Abstract
The comorbidity of peripheral arterial disease (PAD) and chronic obstructive pulmonary disease (COPD) is obvious from a clinical point of view, especially as smoking is an important risk factor for both. Another factor connecting these two clinical conditions is chronic inflammation, which plays a crucial role in their pathophysiology. The aim of this study was to present the prevalence of COPD in patients with PAD, as well as the prevalence of PAD in COPD patients confirmed in all patients by two reliable methods: spirometry and ankle-brachial index (ABI), respectively. The MEDLINE, EMBASE, and Cochrane Database of Systematic Reviews were searched to identify the potentially eligible publications from the previous 10 years. The published characteristics of different PAD and COPD populations were analyzed. A database search identified 894 records. Reliable criteria of both COPD and PAD diagnosis were used only in seven publications. The prevalence of PAD among patients with COPD ranged from 8.5 to 81.4%. The severity of the disease and the exclusion of nonsmokers or symptomatic patients from the analyses were important factors affecting this parameter. The prevalence of COPD in patients with PAD was measured reliably only in one study and assessed as 27.2%. The comorbidity of COPD and PAD is a relatively common occurrence. There are very few publications addressing this issue based on reliable diagnostic criteria, especially in the field of PAD. In the case of COPD and PAD patients, spirometry and ABI measurements are worth considering as noninvasive screening tests for COPD and PAD, respectively.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disorder characterized by persistent respiratory symptoms and airflow obstruction. In COPD, chronic inflammation is a key component of the pathophysiology and causes structural changes leading to the narrowing of the small bronchi. This airway obstruction can be detected noninvasively by spirometry [Citation1].
Peripheral artery disease (PAD) is a condition in which the supply of oxygen to the tissues of the lower limbs is insufficient due to chronically impaired blood flow in the arteries of the lower extremities. In most cases, the cause of PAD is atherosclerosis with inflammation being an important factor in the atherosclerotic pathomechanism [Citation2]. Narrowness of the arteries can be demonstrated easily by an ankle-brachial index (ABI) measurement [Citation3].
Cigarette smoking is undoubtedly one of the strongest risk factors for both COPD [1] and atherosclerosis [Citation4]. The inflammation observed in the airways of COPD patients appears to be a modified inflammatory response of the respiratory tract to irritants, mainly to cigarette smoke components. This leads to pathological changes, including irreversible airway obstruction [Citation1]. Tobacco smoking also affects endothelial function, enhancing oxidative processes, and inflammation in arteries [Citation5]. As a consequence, cigarette smoking promotes the development of atherosclerosis and PAD.
COPD and PAD relatively often coexist due to common risk factor, generalized character of these diseases and the chronic inflammatory process in their pathophysiology. This phenomenon is quite frequent in clinical practice, although PAD is often asymptomatic. Intermittent claudication is one of the complaints reported by patients with COPD. On the other hand, patients with PAD not infrequently report chronic dyspnea, one of the symptoms of COPD. The early symptoms of both diseases are triggered by physical effort. Because COPD and PAD significantly limit exercise capacity, the coexisting disease is very often masked [Citation6]. This fact indicates the difficulty that exists in diagnosing both diseases. There are available simple-to-use and reliable noninvasive tests, spirometry, and ABI measurement, which make it possible to recognize a co-existing disease at an early stage. Despite this, the frequency of PAD and COPD coexistence has not been thoroughly investigated.
Objectives (review question)
The main goal of this systematic review was to present a reliable assessment of the prevalence of COPD in patients with PAD, as well as the prevalence of PAD in COPD patients, based on the results of the studies using exclusively spirometry and ABI measurement in diagnostics of COPD and PAD, respectively. An additional objective of this research was to identify the main factors influencing these prevalences.
Methods
To summarize the evidence from multiple studies, a systematic review of the published literature was conducted in line with the recommendations of the PRISMA Statement [Citation7].
Eligibility criteria
To be eligible for inclusion, publications should have reported COPD or PAD prevalence in patients with PAD or COPD, respectively. To be included, publications should have reported the use of spirometry and ABI measurement as diagnostic methods to confirm COPD and PAD diagnosis, respectively, in all participants. Only studies on human subjects published in the English language were included.
Searching
We searched databases for articles indexed from 2007 to 2017 using keywords – MeSH and Emtree terms relating to PAD and COPD in the relevant databases (see Appendix 1). To ensure comprehensive coverage of the literature, the MEDLINE, EMBASE, and Cochrane Database of Systematic Reviews were searched concurrently.
Screening
Two reviewers worked independently and disagreements were resolved through a consensus reached with a third reviewer. Duplicates were excluded from initial references. The publications, that fulfilled eligibility criteria, were analyzed qualitatively and quantitatively.
Data extraction
The data were collected using a standardized data extraction form containing the detailed information categories regarding the analyzed studies (see Appendix 3). The data from each publication were independently extracted by two reviewers. Any discrepancies in the data extracted were resolved via discussion or adjudication by a third reviewer if necessary.
The principal summary measure was the percentage of COPD patients among patients with PAD, and the percentage of patients with PAD among subjects suffering from COPD.
The risk of bias assessment
To assess the methodological quality of the studies selected for the summary review and to determine the extent to which these studies have addressed the possibility of bias in their design, approach, and analysis, we used the Joanna Brigs Institute (JBI) critical appraisal tools dedicated specifically to different study types [Citation8]. The publications were subjected to rigorous appraisal by two critical appraisers and disagreements were resolved through discussion or by establishing a consensus with a third appraiser.
Results
Search results
The database search identified 894 records. After removing duplicates, we screened the titles and abstracts of 852 publications. At this stage, we excluded from further analysis of 781 publications, which did not cover populations of subjects with COPD and PAD diagnoses. We assessed the 71 remaining full-text articles for eligibility. Then we excluded 31 full-text articles because they did not report the prevalence of PAD in a studied COPD group or COPD prevalence in a PAD group. We included 40 publications in our qualitative analysis. Finally, after checking the diagnostic in our final summary synthesis we included seven publications in which spirometry and ABI measurement were used in all patients to confirm a diagnosis of COPD and PAD, respectively ().
Figure 1. Flow chart [Citation7] presenting the study selection process.
![Figure 1. Flow chart [Citation7] presenting the study selection process.](/cms/asset/9d21cd10-7fe5-4311-8f89-658d00ae5354/icop_a_1653271_f0001_c.jpg)
Qualitative synthesis of articles
Of the 40 publications selected, 33 were articles and 7 conference reports. Among these 40 studies, we identified 26 cross-sectional studies, 9 cohort studies, and 5 case–control studies. We did not find any systematic review referring to COPD and PAD prevalence. Although inclusion criteria were described in all 40 studies, only in 22 of these publications were exclusion criteria reported. PAD diagnosis was made exclusively on the basis of ABI measurements in 14 studies, and COPD diagnosis was confirmed based on spirometrical measurements in 19 studies. Among them, the lower limit of normal for FEV1/FVC ratio (<5th percentile) was used as the spirometrical criterion of COPD in two studies [Citation9,Citation10]. In other 17 studies, COPD diagnosis was confirmed by pulmonary function test in line with the GOLD Report [Citation1]. The publications with the use of other, than ABI and spirometry, diagnostic methods in all patients, have been excluded from the final analysis. Both diseases – PAD by ABI measurement and COPD by spirometry – were objectively confirmed in seven studies, and these publications were included in the final summary synthesis ( and Citation2).
Table 1. Studies of COPD patients.
Final synthesis of articles
Six of the papers selected for the final analysis were dedicated to COPD patients. The prevalence of PAD in the studies ranged from 8.5% to 81.4%. In all these studies, the same standard criteria (based on spirometry and ABI measurement) were used to confirm COPD and diagnose PAD. In four of the six studies, COPD severity was reported that showed differences between studies in this confounder. The studied COPD populations were also not homogeneous due to the exclusion of symptomatic PAD patients from two studies and the inclusion of smokers alone in two studies. Differences between the populations of other analyzed studies in terms of tobacco smoking status were also noticed. The original populations from which the study subjects were recruited, if reported, were as follows: patients hospitalized in a pulmonology department, an internal medicine department, a hospital as a whole and patients consulted in an outpatients’ clinic ambulatory ().
Only one publication based on ABI and spirometrical criterion was conducted on PAD patients. The goal of this study was to determine the prevalence of COPD in patients with cerebral and/or peripheral artery disease. After excluding patients with restrictive ventilatory pattern defined as total lung capacity <80% of a predicted value, the prevalence of COPD in PAD patients was 27.2% ().
Table 2. Study of PAD patients.
Characteristics of the patients with coexisting COPD and PAD
Regarding the characteristics of the subjects suffering from both diseases (PAD and COPD), it was presented in five publications [Citation12,Citation13,Citation15–17]. On the basis of the data presented there, it was possible to describe the average profile of such a patient and thus to indicate the main potential risk factors of COPD and PAD coexistence. The total number of COPD-PAD patients was 345. The mean age of the subjects was 70.68 (±8.7) years. Men constituted 74.78% of this group (n = 258). Most of the respondents were tobacco smokers (93.91%) who were smoking in the past (64.34%) or still (29.56%). Only 6.09% of the patients reported abstinence from smoking during their lifetime. Hypertension was found in 243 (70.43%) and diabetes in 75 (23.36%) of subjects. Patients with both diseases in comparison with patients with COPD alone were characterized by higher rate of male sex, tobacco smokers, occurrence of arterial hypertension, hyperlipidemia, increased: SCORE (Systematic COronary Risk Evaluation) index, blood pressure level, fibrinogen and C-reactive protein (CRP) serum concentrations, more severe COPD and more intensive dyspnea perception, as well as lower: FEV1 and DLCO (diffusing capacity of the lung for carbon monoxide) [Citation12,Citation13,Citation16]. Patients with both diseases in comparison with patients with PAD alone were characterized by higher rate of diabetes mellitus, increased IL-8 serum concentration, higher number of pack-years, elevated peripheral neutrophil percentage, lower peripheral lymphocyte percentage, and erythrocyte level [Citation17].
Discussion
In epidemiology, to determine the prevalence of a disease, it is important to use the reliable diagnostic criteria of an analyzed disease entity. To compare the results of different studies in terms of disease prevalence, selecting publications that report the use of the same diagnostic criteria is crucial. The criteria should be objective, standardized, and used in a valid and reliable way. Only studies in which diagnoses of PAD and COPD had been confirmed by methods recommended by current standards [Citation1,Citation3], which are feasible in clinical practice, were included in the final summary analysis.
Although PAD may be confirmed by Doppler ultrasound or computed tomography, only publications reporting the use of ABI parameter were included into our review, which is also recommended in international PAD guidelines [Citation3]. From a practical point of view, the important features of ABI measurement are simplicity and repeatability. Furthermore, the ABI measurement is noninvasive. Therefore, it can be used in the screening of PAD. ABI makes it possible to diagnose PAD at a very early stage when the disease is completely asymptomatic. Two-thirds of COPD patients with confirmed PAD do not report PAD, thus implying the asymptomatic character of the disease and/or its underdiagnosis [Citation18]. Knowledge about the existence of the disease makes it possible to administer treatment that not only impedes the progression of PAD but also reduces the risk of fatal cardiovascular (CV) incidents, such as myocardial infarction and stroke. This is especially important in patients suffering from COPD, because the generalized inflammatory process observed in COPD promotes the progression of atherosclerosis. Moreover, COPD exacerbations significantly increase the risk of acute CV manifestations, such as myocardial infarction, and also the risk of death [Citation19].
Different criteria are employed in numerous studies to diagnose COPD. Some of these are based on the patient’s medical history or questionnaires. In many studies, only information from large medical digital databases is used to qualify patients into a COPD group. The pulmonary function test should be performed to make a COPD diagnosis more objective. Spirometry is a test designed to prove the presence of irreversible airway obstruction, i.e. one of the attributes of COPD. There are differences between national and international guidelines and standards in terms of the spirometric criterion for COPD. In some of them, the lower limit of normal for FEV1/FVC ratio (<5th percentile) is used [Citation20,Citation21]. The rest [Citation1,Citation22], among them GOLD Report, take into account spirometry proving FEV1/FVC < 0.7. In all the studies included for the final analysis, diagnosis of COPD was confirmed in line with the current GOLD Report [Citation1], although this approach may cause misdiagnosis. We must be aware that the ratio decreases with age and using GOLD criteria may cause the overestimation of COPD prevalence in elderly [Citation21,Citation23]. The studies using the 5th percentile [Citation9,Citation10] were earlier excluded from the analysis because they have not met one of the inclusion criterion, which was the use of ABI measurement to confirm PAD in all participants.
Only 15% of assessed publications reported using the assumed criteria for diagnosing COPD and PAD simultaneously. The other papers reported the following diagnostic tools for PAD: information from medical digital record, the patient’s medical history, ultrasound, or computed tomography. In the case of those papers dedicated to COPD patients the diagnosis was based on the following: information from medical digital record, the patient’s medical history, or spirometry results alone (not referred to the patient’s medical history). The authors of some of the analyzed studies did not even report the diagnostic method they used. To make a reliable comparison of the results of different studies, we took into account only those studies reporting the use of the same diagnostic methods, that are objective, simple, reliable and easy to be used in screening. That is why only 7 of the 40 studies were actually qualified for the final analysis.
The risk factors of COPD and PAD coexistence may be not only smoking status, but also advanced age, male sex, other comorbidities such as e.g. hypertension, hyperlipidemia or diabetes mellitus, functional impairment of respiratory system, and also increased serum concentrations of inflammatory markers, such as fibrinogen, CRP, and neutrophil percentage in blood. The elevated levels of the markers indicate the important role of generalized inflammation in the pathophysiology of both analyzed diseases. The heterogeneity of the studied populations in terms of above factors can explain the observation, that the range of PAD prevalence between the different studies included in the review’s final analysis was relatively wide. The studied populations differed also in terms of other features, including disease severity and the place of recruitment, which is important confounder because the type of health care unit can significantly determine the profile of the recruited patients. The heterogeneity of the populations and no enough detailed data (presented partially only in 5 of 40 studies) made it impossible to analyze statistically the significance of the proposed above potential risk factors.
Taking into account the risk of bias in the publications that qualified for the final analysis, we used JBI tools [Citation8] intended for the critique or appraisal of the research evidence. The risk of bias according to JBI tools was largely assessed as low (see Appendix 2).
Because only one study focused on COPD prevalence among PAD patients, when spirometry as a diagnostic tool was used, there is a need for more research on this group of patients specifically with the aim of providing a precise characterization of the comorbidities. If the significant prevalence of COPD in this group of patients, as noted in the abovementioned study, is confirmed in future studies, spirometry should be considered as a tool for COPD screening in all PAD patients.
Conclusions
Based on the results of six papers selected for the final analysis, the prevalence of PAD in the COPD population was assessed as significantly high, ranging from 8.5 to 81.4%. Only in one study the prevalence of COPD in PAD patients was verified by reliable criterion and assessed as 27.2%.
In our review, we found that the co-existence of COPD and PAD is a common occurrence and greatly depends on the characteristics of the group being studied, with the potential influence of factors such as e.g. age, sex, tobacco smoking status, or other comorbidities. The most credible epidemiological surveys are those based on reliable diagnostic criteria for PAD and COPD. To make the results of the studies more representative, the inclusion criteria for such studies should be as broad as possible to cover a wide spectrum of manifestations of the disease, without excluding from the analyzed group (COPD or PAD) any subgroup of patients, for example, nonsmokers, asthmatics, or symptomatic PAD patients. More well-planned studies are needed, especially those conducted on PAD populations.
Disclosure of interest
The authors report no conflict of interest.
Supplemental Material
Download Zip (603.2 KB)References
- Global Initiative for Chronic Obstructive Lung Disease Inc. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2018 report. 2018. Available from: http://www.goldcopd.org
- Januszek R, Mika P, Konik A, et al. The effect of treadmill training on endothelial function and walking abilities in patients with peripheral arterial disease. J Cardiol. 2014;64:145–151. doi:10.1016/j.jjcc.2013.12.002.
- Aboyans V, Ricco J-B, Bartelink M-L, et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. doi:10.1093/eurheartj/ehx095.
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J.. 2016;37:2315–2381. doi:10.1093/eurheartj/ehw106.
- Wozniak K, Sleszycka J, Safianowska A, et al. Systemic inflammation in peripheral arterial disease with or without coexistent chronic obstructive pulmonary disease: analysis of selected markers. Arch Med Sci. 2012;8:477–483. doi:10.5114/aoms.2012.29525.
- Wilke S, Watz H, Bals R, et al. Impact of peripheral artery disease on functional capacity and health status in patients with chronic obstructive pulmonary disease: results of the COSYCONET study. Am J Respir Crit Care Med. 2015;191:A6204.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi:10.7326/0003-4819-151-4-200908180-00135.
- Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute, 2017. Available from: https://reviewersmanual.joannabriggs.org/
- Tschopp J, Dumont P, Hayoz D. True prevalence of COPD and its association with peripheral arterial disease in the internal medicine ward of a tertiary care hospital. Swiss Med Wkly. 2017;147:w14460. doi:10.4414/smw.2017.14460.
- Sleszycka J, Wozniak K, Banaszek M, et al. Prevalence and difficulties in chronic obstructive pulmonary disease diagnosis in patients suffering from severe peripheral arterial disease. Pol Merkur Lekarski. 2009;27:92–96.
- Castagna O, Boussuges A, Nussbaum E, et al. Peripheral arterial disease: an underestimated aetiology of exercise intolerance in chronic obstructive pulmonary disease patients. Eur J Cardiovasc Prev Rehabil. 2008;15:270–277. doi:10.1097/HJR.0b013e3282f009a9.
- Pecci R, De La Fuente Aguado J, Sanjurjo Rivo AB, et al. Peripheral arterial disease in patients with chronic obstructive pulmonary disease. Int Angiol. 2012;31:444–453.
- Chen W, Lin M-S, Sun K-S, et al. Is asymptomatic peripheral arterial disease associated with walking endurance in patients with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:1487–1492.
- Pizarro C, Linnhoff F, van Essen F, et al. Lower extremity and carotid artery disease in COPD. ERJ Open Res. 2016;2:00037–02016. doi:10.1183/23120541.00037-2016.
- Houben-Wilke S, Jörres RA, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am J Respir Crit Care Med. 2017;195:189–197. doi:10.1164/rccm.201602-0354OC.
- Crisafulli E, Scelfo C, Tzani P, et al. Asymptomatic peripheral artery disease can limit maximal exercise capacity in chronic obstructive pulmonary disease patients regardless of airflow obstruction and lung hyperinflation. Eur J Prev Cardiolog. 2017;24:990–999. doi:10.1177/2047487317695629.
- Tuleta I, Farrag T, Busse L, et al. High prevalence of COPD in atherosclerosis patients. Int J Chron Obstruct Pulmon Dis. 2017;12:3047–3053. doi:10.2147/COPD.S141988.
- Brusselle G, Bracke K, De Pauw M. Peripheral artery disease in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:148–150. doi:10.1164/rccm.201608-1712ED.
- Finkelstein J, Cha E, Scharf SM. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis. 2009;4:337–349.
- Śliwiński P, Górecka D, Jassem E, et al. [Polish respiratory society guidelines for chronic obstructive pulmonary disease]. Pneumonol Alergol Pol. 2014;82:227–263.
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi:10.1183/09031936.05.00035205.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051. doi:10.1136/thx.2008.098483.
Appendix 1. Electronic search of the MEDLINE, EMBASE and Cochrane Database of Systematic Reviews – publication date from 1 January 2008 to 31 December 2017
a. MEDLINE
b. EMBASE
c. Cochrane Database of Systematic Reviews
Appendix 2. Risk of bias analysis
A. Risk of bias summary: review of authors’ judgments about each risk of bias question for each study included in the project.
Table
B. Risk of bias graph: review of authors’ judgments regarding each risk of bias question presented as percentages across all studies included in the project.
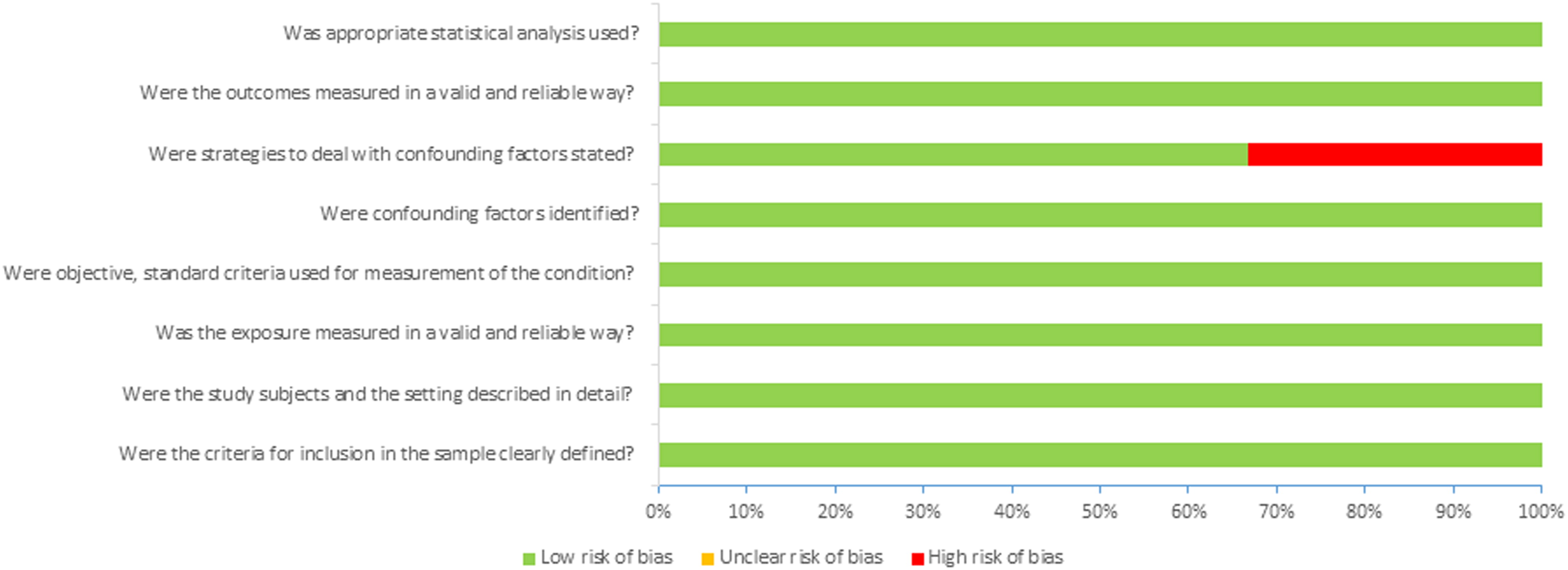
Appendix 2. References
- Castagna O, Boussuges A, Nussbaum E, et al. Peripheral arterial disease: an underestimated aetiology of exercise intolerance in chronic obstructive pulmonary disease patients. Eur J Cardiovasc Prev Rehabil. 2008;15:270–277. doi:10.1097/HJR.0b013e3282f009a9. <!--${if: isGetFTREnabled}-->
- Pecci R, De La Fuente AJ, Sanjurjo Rivo AB, et al. Peripheral arterial disease in patients with chronic obstructive pulmonary disease. Int Angiol. 2012;31:444–453. <!--${if: isGetFTREnabled}--><!--${/if:}--> <!--${ifNot: isGetFTREnabled}--><!--${/ifNot:}--><!--${if: isGetFTREnabled}--><!--${/if:}-->PubMed Web of Science ®<!--${googleScholarLinkReplacer: %00empty%00 journal Peripheral+arterial+disease+in+patients+with+chronic+obstructive+pulmonary+disease author%3DR+Pecci%26author%3DAJ+De+La+Fuente%26author%3DAB+Sanjurjo+Rivo 2012 Pecci+R%2C+De+La+Fuente+AJ%2C+Sanjurjo+Rivo+AB%2C+et%C2%A0al.+Peripheral+arterial+disease+in+patients+with+chronic+obstructive+pulmonary+disease.+Int+Angiol.+2012%3B31%3A444%E2%80%93453. 444-453 Int+Angiol 31 %00null%00 %00empty%00 %00empty%00 %00null%00 getFTREnabled FULL_TEXT %00empty%00}--><!--${sfxLinkReplacer: e_1_3_3_2_3_3_1 %00empty%00 url_ver%3DZ39.88-2004%26rft.genre%3Darticle%26rfr_id%3Dinfo%3Asid%2Fliteratum%253Atandf%26rft.aulast%3DPecci%26rft.aufirst%3DR%26rft.atitle%3DPeripheral%2520arterial%2520disease%2520in%2520patients%2520with%2520chronic%2520obstructive%2520pulmonary%2520disease%26rft.jtitle%3DInt%2520Angiol%26rft.date%3D2012%26rft.volume%3D31%26rft.spage%3D444%26rft.epage%3D453 %00empty%00}-->
- Chen W, Lin M-S, Sun K-S, et al. Is asymptomatic peripheral arterial disease associated with walking endurance in patients with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:1487–1492. <!--${if: isGetFTREnabled}-->
- Pizarro C, Linnhoff F, van Essen F, et al. Lower extremity and carotid artery disease in COPD. ERJ Open Res. 2016;2:00037–02016. doi:10.1183/23120541.00037-2016. <!--${if: isGetFTREnabled}-->
- Houben-Wilke S, Jörres RA, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am J Respir Crit Care Med. 2017;195:189–197. doi:10.1164/rccm.201602-0354OC. <!--${if: isGetFTREnabled}-->
- Crisafulli E, Scelfo C, Tzani P, et al. Asymptomatic peripheral artery disease can limit maximal exercise capacity in chronic obstructive pulmonary disease patients regardless of airflow obstruction and lung hyperinflation. Eur J Prev Cardiolog. 2017;24:990–999. doi:10.1177/2047487317695629. <!--${if: isGetFTREnabled}-->
- Tuleta I, Farrag T, Busse L, et al. High prevalence of COPD in atherosclerosis patients. Int J Chron Obstruct Pulmon Dis.. 2017;12:3047–3053. doi:10.2147/COPD.S141988. <!--${if: isGetFTREnabled}-->
Appendix 3. Characteristics of the analyzed studies.
Appendix 3. References
- Castagna O, Boussuges A, Nussbaum E, et al. Peripheral arterial disease: an underestimated aetiology of exercise intolerance in chronic obstructive pulmonary disease patients. Eur J Cardiovasc Prev Rehabil. 2008;15:270–277. doi:10.1097/HJR.0b013e3282f009a9. <!--${if: isGetFTREnabled}-->
- Pecci R, De La Fuente AJ, Sanjurjo RA, et al. Peripheral arterial disease in patients with chronic obstructive pulmonary disease. Int Angiol. 2012;31:444–453. <!--${if: isGetFTREnabled}--><!--${/if:}--> <!--${ifNot: isGetFTREnabled}--><!--${/ifNot:}--><!--${if: isGetFTREnabled}--><!--${/if:}-->PubMed Web of Science ®<!--${googleScholarLinkReplacer: %00empty%00 journal Peripheral+arterial+disease+in+patients+with+chronic+obstructive+pulmonary+disease author%3DR+Pecci%26author%3DAJ+De+La+Fuente%26author%3DRA+Sanjurjo 2012 Pecci+R%2C+De+La+Fuente+AJ%2C+Sanjurjo+RA%2C+et%C2%A0al.+Peripheral+arterial+disease+in+patients+with+chronic+obstructive+pulmonary+disease.+Int+Angiol.+2012%3B31%3A444%E2%80%93453. 444-453 Int+Angiol 31 %00null%00 %00empty%00 %00empty%00 %00null%00 getFTREnabled FULL_TEXT %00empty%00}--><!--${sfxLinkReplacer: e_1_3_3_3_2_3_1 %00empty%00 url_ver%3DZ39.88-2004%26rft.genre%3Darticle%26rfr_id%3Dinfo%3Asid%2Fliteratum%253Atandf%26rft.aulast%3DPecci%26rft.aufirst%3DR%26rft.atitle%3DPeripheral%2520arterial%2520disease%2520in%2520patients%2520with%2520chronic%2520obstructive%2520pulmonary%2520disease%26rft.jtitle%3DInt%2520Angiol%26rft.date%3D2012%26rft.volume%3D31%26rft.spage%3D444%26rft.epage%3D453 %00empty%00}-->
- Chen W, Lin M-S, Sun K-S, et al. Is asymptomatic peripheral arterial disease associated with walking endurance in patients with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:1487–1492. <!--${if: isGetFTREnabled}-->
- Pizarro C, Linnhoff F, van Essen F, et al. Lower extremity and carotid artery disease in COPD. ERJ Open Res. 2016;2:00037–02016. doi:10.1183/23120541.00037-2016. <!--${if: isGetFTREnabled}-->
- Houben-Wilke S, Jörres RA, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am J Respir Crit Care Med. 2017;195:189–197. doi:10.1164/rccm.201602-0354OC. <!--${if: isGetFTREnabled}-->
- Crisafulli E, Scelfo C, Tzani P, et al. Asymptomatic peripheral artery disease can limit maximal exercise capacity in chronic obstructive pulmonary disease patients regardless of airflow obstruction and lung hyperinflation. Eur J Prev Cardiol. 2017;24:990–999. doi:10.1177/2047487317695629. <!--${if: isGetFTREnabled}-->
- Tuleta I, Farrag T, Busse L, et al. High prevalence of COPD in atherosclerosis patients. Int J Chron Obstruct Pulmon Dis. 2017;12:3047–3053. doi:10.2147/COPD.S141988. <!--${if: isGetFTREnabled}-->
- Lin M-S, Hsu K-Y, Chen Y-J, et al. Prevalence and risk factors of asymptomatic peripheral arterial disease in patients with COPD in Taiwan. PLoS One. 2013;8:e64714. doi:10.1371/journal.pone.0064714. <!--${if: isGetFTREnabled}-->
