacerbation. The primary aim of this study was therefore to evaluate if a reduction in performance during rehabilitation sessions could predict exacerbation.
Method
Study population and patient selection
This was a retrospective study of patients with COPD who participated in a pulmonary rehabilitation programme between January 2015 and October 2018 in two centres: Le Havre Hospital and ADIR Association, Rouen University Hospital. The study was approved by the local committee for non-interventional research (Comité d’éthique pour la recherche non interventionnelle du CHU de Rouen, CERNI E2018-70). In accordance with current French legislation, written informed patient consent was not required.
Inclusion criteria
Patients with clinical diagnosis of COPD and spirometry obstruction (FEV1/FVC < 5th percentile of the predicted value) were included. Severity of airflow limitation post bronchodilator was rated according to the GOLD classification [GOLD] [Citation1]. Patients had to be 18 years of age or older, have not had an exacerbation during the previous month, to have experienced at least one exacerbation during their pulmonary rehabilitation programme and also to have participated in at least five rehabilitation sessions before the exacerbation occurred.
Exclusion criteria
Patients who did not experience any exacerbation or who were poorly compliant with the pulmonary rehabilitation programme (less than one session per week) were not included in the analysis. Patients who had participated in <5 sessions before an exacerbation were not included.
Rehabilitation programme
In both rehabilitation centres, patients underwent 24 sessions of rehabilitation. The programmes were standardised to include, as a minimum for each session, endurance exercise on a cycle-ergometer or treadmill and both upper- and lower-limb strengthening exercises during each session. Sessions were carried out at least twice weekly in both centres. No supervised home-based training was provided.
All patients underwent a cardiopulmonary exercise test (CPET) and a six-minute walk test (6MWT) prior to beginning the rehabilitation programme. The Modified Borg scale (0–10) was used to set the intensity on the cycle ergometer or treadmill for the first rehabilitation session. Patients were encouraged to carry out 30–40 minutes of exercise at each session. The intensity was increased at each session as tolerated.
The following variables were recorded during each session:
Treadmill: mean speed (km/hours), duration of the exercise (minutes), distance covered (km), maximum dyspnoea using the modified Borg scale (0–10).
Cycle ergometer: mean work rate output (Watts), duration of the exercise (minutes), distance covered (km), maximum dyspnoea using the modified Borg scale (0–10).
The performance variable on the cycle ergometer was defined as: work rate output (Watts) × distance covered (km) (). This reflected the balance between the task difficulty, pedalling speed and the exercise duration. Performance on the treadmill was simply defined as the distance covered in order to reflect the balance between exercise speed and the duration.
Data extraction
The following data were collected: demographic data, co-morbidities, pulmonary function, initial exercise capacity (maximal work rate output (Wpeak) during CPET and the 6MWT) and performance during the five sessions prior to the exacerbation (S-1, S-2, S-3, S-4, S-5) and the three sessions following the exacerbation (S + 1, S + 2, S + 3).
Exacerbation was defined as a non-scheduled attendance at the emergency department or consultation with the pulmonologist that resulted in a change in therapy. Admissions to intensive care or the pulmonary department were considered if the reason for admission was decompensation of COPD. Consultation with the pulmonologist with change in therapy was defined as “moderate exacerbation”. Admissions to intensive care or the pulmonary department were defined as a “severe exacerbation”. Exacerbations were considered that occurred outside the period of pulmonary rehabilitation were not included in the analyses.
Statistical analysis
The normality of the distribution was assessed using the Kolmogorov-Smirnov test. Categorical data were expressed as counts and percentage (%) and continuous data were expressed as either means (±SD) or medians (25th–75th percentile), according to the normality of the data distribution. Repeated measures ANOVA were used to compare differences in performance between sessions. If the results were significant, post-hoc analysis was carried out using a Bonferroni Multiple Comparison test. If the results were significant, a Receiver-Operating-Characteristic (ROC) curve analysis was used to determine if the parameters were predictive of the exacerbation. The session S-2 was chosen for the ROC analysis. A two-tailed p value of 0.05 was considered as significant for all analyses. Statistical analyses were performed using XLSAT 2018.1 (Addinsoft. 2016. XLSTAT 2016: Data Analysis and Statistical Solution for Microsoft Excel. Paris, France, 2016) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Sub-group analyses were also carried out for moderate (non-scheduled consultation with pulmonologist or emergency department) and severe (hospital admission) exacerbations.
Results
During the study period, 548 patients participated in a pulmonary rehabilitation programme at one of the two participating care centres. Four hundred and two of these patients had a diagnosis of COPD and, of this number, 50 patients had a combined total of 76 exacerbations. Six patients were excluded because they performed less than five sessions before their exacerbation. Seventy exacerbations could be analysed for 44 patients.
The characteristics of the 44 patients who had at least one exacerbation during the programme are presented in . Briefly, 36% were female, their mean age was 67.2 years, body mass index was 24.9 kg/m2 and mean FEV1 was 36% of the predicted value. Mean distance covered on the 6MWT was 392 metres (73% of the predicted distance).
Table 1. Patients characteristics.
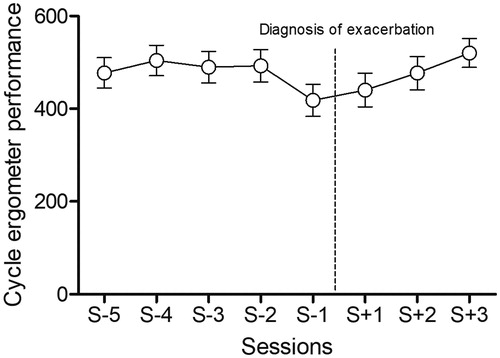
Performance on the cycle ergometer was significantly lower at S-1. Post-hoc analysis showed a statistically significant difference in performance on the cycle ergometer between S-1 and S-4, S-3, S-2 and S + 3 (See ).
Table 2. Mean difference in performance variables between sessions.
There were no significant differences in mean work rate output (Watts) on the ergometer, speed or distance walked on the treadmill or dyspnoea at S-1 compared with the other sessions (See ).
The median (IQR) time between the last session and the diagnosis of exacerbation was 6 [Citation4–11, Citation17] days.
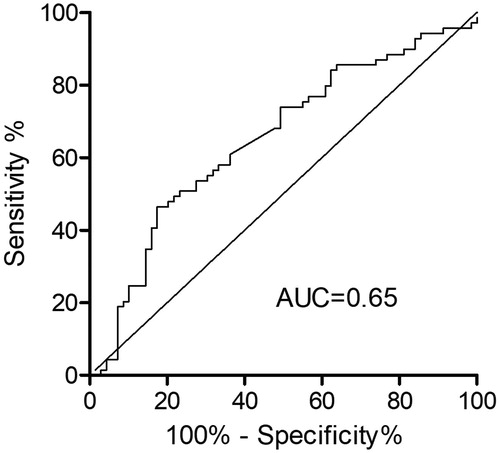
The ROC curve for the value of performance on the cycle ergometer was used to predict exacerbation and is presented in . The ROC curve-derived optimal threshold value revealed a 17% decrease in performance compared to the previous training session. Sensitivity was 0.46 (95%CI 0.34–0.59), specificity was 0.83 (95%CI 0.72–0.91) and the area under the curve was 0.65 (95%CI 0.56–0.74) (p < 0.01).
Figure 2. ROC curve for the prediction of exacerbation by cycle ergometer performance. AUC: Area under the curve.
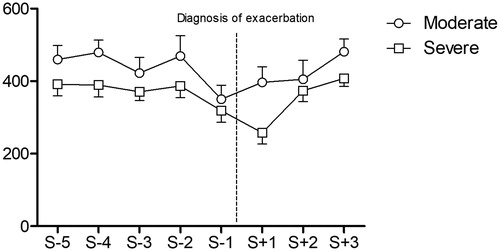
Sub-group post hoc analysis showed that the performances of the patients who had moderate (n = 22) or severe (n = 22) exacerbation were both significantly lower during S-1 compared with the preceding sessions (respectively 469 ± 264 vs. 350 ± 180; p = 0.01 and 387 ± 150 vs. 319 ± 149; p = 0.02). The performance of the group with severe exacerbation remained significantly reduced at S + 1 (319 ± 149 vs. 258 ± 146; p = 0.04) (See and ).
Discussion
This study showed that (Citation1) the cycle ergometer performance of patients with COPD was reduced prior to the diagnosis of an exacerbation; (Citation2) the threshold value of performance deterioration on the cycle ergometer that predicted that a potential exacerbation was going to occur was 17% and (Citation3) performance remained reduced longer when exacerbations were severe.
To our knowledge, this is the first study to evaluate the value of exercise performance in patients undergoing pulmonary rehabilitation in order to predict future exacerbations. The results confirmed our hypothesis that the progressive increase of symptoms prior to an exacerbation would reduce exercise tolerance and thus performance during a rehabilitation session.
These results are in line with those of Alahmari et al. and Borges et al., who evaluated daily activity level in patients with COPD using pedometers and accelerometers. Patients were found to have reduced their activity levels before exacerbations occurred; furthermore, patients who reported the increase in their symptoms earlier also received earlier therapy and were able to increase their activity levels again more quickly post-exacerbation than those who received treatment later. Moreover, the results of the present study showed a greater loss of performance in patients who were hospitalised [Citation14–16, Citation18]. The results of these three studies therefore suggest that early detection of an exacerbation and early initiation of therapy may avoid both hospitalisation and excessive loss of performance.
Several other studies have attempted to determine predictors of exacerbation in patients with COPD. However, these indices (such as the BODE or SCOPEX) are multifactorial and lack sensitivity to change [Citation19]. More recently, Borel et al. evaluated progressive factors such as variables recorded on home ventilators (respiratory rate, %Trigger and daily usage (hours/day)). They found that an increased respiratory rate over the last five days predicted an exacerbation (sensitivity and specificity: 46.2% and 89.7%), as did the respiratory trigger percentage (sensitivity and specificity: 53.8% and 73.2%). The present study found similar sensitivity and specificity (46% and 83%) values for data from performance on the cycle ergometer [Citation20].
Surprisingly, there was no change in perceived dyspnoea (rated on the Borg scale) during exercise prior to exacerbation. We believe this could be attributed to the reduction in physical effort that ensured that dyspnoea remained at a similar, tolerable level. The lack of change in the distance covered on the treadmill may be due to the fact some patients took breaks by putting their feet on the sides of the treadmill but without stopping the treadmill. The performance values at the end of a session were therefore likely overestimated.
There is currently no consensus in the literature regarding how to predict exacerbation [Citation21]. Therapy is usually initiated or changed when the exacerbation actually occurs or if the symptoms worsen (patient-reported outcomes) [Citation22]. Moreover, several studies have evaluated the utility of telemetric monitoring of simple parameters such as SpO2 or heart rate, but the majority of results have been inconclusive [Citation23–25]. In contrast, monitoring of exercise performance during pulmonary rehabilitation is an interesting approach that should be developed further because, not only are pulmonary rehabilitation programmes currently being encouraged by health initiatives but monitoring exercise performance is simple and can be undertaken in any care setting (e.g. ambulatory, outpatient or the patient’s home).
This study has several limitations. Firstly, the low sensitivity and AUC could be explained by the large day-to-day variability of COPD symptoms as well as variability in the motivation to exercise. Motivated subjects may fight their symptoms for several days before looking for medical help, while others may immediately lose motivation. Unfortunately, due to the retrospective design of the study, no specific measures were used to evaluate COPD symptoms, such as the EXACT tool. Secondly, although the performance measure mean work rate output (watts) × distance covered) was simple to calculate, it cannot be adapted to other activities and is only relevant during the time the patients are involved in a pulmonary rehabilitation programme. Moreover, it would have been useful to evaluate SpO2 and heart rate during exercise to correlate physiological data to exercise performance. Although attempts were made to standardise the pulmonary rehabilitation programmes between the centres, we know that a few differences existed, in particular with regard to the frequency of rehabilitation sessions and their duration. Finally, some exacerbations evaluated were for the same patients at different time-points and it could be overestimated the sensitivity of prediction.
The strong points of this study are that (Citation1) the approach described to predict the risk of an exacerbation is simple to use and can be applied in any care setting; (Citation2) the risk could be approached as a “red flag” by making patients aware that they may experience an exacerbation within the next few days and by encouraging them to pay particular attention to their symptoms over the following days; (Citation3) it involves no extra costs or equipment to record the necessary data; (Citation4) this method could be developed and adapted to be used with other types of exercise and (Citation5) unlike questionnaires that are used to predict exacerbation, patient comprehension is not required.
Further prospective multicenter studies are needed to identify and evaluate an equally practical criterion that could be used during both activities of daily living and rehabilitation. The predictive value of exercise performance could also be improved, perhaps by the addition of variables such as SpO2 and heart-rate during exercise. These variables could also easily be monitored in any setting, including at home, via telemonitoring [Citation26].
Conclusion
The analysis of performance data from a pulmonary rehabilitation programme, in particular cycle ergometer (power/distance ratio) data, is a potentially useful method to apply a “red flag” and alert for the risk of occurrence of exacerbation. Further prospective multicenter studies are required to confirm these data and to ensure the clinical utility of the method.
Declaration of interest
The authors report no conflict of interest.
References
- GOLD, Global Initiative for Chronic Obstructive Lung Disease (GOLD).
- Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706.
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi:10.1016/S0140-6736(07)61382-8.
- The economic burden of lung disease - ERS [Internet]. [cited 2019 Feb 10]. Available from: https://www.erswhitebook.org/chapters/the-economic-burden-of-lung-disease/#alternate.
- Toy EL, Gallagher KF, Stanley EL, et al. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD J Chronic Obstr Pulm Dis. 2010;7:214–228. doi:10.3109/15412555.2010.481697.
- Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19:113–118. doi:10.1183/09059180.00002610.
- Groenewegen KH, Schols A, Wouters E. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124:459–467. doi:10.1378/chest.124.2.459.
- Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147:999–1007. doi:10.1378/chest.14-0655.
- Donaldson GC, Law M, Kowlessar B, et al. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:943–950. doi:10.1164/rccm.201412-2269OC.
- Wilkinson TMA, Donaldson GC, Hurst JR, et al. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:1298–1303. doi:10.1164/rccm.200310-1443OC.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Airways Group, editor. Cochrane Database Syst. Rev. [Internet]. [cited 2019 Apr 2]; Available from: http://doi.wiley.com/10.1002/14651858.CD003793.pub3.
- Horton EJ, Mitchell KE, Johnson-Warrington V, et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax. 2018;73:29–36. doi:10.1136/thoraxjnl-2016-208506.
- Abdulai RM, Jensen TJ, Patel NR, et al. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:433–449. doi:10.1164/rccm.201703-0615CI.
- Borges RC, Carvalho C. Physical activity in daily life in Brazilian COPD patients during and after exacerbation. COPD J Chronic Obstr Pulm Dis. 2012;9:596–602. doi:10.3109/15412555.2012.705364.
- Kahnert K, Alter P, Welte T, et al. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: a multi-dimensional approach. Respir Res. [Internet]. 2018;19:110. Available from: https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-018-0815-y.
- Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi:10.1378/chest.129.3.536.
- Raluy-Callado M, Merinopoulou E, Ramagopalan S, et al. COPD exacerbations by disease severity in England. Int J Chron Obstruct Pulmon Dis. 2016;11:697–709.
- Alahmari AD, Patel AR, Kowlessar BS, et al. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. [Internet]. 2014;14:98. Available from: http://bmcpulmmed.biomedcentral.com/articles/10.1186/1471-2466-14-98.
- Herer B, Chinet T. Acute exacerbation of COPD during pulmonary rehabilitation: outcomes and risk prediction. Int J Chron Obstruct Pulmon Dis. 2018;13:1767–1774. doi:10.2147/COPD.S163472.
- Borel J-C, Pelletier J, Taleux N, et al. Parameters recorded by software of non-invasive ventilators predict COPD exacerbation: a proof-of-concept study. Thorax. 2015;70:284–285. doi:10.1136/thoraxjnl-2014-206569.
- Guerra B, Gaveikaite V, Bianchi C, et al. Prediction models for exacerbations in patients with COPD. Eur Respir Rev. 2017;26:160061. doi:10.1183/16000617.0061-2016.
- Strassmann A, Frei A, Haile SR, et al. Commonly used patient-reported outcomes do not improve prediction of COPD exacerbations: a multicenter 4½ year prospective cohort study. Chest. 2017;152:1179–1187. doi:10.1016/j.chest.2017.09.003.
- Jensen MH, Cichosz SL, Dinesen B, et al. Moving prediction of exacerbation in chronic obstructive pulmonary disease for patients in telecare. J Telemed Telecare. 2012;18:99–103. doi:10.1258/jtt.2011.110607.
- Sanchez-Morillo D, Fernandez-Granero MA, Jiménez AL. Detecting COPD exacerbations early using daily telemonitoring of symptoms and k-means clustering: a pilot study. Med Biol Eng Comput. 2015;53:441–451. doi:10.1007/s11517-015-1252-4.
- Price D, Kerkhof M, Freeman D, et al. Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis. 2015;10:2439–2450.
- Bonnevie T, Gravier F-E, Elkins M, et al. People undertaking pulmonary rehabilitation are willing and able to provide accurate data via a remote pulse oximetry system: a multicentre observational study. J Physiother. 2019;65:28–36. doi:10.1016/j.jphys.2018.11.002.