?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chronic Obstructive Pulmonary Disease (COPD) is associated with increased inflammatory responses to noxious particles, which can be further enhanced during Acute Exacerbation of COPD (AECOPD). Considering the important immunoregulatory function of vitamin D, high prevalence of Vitamin D Deficiency (VDD) in COPD patients and a negative link between vitamin D levels and inflammatory biomarkers, suggests the seemingly interesting mechanism of vitamin D effects on inflammation resolution during the conventional treatment of AECOPD. The admitted AECOPD patients with VDD were recruited and randomly allocated to receive either 300,000 IU of intramuscular vitamin D (n = 35) or placebo (n = 35). Primary outcomes included inflammation resolution dynamics, which were assessed by monitoring the serum levels of IL-6, IL-8, and hs-CRP. Symptom recovery was evaluated based on the modified Medical Research Council (mMRC) dyspnea scale on the 1st and 6th days of admission. Secondary outcomes included the length of hospital stay (LOS) and 30-day mortality rates. Inflammatory biomarkers were highest at Day 1. Baseline vitamin D levels were 11.25 ± 3.09 and 10.59 ± 3.90 ng/ml (P = 0.45), which reached 11.35 ± 3.16 and 18.17 ± 4.24 by Day 6 (P < 0.001) in the placebo and, vitamin-D groups, respectively. IL-6 levels significantly decreased in the vitamin-D vs. placebo group on the 6th day (P = 0.02); however, no significant differences were observed in IL-8 (P = 0.15) and hs-CRP (P = 0.24) levels, mMRC scale (P = 0.45), LOS (P = 0.20), and mortality rates (P = 0.61). Vitamin D replacement as adjunctive therapy may accelerate inflammation resolution in hospitalized AECOPD patients. Further studies were needed to establish vitamin D exact role on inflammation resolution in AECOPD.
Introduction
According to the Global Initiative on Obstructive Lung Disease (GOLD) guidelines for Chronic Obstructive Pulmonary Disease (COPD), COPD is a common preventable and treatable disease, which is associated with enhanced chronic inflammatory responses in the airways and the lung to noxious particles or gases [Citation1]. The prevalence and burden of COPD as a third leading cause of deaths are substantially increasing, and the majority of COPD burden is attributed to the disease exacerbations and hospitalizations [Citation2]. During Acute Exacerbation of COPD (AECOPD), several mechanisms precipitate chronic inflammation. A further increase in inflammation deteriorates lung function, impairs the involved patients’ quality of life, and enhances mortality rates and COPD socio-economic costs [Citation3,Citation4].
Considering the high prevalence of Vitamin D Deficiency (VDD) in COPD patients, especially those with COPD exacerbation [Citation5], the negative link between vitamin D levels and inflammatory components such as C-Reactive Protein (CRP), interleukin 6 (IL-6), and interleukin 8 (IL-8) [Citation6–8], together with the important immune-regulatory function of vitamin D in human bronchial epithelial cells [Citation9], have suggested the seemingly interesting mechanism of vitamin D affecting inflammation resolution during the conventional treatment of AECOPD. To the best of our knowledge, no research on vitamin D effects on inflammation resolution in AECOPD patients has been reported so far. To fill this gap, we assessed the potential effects of the rapid normalization of vitamin D levels on the dynamics of inflammation resolution by using a safe and efficient high dose of vitamin D (300,000 IU) [Citation10], in AECOPD patients with concurrent VDD.
Materials and methods
Study design and participants
The study was designed as a randomized, double-blind placebo-controlled trial and conducted at the referral teaching hospital located in Shahid Beheshti University of Medical Sciences, Tehran, Iran, from November 2017 to January 2018. It was registered in the Iranian Registry of Clinical Trials (IRCT 2016031425726N4) and approved by the University Ethics committee. Seventy patients (35 participants in each group) with the COPD stages of II-IV according to the GOLD report (post-bronchodilator ratio of <0.7 and FEV1 < 80% predicted) entered the study. The participants were included based on AECOPD diagnosis and the inclusion criteria of having VDD (serum 25(OH) vitamin D level of <20 ng/ml) and an age of 40 years. The exclusion criteria were as follows: using a maintenance dose of oral corticosteroids in the last 3 months and having a serum 25(OH) vitamin D level of <5 ng/ml, 3 months of vitamin D supplementation before admission, a history of asthma, osteoporosis, renal failure (serum creatinine level of ≥2.5), nephrolithiasis, uncompensated liver failure (Child-Pough classes of B and C), hypercalcemia (ionized calcium level of >1.3 mmol/L), conditions associated with pathological 1-alpha hydroxylase activity, such as sarcoidosis, lymphoma, multiple myeloma, pregnancy and lactation, immuno-compromised states, and coagulopathy (platelet count of <30,000 per mm3 or international normalized ratio of >3 as a contraindication for intramuscular administration). Informed consent was obtained from all the participants before enrollment.
Randomization and treatment design
A permuted (balanced)-block randomization design was utilized for the treatment allocations in this trial. The patients were randomly allocated to receive 300,000 IU of vitamin D (25-hydroxycholecalciferol) (Daropakhsh-Iran) or placebo as a single intramuscular injection. Both groups received a standard treatment procedure as follows: 1) short-acting inhaled beta2-agonists with or without a short-acting inhaled anticholinergic bronchodilator; 2) a dose of 40 mg of prednisone per day for 5 days or nebulizer budesonide; and 3) antibiotics for 5–10 days in the case of having the three cardinal symptoms of increased dyspnea, sputum volume, and sputum purulence or increased sputum purulence associated with one of the other two symptoms or requiring mechanical ventilation according to the GOLD report [Citation1].
Study outcomes
The primary outcomes included systemic inflammation resolution and symptom recovery, which were assessed by monitoring the serum levels of IL-6, IL-8, and hs-CRP and based on the modified Medical Research Council (mMRC) dyspnea scale, respectively. The secondary end-points were Length Of hospital Stay (LOS) and 30-day mortality rates.
Sample collection and measurements
Peripheral venous blood (5 mL) was collected into a vacutainer tube and centrifuged at 3,000 rpm for 10 min. The isolated serum was stored at −70 °C until the final analysis. The serum levels of IL-6 (Human IL6, DuoSet®, R&D Systems®, USA), IL-8 (Human CXCL8/IL8DuoSet®, R&D Systems®, USA), and hs-CRP (Human hs-CRP Diclone, Germany) were quantified using commercial sandwich ELISA kits.
The levels of systemic inflammatory biomarkers (IL-6, IL-8, and hs-CRP) and symptom recovery (mMRC dyspnea score) were measured at the baseline on the day of admission (Day 1) prior to vitamin D administration and the 6th day of AECOPD conventional treatment, respectively. Vitamin D, phosphor, and calcium levels were also measured at the mentioned time points. The patients' demographic and medical histories, including age, gender, exposure to such risk factors as smoking, occupational or environmental exposure, disease exacerbation and subsequent hospitalization for a respiratory disorder, presence of comorbidity, and any relevant paraclinical or laboratory findings, were also collected for further analysis.
Statistical analysis
Data analysis was conducted with IBM SPSS Statistics v 21.0 software. Continuous variables were assessed for normality by “Kolmogorov-Smirnov” test, and according to its results, t-test (normal distribution) or Mann–Whitney U-test (non-normal distribution) was used for between group's comparisons. Continuous variables expressed as Mean ± Standard Deviation (SD) or median (interquartile range) when appropriate. Categorical and nominal variables were expressed as frequency (%) and were analyzed using the Chi-square test or Fisher's-exact test. Pearson and Spearman's correlations were applied to assess the association between two variables as appropriate. P-values <0.05 were considered statistically significant.
Results and analyses
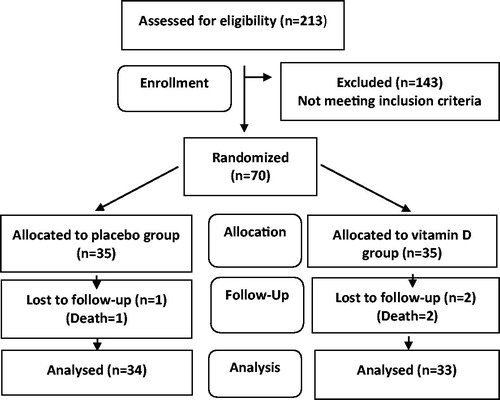
Out of 213 patients assessed for eligibility, 85 patients met the primary criteria. They were evaluated for vitamin D level. A total of 6 and 9 patients with the serum 25(OH) vitamin D levels of less and more than 5 and 20 ng/ml were excluded from the study, respectively. Therefore, 70 patients with VDD were equally allocated to the mentioned study groups. Finally, 33 and 34 patients in the vitamin-D and placebo groups accomplished the clinical trials, respectively ()
The baseline characteristics of the 67 patients included in the placebo and vitamin-D groups are shown in . There were no statistically significant differences between the groups in terms of the mentioned baseline variables.
Table 1. Demographic and baseline characteristics of the recruited patients.
Changes in vitamin D levels
At the baseline, the Mean ± SD values of the serum 25(OH) vitamin D levels were 11.25 ± 3.09 and 10.59 ± 3.90 ng/ml in the placebo and vitamin-D groups, respectively, The baseline vitamin D levels were not significantly different between the two groups (P = 0.45).
There is moderate correlation between baseline vitamin D level with disease severity (FEV1 value) (r= −0.37, P < 0.01), hs-CRP (r= −0.32, P = 0.01), IL6 (r= −0.14, P = 0.25) and IL8(r= −0.10, P = 0.59) in the study groups.
The vitamin D levels reached 11.35 ± 3.16 and 18.17 ± 4.24 in the placebo and vitamin-D groups by Day 6, respectively (P < 0.01).
Primary outcomes
Changes in the levels of the inflammatory biomarkers
The baseline levels of the inflammatory biomarkers were similar in the placebo and vitamin-D groups (). The systemic inflammatory biomarkers were at their highest levels on the day of admission (Day 1), while their levels significantly reduced during the conventional treatment of AECOPD in both groups by Day 6 ().
Table 2. Levels of systemic inflammatory biomarkers at baseline and day 6 in the study groups.
The median (interquartile range) and Mean ± SD differences in the levels of the systemic biomarkers occurring from the baseline through the 6th day and comparisons of the changes between the groups are given in . IL-6 level significantly lowered in the vitamin-D compared to the placebo group (P = 0.02), but no statistically significant differences in IL-8 (P = 0.15) and hs-CRP (P = 0.24) levels were observed by Day 6.
Table 3. Changes in the levels of the systemic biomarkers from the baseline through the 6th day and comparisons of changes between the groups.
Notably, a significant correlation was found between the change of the vitamin D levels and the degree of decrease in the level of inflammatory biomarkers [IL-6 (r = 0.44, P = 0.01), IL-8 (r = 0.40, P = 0.03), and hs-CRP (r = 0.56, P < 0.01)], in the vitamin-D group by Day 6.
Changes in dyspnea severity scale (mMRC)
The Mean ± SD values of the baseline mMRC scores were 3.71 ± 0.46 and 3.79 ± 0.42 in the placebo and vitamin-D groups, respectively (P = 0.44). The Mean ± SD values of mMRC scores for the changes occurring from the baseline through the 6th day were 0.82 ± 0.81 and 0.97 ± 0.86 in the placebo and vitamin-D groups, respectively. Therefore, no statistically significant differences were seen between the groups [P = 0.45].
Secondary outcomes
The Mean ± SD values for LOS were 8.09 ± 3.38 and 7.48 ± 3.02 days in the placebo and vitamin-D groups, respectively, thus indicating no significant differences between the groups (P = 0.20). The mortality rates were 1 and 2 patients in the placebo and vitamin-D groups, respectively, which were not significantly different between the groups (P = 0.61).
Discussion
Despite well-established role of Inflammation in COPD pathogenesis and significant anti-inflammatory effect of vitamin D in several hyper-inflammatory disease states [Citation4,Citation11,Citation12], studies on vitamin D supplementation in COPD patients have mostly focused on clinical outcomes with promising results such as improved lung function, health-related quality of life, exercise capacity, and dyspnea scale and reduced numbers of COPD exacerbation specially in patients with concurrent VDD [Citation13–16].
Previous research regarding the systemic inflammatory biomarkers, on COPD patients, has shown a significant elevation in the levels of inflammatory biomarkers (CRP, IL-6, and IL-8) with further inflammation during the disease exacerbation and in patients with VDD [Citation17–Citation19]. Additionally, a significant association between the levels of the mentioned inflammatory biomarkers and COPD severity (FEV1 value) has been reported in several studies on COPD patients as well [Citation20–22]. Following the previous finding, our results revealed a high prevalence of VDD (89%) and a significant correlation between vitamin D level and COPD severity (FEV1) (r= −0.37, P < 0.01) and hs-CRP (r= −0.32, P = 0.01).
In the study by Chang et al. on hospital admitted AECOPD patients, highest inflammation (serum levels of IL-8, CRP) was reported on day of admission which significantly reduced during conventional treatment of AECOPD, by day 4, consistent with clinical improvement in lung function and dyspnea scale [Citation17].
In the present study, in accordance with previous finding [Citation17], the serum levels of inflammatory biomarkers, including IL-6, IL-8, and hs-CRP were highest at the day of admission (), while significantly declining in both groups during the treatment up to the 6th day ().
Despite vitamin D supplementation on highly inflammatory conditions (infantile acute congestive heart failure, multiple sclerosis, inflammatory bowel disease, cystic fibrosis, systemic lupus erythematous, active tuberculosis, and evolving myocardial infarction) in 6 out of 7 clinical trials, significant reduction in inflammation was reported [Citation11]. Moreover, significant alleviation of inflammation by vitamin D was detected in animal models of COPD, by decreasing the serum levels of inflammatory biomarkers (Tumor necrosis factor-α (TNF-α) and interleukin 1) , and increasing anti-inflammatory cytokines (interleukin -10 (IL-10)) [Citation23]. To the best of our knowledge, no study has aimed at exploring vitamin D effects on the dynamics of inflammation resolution during conventional treatment of AECOPD. Only one study by Rezk et al. in stable COPD patients, reported statistically significant decreases in the levels of CRP in the vitamin-D vs. placebo group after one year of supplementation [Citation15].
Nonetheless, in the present research, vitamin D replacement with safe and efficient high dose of vitamin D (300,000 IU) [Citation10] resulted in rapid normalization of vitamin D levels and acceleration of systemic inflammation resolution as detected through the reductions of the serum levels of inflammatory biomarkers [hs-CRP (P = 0.24), IL-6 (P = 0.02), and IL-8 (P = 0.15)], however, only IL-6 levels displayed statistically significant difference in between groups compared.
Interestingly, the increases in vitamin D levels and decreases in the inflammatory biomarkers by Day 6 were statistically significantly correlated in the vitamin-D group [IL-6 (r = 0.44, P = 0.01), IL-8 (r = 0.40, P = 0.03), and hs-CRP (r = 0.56, P < 0.01)].
Increased inflammation in COPD patients negatively affected disease progression and exacerbation and could worsen comorbidities, such as cardiovascular diseases, diabetes, and osteoporosis [Citation12].
Augmented inflammation in COPD characterized by increased numbers of different immune cells both innate immunity (neutrophils, macrophages, eosinophils, mast cells, natural killer cells, innate lymphoid cells, and dendritic cells) and adaptive immunity (T and B lymphocytes), and caused the elevated levels of pro-inflammatory mediators including cytokines (TNF- α), chemokines (IL6, IL-8) , growth factors, lipid mediators (prostaglandin and leukotriene levels), as well as enhanced oxidative stress and protease–antiprotease imbalance [Citation12,Citation17,Citation18]. Several recent studies may explain vitamin D effect on the acceleration of inflammation resolution in AECOPD patients, however further studies are needed to clarify the exact mechanisms. In a study by Pfeffer et al. in human bronchial epithelial cells (HBEC) vitamin D suppressed the expression of a subset of particulate matter-induced genes, including IL-6, IL-8 and increased expression of the antioxidant pathway gene (glucose-6-phosphate dehydrogenase) [Citation9].
In other study by Dimeloe et al. vitamin D induced α-1-antitrypsin synthesis by CD4+ T cells,which regulated T cell function, increased anti-inflammatory cytokines (IL-10) besides its antiprotease activity [Citation24].
In the recent published study by Zhou et al. vitamin D suppressed the inflammatory response in lipopolysaccharide (LPS) induced by vascular smooth muscle cells through p38 MAPK signaling pathway via inhibition the significant up-regulation of cyclooxygenase-2, prostaglandin E, TNF-α and IL-6 and p38 phosphorylation [Citation25]. In summary, essential immunoregulatory functions of vitamin D in different immune cells (differentiation and proliferation), several signal pathways, expressions of several pro-inflammatory cytokines, increased numbers of antioxidant pathway genes and protective role of vitamin D against tissue injury in the lungs in response to oxidative stress, could explain our result in AECOPD patient with concurrent VDD [Citation9,Citation23–26].
Study limitations
This study had certain limitations. Firstly, since we chose to focus on vitamin D effects on AECOPD patients with VDD, our results could not be generalized to all AECOPD patients. Secondly, our relatively short duration of monitoring changes might have been inadequate for evaluating the maximum effects of vitamin D on the inflammation resolutions and clinical outcome improvements, including symptom recoveries and mortality rates. We recommend that further studies will be needed with larger sample sizes, longer follow-up durations, and more extensive profiles of inflammatory cytokines via both systemic and airway (sputum and Bronchoalveolar lavage) inflammatory biomarkers so as to substantiate vitamin D effects on inflammation resolution and provide an adjunctive therapy for AECOPD patients with deficient vitamin D levels.
Conclusion
The results of the current investigation revealed significantly alleviated systemic inflammations during the conventional treatments of AECOPD patients. Vitamin D replacement may be an effective intervention to accelerate inflammation resolution. Nevertheless, further studies with larger sample size and longer duration were required to establish the precise effects of vitamin D replacement on systemic inflammation and clinical outcomes in AECOPD.
Declaration of interest
The authors declare that they do not have conflicts of interest related to the content of this manuscript.
Acknowledgments
We greatly thank all the study participants for taking part in this investigation. We appreciate the medical team for their cooperation and assistance as well.
Additional information
Funding
References
- Global initiative of chronic obstructive lung disease: global strategy for the diagnosis and management and prevention of chronic obstructive lung disease. 2017. Available from: https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/
- Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom. 2018;3:e4.doi:10.1017/gheg.2018.1.
- Perng DW, Chen PK. The relationship between airway inflammation and exacerbation in chronic obstructive pulmonary disease. Tuberc Respir Dis. 2017;80(4):325–335. doi:10.4046/trd.2017.0085.
- Comes A, Ianoşi ES, Jimborean G. Inflammatory biomarkers in chronic obstructive pulmonary disease. J. Interdiscip Med. 2016;1(1):12–17. doi:10.1515/jim-2016-0004.
- Zhu M, Wang T, Wang C, et al. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:2597–2607. doi:10.2147/COPD.S101382.
- Laird E, McNulty H, Ward M, et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J Clin Endocrinol Metab. 2014;99(5):1807–1815. doi:10.1210/jc.2013-3507.
- Gonçalves de Carvalho CMR, Ribeiro S. Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr. 2017;71(4):434–440. doi:10.1038/ejcn.2016.177.
- Herr C, Greulich T, Koczulla RA, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12(1):31–31. doi:10.1186/1465-9921-12-31.
- Pfeffer PE, Lu H, Mann EH, et al. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS One. 2018;13(8):e0200040. doi:10.1371/journal.pone.0200040.
- McNally JD, Iliriani K, Pojsupap S, et al. Rapid normalization of vitamin D levels: a meta-analysis. Pediatrics. 2015;135(1):e152. doi:10.1542/peds.2014-1703.
- Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermatoendocrinology. 2014;6(1):e983401. doi:10.4161/19381980.2014.983401.
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi:10.1016/j.jaci.2016.05.011.
- Zendedel A, Gholami M, Anbari K, et al. Effects of vitamin D intake on FEV1 and COPD exacerbation: a randomized clinical trial study. Glob J Health Sci. 2015;7(4):243–248. doi:10.5539/gjhs.v7n4p243.
- Pourrashid MH, Dastan F, Salamzadeh J, et al. Role of vitamin D replacement on health related quality of life in hospitalized patients with “acute exacerbation of chronic obstructive pulmonary disease. Iran J Pharm Res. 2018;17(2):801–810.
- Rezk N, Aly NYA, Hewidy A. Effect of vitamin D replacement in chronic obstructive pulmonary disease patients with vitamin D deficiency. Egypt J Chest Dis Tuberc. 2015;64(2):353–357. doi:10.1016/j.ejcdt.2015.01.002.
- Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019;74(4):337–345. doi:10.1136/thoraxjnl-2018-212092.
- Chang C, Guo Z, Shen N, et al. Dynamics of inflammation resolution and symptom recovery during AECOPD treatment. Sci Rep. 2015;4(1):5516. doi:10.1038/srep05516.
- Su B, Liu T, Fan H, et al. Inflammatory markers and the risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0150586. doi:10.1371/journal.pone.0150586.
- Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. doi:10.7326/0003-4819-156-2-201201170-00004.
- Huang AX, Lu LW, Liu WJ, et al. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-alpha levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome. Med Sci Monit. 2016;22:2800–2808. doi:10.12659/MSM.896458.
- Singh S, Verma SK, Kumar S, et al. Association between serum cytokine levels and severity of chronic obstructive pulmonary disease in Northern India. Int J Innovation Sci Res. 2015;18(2):357–361.
- Gupta R, Kaur R, Singh V, et al. Serial estimation of serum CRP levels in patients of COPD with acute exacerbation. Gobal J Med Public Health. 2012;1(6):1–10.
- Han J, Cheng C, Zhu Z, et al. Vitamin D reduces the serum levels of inflammatory cytokines in rat models of periodontitis and chronic obstructive pulmonary disease. J Oral Sci. 2019;61(1):53–60. doi:10.2334/josnusd.17-0357.
- Dimeloe S, Rice LV, Chen H, et al. Vitamin D (1,25(OH)2D3) induces α-1-antitrypsin synthesis by CD4+ T cells, which is required for 1,25(OH)2D3-driven IL-10. J Steroid Biochem Mol Biol. 2019;189:1–9. doi:10.1016/j.jsbmb.2019.01.014.
- Zhou W, Wang Q. Vitamin D suppresses lipopolysaccharide-induced inflammatory response in vascular smooth muscle cells via inhibition of the p38 MAPK signaling pathway. Pharmazie. 2019;74(3):168–174. doi:10.1691/ph.2019.8818.
- Ke CY, Yang FL, Wu WT, et al. Vitamin D3 reduces tissue damage and oxidative stress caused by exhaustive exercise. Int J Med Sci. 2016;13(2):147–153. doi:10.7150/ijms.13746.