Abstract
Few studies have tried to assess prognostic variables in chronic obstructive pulmonary disease (COPD) patients requiring mechanical ventilation (MV). We evaluated serum C reactive protein, (CRP) pre-albumin (PA) and transferrin (TR) levels in AE-COPD patients requiring MV as prognostic markers of in hospital mortality. 93 AE-COPD patients on MV were evaluated. Detailed clinical evaluation was done daily. Serum CRP & PA were measured on admission, 3rd, 8th and 16th day; TR was measured on admission, 8th and 16th day. Demographics, baseline parameters, CRP, PA and TR were correlated with mortality. Of 93 patients, 49 (52.69%) survived whereas 44 patients (47.31%) died. APACHE II, serum urea & albumin were similar in survivors & non-survivors. Baseline CRP (≥10.5 mg/dl) had sensitivity of 60.5%, specificity of 60.2%, with area under curve (AUC) of 0.62 as predictor of mortality. CRP (≥7 mg/dl) on day 3 had sensitivity (65.5%) and specificity (63.3%) with AUC 0.70 as predictor of mortality. Baseline serum prealbumin was 11.00 (0.09–29.26) mg/dl, and similar in survivors & non-survivors (p = 0.7). Prealbumin at day 8 (n = 50) < 13.5 mg/dl had sensitivity 54.6%, and specificity 51.4% with AUC 0.54 (95% CI 0.34–0.75) as predictor of mortality. Transferrin at day 8 (n = 50) of <148.9 had sensitivity 63.4% and specificity 61.4% with AUC 0.61 with respect to mortality. High CRP levels at baseline, persistently elevated CRP (on day 3) may predict mortality in AE-COPD patients requiring MV. Further studies are required to establish prognostic variables in this patient population.
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by airflow limitation, chronic lung inflammation, and extra-pulmonary effects, is a major cause of morbidity and mortality throughout the world [Citation1]. The prevalence of COPD is increasing worldwide and in India due to risk factors such as ageing and particulate matter exposure. According to Global burden of disease (2015) estimates, crude prevalence of COPD in India is 4.2%, an increase of 29.2% from 1990. COPD is the second leading cause of death in India, after ischemic heart disease. Nearly 32% of global DALYS due to chronic respiratory diseases are from India, with 76% of these attributable to COPD [Citation2, Citation3]. Each episode of exacerbation of COPD (AECOPD) entails a faster decline in lung function and subsequent poorer prognosis for the patient. Severe exacerbations of COPD account for only 10% of exacerbations [Citation4]. However, in most health care systems, hospitalization due to AECOPD accounts for the major socioeconomic impact [Citation5, Citation6]. Exacerbations with respiratory acidosis and ventilatory requirement have been associated with increased in hospital mortality and risk of readmissions [Citation7, Citation8]. In a recent audit of 422 hospitals from 13 European countries (n = 16016 patients), COPD exacerbation requiring admission was associated with 45.7% in-hospital mortality, highlighting the high risk of mortality even in well-organized institutional settings [Citation7].
As AECOPD is associated with increased local and systemic inflammation [Citation9, Citation10], there has been a constant interest in finding systemic biomarkers which can predict disease severity and outcome. Various biomarkers have been studied, but there is little information about correlation between serum biomarkers and clinical outcomes such as length of hospital stay, need for ICU treatment, and mortality.
C-reactive protein (CRP) is an acute phase reactant high sensitivity, and has found use in diagnosis of various infectious and inflammatory conditions [Citation11–13], including COPD [Citation14, Citation15], both during stable phase [Citation16] and AECOPD [Citation17]. The value of CRP as a prognostic biomarker in AECOPD patients has been extensively investigated [Citation18, Citation19]. A short half-life (19 h)makes it useful for follow-up of inflammatory response and infection. However, CRP levels are affected by numerous factors such as cardiovascular disease, diabetes, renal disease, metabolic syndrome, obstructive sleep apnea, hypertension, smoking status, and medications [Citation14].
Malnutrition is common in COPD, with prevalence of 22% to 24% in outpatients and 34% to 50% in hospitalized patients [Citation20]. Body mass index and body anthropometry have been widely used as indicators of nutritional state. Low BMI has been associated with greater severity, poorer quality of life and higher mortality in patients with COPD [Citation21]. Serum levels of pre-albumin and transferrin reflect the state of the visceral protein reserves and are more sensitive and specific than anthropometric parameters in evaluating nutritional state [Citation22]; their rapid turnover and short half-life can make them indicators sensitive to acute changes in nutritional state. Prealbumin has emerged as the preferred marker for malnutrition in critically ill patients [Citation23] and those with chronic disease [Citation24] as it is less affected by liver disease. The prealbumin level transiently decreases in presence of inflammation, post-surgery, protein malnutrition, malignancy, cirrhosis, and zinc deficiency [Citation24]. Serum transferrin, a beta-globulin, with a short half-life (7–10 days), transports iron in the plasma, and has been used in assessing nutritional status in various clinical conditions. Serum levels of transferrin are affected by nutritional factors (as are serum levels of albumin during a stress response) and iron metabolism [Citation25, Citation26]. Thus, assessing C-reactive protein, prealbumin and transferrin levels may be an effective method for assessing severity of the disease and predicting outcome in these patients.
The aim of our study was to investigate the utility of acute phase proteins (C-reactive protein, prealbumin and transferrin) to predict in-hospital mortality in mechanically ventilated patients with AECOPD.
Materials and methods
All patients with acute exacerbation of COPD requiring mechanical ventilation during the duration of the study (August 2006 to October 2009) were screened, and 93 patients satisfying inclusion criteria were enrolled. Diagnosis of COPD was based on the Global Initiative for chronic obstructive Lung Disease (GOLD). Patients with a clinical history suggestive of asthma, pulmonary emboli, congestive heart failure, lung cancer, or other lung disease were excluded; COPD patients on oxygen supplementation or noninvasive ventilation were also excluded. This study was performed in full adherence to the guidelines of the Declaration of Helsinki. Study was approved by ethics committee of the All India Institute of Medical Sciences, New Delhi. Written informed consent was obtained from legally acceptable representative of each participant before inclusion in the study.
All patients were followed till discharge or death. Demographic and clinical data were collected for all patients including age, sex, socio-economic and marital status, smoking pack years and duration of COPD. Baseline clinical and laboratory assessment including arterial blood gas analysis was recorded at baseline and subsequent follow up. Anthropometric measurement including body mass index (BMI), waist/hip ratio (WHR), upper arm, skin fold thickness (biceps, triceps and suprailiac), and serum levels of acute phase proteins (prealbumin, C-reactive and transferrin) were estimated at baseline and on subsequent follow up. Length of hospital stay was also noted for each patient. All observations including serum acute phase protein levels were recorded at five different times: within 24 h of admission (day 1) and on days 3, 8, 16 and 28 days of ICU stay.
Serum assays
Peripheral blood samples were processed on the same day by centrifugation at 3000 rpm for 15 min. Serum thus obtained was stored at −80 °C until analysis. Serum CRP was measured by Latex Enhanced Immunoturbidimetry method (LEIT) with a lower limit of detection 0.3 mg/dl using commercial available kit from Agappe (Kerala, India). Serum prealbumin was measured by Quantitative turbidimetric method with a lower limit of detection 6.7 mg/dl using commercial available kit from Spinreact (Spain). Serum transferrin was measured by quantitative turbidimetric method using commercial available kit from Spinreact (Spain).
Statistical analysis
Discrete variables were expressed as counts (percentages) and continuous variables as mean ± SD or median (inter quartile range, IQR). Comparability of groups was analyzed by chi-square test, student t-test, or Mann-Whitney test as appropriate. The changes over the period of time were estimated repeated measure ANOVA with Bonferroni correction for multiple comparisons. Friedman test was applied to see changes over time for non-parametric values. Correlation between serum markers and baseline parameters was assessed using Spearman correlation coefficient. Receiver operator curves (ROC) were made for serum markers or changes as compared to baseline. All tests were two tailed; p < 0.05 was defined as significant. Logistic regression analysis was performed to look for predictors of mortality. Data was analyzed using statistical software (Statistical Package for Social Sciences, version 15 for Windows; SPSS; Chicago IL).
Results
Detailed baseline characteristics of the study population are presented in . The mean age of the patients was 61.67 (±11.06) years. Out of 93 patients, 32(34.41%) were current smokers, 49(52.60% were ex-smokers, and 9(9.68%) had never smoked. Three patients (3.23%) had a history of hukka use or biomass fuel exposure. Comorbidities in recruited patients included previous history of pulmonary tuberculosis (17.2%), diabetes mellitus (61.5%) and coronary artery disease (18.3%). Of recruited patients, 80 patients were males (84%); only 13 patients (16%) were females. Thirteen (14%) patients had GOLD stage II, 34 (36.6%) patients had GOLD stage III whereas 4 (4.3%) patients had GOLD Stage IV COPD. Recent PFT was not available for 51 patients.
Table 1. Baseline characteristics and comparison of baseline demographics and laboratory tests between survivors and non-survivors.
BMI of patients was 19.05 (IQR 13.52–37.77) kg/m2. Baseline mid upper arm circumference (MUAC) was 22.50(IQR 15.00–37.00) cm. There was no significant variation in anthropometric variables on follow up measurements. At baseline, serum total proteins were 6.40 (IQR 2.8–8.9) g/dl and serum albumin was 3.30 (±0.5) g/dl. There was statistically significant decrease in serum protein (p = 0.03) and serum albumin levels during hospital stay at days 3rd, 8th, and 16th.
Arterial blood gas analysis of all the patients revealed higher paCO2 on admission. 3rd, 8th, and 16th day. There was statistically significant decrease (p < 0.001) in PaCO2 during ICU stay; on pairwise comparison, the significance was observed between day 1st with 8th (p = 0.005), day 1st with 16th (p = 0.002) and day 3rd with 16th (p = 0.001). pH was also low at baseline, with statistically significant improvement with time. Significance was observed on day 1st with 8th (p = 0.001) and day 1st with 16th (p < 0.001). Serum bicarbonate levels were higher than normal at baseline, with no statistically significant difference on subsequent follow up (p = 0.46).
Baseline serum CRP was high i.e. 10.40 (IQR, 0.7–30.4) mg/dl. There was statistically significant decline in CRP levels on follow up to 2.0(0.3–27.6) mg/dl till 16th day (p < 0.001). On pairwise comparison, the significance was observed between day 1st with 8th (p = 0.002), day 1st with 16th (p = 0.001) and day 3rd with 16th day (p = 0.041) serum levels.
Baseline serum prealbumin levels were 11.00 (0.09–29.26) mg/dl, well below normal range (reference range 19–38 mg/dl) in all patients included in the study. There was marginal albeit statistically significant increase (p < 0.001) in the prealbumin levels on days 8th and 16th i.e. 12.93 (0.01–43.43) mg/dl and 14.48 (4.78–34.23) mg/dl respectively.
The level of serum transferrin (reference range 200–400 mg/dl) was low on admission, i.e. 163.29 (IQR 19.0–480.0) mg/dl and follow up. The difference observed in the levels of transferrin with time was not significant (p = 0.1).
There was no significant correlation of COPD stage with baseline serum CRP p = (0.1), transferrin (p = 0.6) and prealbumin (0.7) levels. Similarly, there was no significant correlation between serum CRP, prealbumin and transferrin with respect to age of patient, BMI, baseline pH, pCO2, serum albumin, APACHE II score or smoking status. There was no significant difference in baseline serum CRP, prealbumin and transferrin with respect to current smokers and former smokers, or comorbidities. There was significant negative correlation between baseline prealbumin and CRP levels (Spearman coefficient −0.27; p < 0.01)
Of 93 patients enrolled in the study, 49 patients (52.7%) survived whereas 44 patients (47.3%) died. Median duration of hospital stay in enrolled patients was 5 (IQR 3–10) days. shows the differences in demographic parameters and laboratory variables between survivors and non survivors. Age, gender, baseline ABG parameters, serum proteins and albumin levels were not significantly different between the two groups. Baseline APACHE scores were high 24.2 (5.2) and were not significantly different between survivors and non- survivors. BMI and mid upper arm circumference were not significantly different between survivors and non-survivors. Median neutrophil to lymphocyte (N/L) ratio was 3.8 (min-max 0.9–23.8) in enrolled subjects. There was no significant difference in the median N/L ratio of survivors as compared to non-survivors (3.8; 1.9–14.8 vs 3.5; 0.9–23.8) with a p value of 0.3 ().
Table 2. Change in various serum markers in survivors and non-survivors from baseline on subsequent follow up.
Serum CRP was elevated at baseline (median 10.40, IQR 0.7–30.4) in both groups, but levels were significantly higher in non-survivors (12.30, IQR 0.9–30.4) as compared to survivors (7.65, IQR 0.7–29.2) (p = 0.045). CRP levels were significantly high on follow up i.e. days 3rd, 8th and 16th when compared between survivors and non-survivors. Also, serum CRP levels decreased significantly (p < 0.001) in survivors on day 3rd, 8th and 16th post admission, whereas there was no significant decline for the same noted in non-survivors. Changes in various serum markers and correlation with mortality are shown in .
Table 3. Receiver operated curve characteristics (ROC) of studied acute phase proteins with respect to survival in study subjects.
Serum prealbumin was found to be low at baseline with modest increase on follow up; there was statistically significant improvement in prealbumin levels on follow up in survivors (p < 0.001); no statistically significant improvement was noted in serum prealbumin levels in non-survivors on follow up visits.
Serum transferrin levels were not significantly different between survivors and non-survivors at baseline (p = 0.43). Serum transferrin levels at day 16th were significantly lower (p = 0.04) in non survivors as compared to survivors (161.63, IQR 124.0–174.52 mg/dl vs. 83.00, IQR 56.85–132.36 mg/dl). Similarly, serum transferrin levels at baseline and day 8 were not significantly associated with survival in enrolled patients.
Receiver operated curve (ROC) characteristics for studied acute phase reactants with respect to survival are shown in . Baseline CRP at a cutoff of 10.5 mg/dl, had a sensitivity of 60.5% and specificity of 60.2%, for survival in patients requiring MV due to AECOPD, with AUC 0.62 & 95% CI of 0.50–0.73. CRP at day 3 at a cutoff of 7.0 mg/dl had a sensitivity of 65.5% and specificity of 63.3%, for survival in patients requiring MV due to AECOPD, with AUC 0.70 & 95% CI of 0.58–0.84. shows the ROC curves for baseline and day 3 CRP with respect to mortality.
Figure 1. A) Baseline CRP at a cutoff of 10.5 mg/dl, had a sensitivity of 60.5% and specificity of 60.2%, for survival in patients requiring MV due to AECOPD, with AUC 0.62 & 95% CI of 0.50–0.73. B) CRP at day 3 at a cutoff of 7.0 mg/dl, had a sensitivity of 65.5% and specificity of 63.3% for survival in patients requiring MV due to AECOPD, with AUC 0.70 & 95% CI of 0.58–0.84.
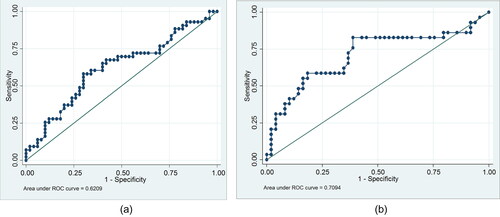
There was no significant improvement in ROC characteristics of CRP even when change in CRP from baseline to days 3, 8 and 16 were evaluated. Change in prealbumin (from day 1 to day 3) of > −0.71 mg/dl had sensitivity (69.4%) and specificity (62.1%) with an AUC of 0.66 (95% CI 0.53–0.79) for survival as compared to absolute prealbumin at day 3. Similarly, change in prealbumin > −3.6 mg/dl had sensitivity (86.5%) with specificity (45.4%) with a better AUC characteristic 0.61 (95% CI 0.39–0.83) as compared to absolute prealbumin value at day 8. Similarly, change of > −59.5 mg/dl in transferrin from day 1 to day 8 had 89.7% sensitivity and 45.4% specificity with better AUC of 0.65 (95% CI 0.43–0.86) as compared to absolute transferrin value at day 8. There was some improvement in ROC characteristics for prealbumin and transferrin when change in values from day 1 to day 16 were compared to absolute values. However, due to discharge or death of enrolled subjects, only 20 paired samples were available for analysis at day 16, limiting the validity of this result ().
Baseline APACHE II scores had AUC of 0.54 (SE 0.06) for mortality, with 95%CI 0.42–0.66; p value was 0.5 and not statistically significant. Similarly, baseline serum albumin had a AUC of 0.46 (SE 0.06, 95%CI 0.34–0.57) for mortality (p = 0.40). None of the variables were found to be independent predictors of mortality on regression analysis ().
Table 4. Multiple regression analysis of various factors with mortality in studied patients with AE-COPD.
Discussion
This study demonstrates the utility of elevated day 3 and day 8 serum CRP as a predictor of mortality in patients with AE-COPD requiring mechanical ventilation, with fair ROC characteristics.
Baseline APACHE II scores were high (24); APACHE II could not predict survival in patients included in our study. This finding is different from previous studies [Citation27, Citation28]. This difference may be due to the lower baseline APACHE II scores in previous studies; In the study by Khilnani et al., the APACHE II was 13 (±6), whereas in another study by Kun et al., survivors had APACHE II scores of 19 (±7) versus 28 (±8) in non survivors (p < 0.001). The other factor could be relatively less severe exacerbations with 16% to 42% patients managed with noninvasive ventilation [Citation27, Citation28].
CRP is an acute phase protein with significantly higher levels during AECOPD, but CRP levels on hospital admission have not been able to predict reliably the short-term or long-term outcome of the exacerbation including need for intensive care in previous studies [Citation18]. However, no studies have examined the utility of serum CRP to ascertain prognosis in subset of AECOPD requiring mechanical ventilation. The most important finding of this study is that serum CRP levels of non survivors with AECOPD requiring mechanical ventilation was significantly higher at baseline (p = 0.037), day 3rd (p = 0.002), 8th (p = 0.044) and 16th (p = 0.099) than that of survivors.
In agreement with previous studies [Citation29–32], CRP levels were significantly elevated at the exacerbation and decreased substantially thereafter, at 8th and 16th day. Also, serum CRP levels did not correlate significantly with age, gender, GOLD stage of COPD, duration of COPD or history of prior exacerbations. CRP levels also did not correlate significantly with ABG parameters like pH, pCO2 and PO2 at baseline or subsequent follow up.
CRP increases with respect to COPD stage as previously reported [Citation15, Citation16, Citation33]. CRP has also been variably associated with severity of exacerbation and prediction of requirement of MV [Citation29]; however, as all recruited patients had severe enough exacerbation requiring MV, we could not study this association.
In this study, serum transferrin levels were low at baseline and follow up. However, serum transferrin levels in mechanically ventilated patients with AECOPD have been found to be variable but similar to controls in previous studies, with lower BAL transferrin. The same study also showed decreased transferrin in lower respiratory tract fluid of COPD patients [Citation34]. Low transferrin levels in our study could be due to suboptimal nutritional status of our patients, with mean BMI 19.71(±3.90) kg/m2, and median serum albumin was 3.30 (2.1–5.8) g/dl at baseline. However, owing to small half-life (10 days), serum transferrin levels at day 16th did have prognostic significance, and were significantly lower (p = 0.04) in non-survivors as compared to survivors.
In our study, the low baseline prealbumin could be due to low BMI of the subjects included in this study, significant number of moderate to severe COPD cases, and catabolic state related to critical illness. There was significant improvement in prealbumin levels on follow up in survivors as compared to non survivors which indicate a potential prognostic value for this marker. We have previously demonstrated the utility of serum prealbumin for monitoring malnutrition and disease severity in stable COPD patients [Citation33]. Prealbumin has been said to be the earliest laboratory indicator of nutritional status and preferred marker for malnutrition as it correlates with patient outcome in a wide variety of clinical conditions [Citation24, Citation35]. Prealbumin levels have been reported to be higher (2 fold) at day 16 in survivors versus non survivors in mechanically ventilated patients with AECOPD (n = 336) in a recent study (only abstract available) [Citation30]. However, serum prealbumin levels could not reliably predict duration of hospital stay or mechanical ventilation in a previous study from our center [Citation36].
This study is one of the few to explore prognostic markers in mechanically ventilated patients with acute exacerbation of COPD. Serum CRP levels at baseline predict in-hospital mortality in AECOPD patients requiring mechanical ventilation. Also, serum prealbumin and serum transferrin levels have been shown to be significantly higher in survivors than non-survivors, albeit not significantly different at baseline. CRP has the advantage of being readily available and cost-effective investigation which can be repeated on follow up.
There are certain limitations to this study. In-hospital mortality in our study was higher (47%) as compared to previous studies by Khilnani et al. (37%) [Citation27]. However, higher mortality was reported in another study by Kun et al. (61.4%) [Citation28]. Also, recent European audit reported in-hospital mortality 45% in patients of AECOPD, with 45.7% mortality in hospitalized COPD patients, with increased odds of mortality 3.667 (2.997–4.486) with ventilatory support [Citation7]. A low mean BMI of enrolled subjects and severe exacerbation requiring mechanical ventilation meant higher CRP, and lower than normal transferrin and prealbumin levels. Systemic corticosteroids were administered to all patients, which could have confounded change in CRP levels. SOFA scores, recently reported to have high AUC for mortality prediction in AE-COPD, as well as the preferred modality in recent sepsis guidelines, were not utilized. As most of the mortality occurred upto 14 days of admission, we could not reliably derive prognostic value of follow up prealbumin and transferrin, though we did report receiver operated curve characteristics for these markers. Also, patients were not followed up beyond discharge, so we could not determine the prognostic value of variables tested to predict long term outcome of exacerbation or recurrence. We did not do detailed iron profile studies in our patients to rule out iron deficiency anemia or overload, which could confound transferrin levels in our study.
Conclusion
In mechanically ventilated patients with severe acute exacerbation of COPD, elevated serum CRP levels correlated positively with mortality and were significantly higher on admission, 3rd, 8th and 16th day. An increase in the prealbumin level was observed in survivors. Persistently high CRP levels at along with low prealbumin and transferrin levels in mechanically ventilated AECOPD patients were associated with poor prognosis. CRP at day 3. 8 and 16 had fair AUC as a predictor of mortality. Change in serum prealbumin and transferrin from day 1 to day 16 also had fair AUC as predictors of mortality, but with limited reliability in view of small number of paired samples. Further studies are required to establish prognostic variables in this subset of AECOPD patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
Funded by Department of Science and Technology, Government of India.
References
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP.
- Salvi S, Kumar GA, Dhaliwal RS, et al. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the global burden of disease study 1990–2016. Lancet Glob Health 2018;6(12):e1363–e1374.
- Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5(9):691–706.
- Halpin DM, Miravitlles M, Metzdorf N, et al. Impact and prevention of severe exacerbations of COPD: a review of the evidence. COPD. 2017;12:2891–2908. doi:10.2147/COPD.S139470.
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117(2):5S–9S. doi:10.1378/chest.117.2_suppl.5S.
- Dalal AA, Shah M, D’Souza AO, et al. Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med. 2011;105(3):454–460. doi:10.1016/j.rmed.2010.09.003.
- Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD audit. Eur Respir J. 2016;47(1):113–121. doi:10.1183/13993003.01391-2014.
- Perera PN, Armstrong EP, Sherrill DL, et al. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD J Chronic Obstr Pulm Dis. 2012;9(2):131–141. doi:10.3109/15412555.2011.650239.
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi:10.1164/rccm.200506-859OC.
- Hurst JR, Perera WR, Wilkinson TMA, et al. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(1):71–78. doi:10.1164/rccm.200505-704OC.
- Malo O, Sauleda J, Busquets X, et al. Systemic inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol. 2002;38:172–176.
- Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(8):867–874. doi:10.1164/rccm.200604-506OC.
- Müller B, Tamm M. Biomarkers in acute exacerbation of chronic obstructive pulmonary disease: among the blind, the one-eyed is king. Am J Respir Crit Care Med. 2006;174(8):848–849. doi:10.1164/rccm.200607-922ED.
- Pinto-Plata VM, Müllerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2005;61(1):23–28. doi:10.1136/thx.2005.042200.
- de Torres JP, Cordoba-Lanus E, López-Aguilar C, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J. 2006;27(5):902–907. doi:10.1183/09031936.06.00109605.
- Aksu F, Capan N, Aksu K, et al. C-reactive protein levels are raised in stable chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure. J Thorac Dis. 2013;5(4):414–421.
- Dev D, Wallace E, Sankaran R, et al. Value of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary disease. Respir Med. 1998;92(4):664–667. doi:10.1016/S0954-6111(98)90515-7.
- Stolz D, Christ-Crain M, Morgenthaler NG, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest 2007;131(4):1058–1067. doi:10.1378/chest.06-2336.
- Perera WR, Hurst JR, Wilkinson TMA, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29(3):527–534. doi:10.1183/09031936.00092506.
- Soler JJ, Sánchez L, Román P, et al. Prevalence of malnutrition in outpatients with stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2004;40(6):250–258. doi:10.1016/S1579-2129(06)70095-7.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322.
- Documentos de consenso | SENPE | Sociedad Española de Nutrición Parenteral y Enteral [Internet]. [cited 2017 Nov 24]. Available from: http://www.senpe.com/documentos-de-consenso.php.
- Davis CJ, Sowa D, Keim KS, et al. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197–204. doi:10.1177/0148607111413896.
- Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Physician 2002;65(8):1575.
- Fuhrman C, Sarter H, Thibaudon M, et al. Short-term effect of pollen exposure on antiallergic drug consumption. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2007;99(3):225–231. doi:10.1016/S1081-1206(10)60657-6.
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf) 2016;4(4):272–280.
- Khilnani G, Banga A, Sharma S. Predictors of mortality of patients with acute respiratory failure secondary to chronic obstructive pulmonary disease admitted to an intensive care unit: a one year study. BMC Pulm Med. 2004;4:12.
- Xiao K, Guo C, Su L, et al. Prognostic value of different scoring models in patients with multiple organ dysfunction syndrome associated with acute COPD exacerbation. J Thorac Dis 2015;7(3):329–336.
- Chen Y-W, Leung JM, Sin DD. A systematic review of diagnostic biomarkers of COPD exacerbation. PloS ONE. 2016;11:e0158843. doi:10.1371/journal.pone.0158843.
- Agmy G, Maghlouf H, Wafy S, et al. Can acute phase proteins predict survival in ventilated patients with acute exacerbation of COPD? Eur Respir J. 2012;40:P4818.
- Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med. 2009;15(2):120–125. doi:10.1097/MCP.0b013e3283218603.
- Bircan A, Gokirmak M, Kilic O, et al. C-reactive protein levels in patients with chronic obstructive pulmonary disease: role of infection. Med Princ Pract. 2008;17(3):202–208. doi:10.1159/000117793.
- Guleria R, Chawla TC, Arora S. Inflammatory markers and BMI in patients with exacerbation of COPD. Chest 2009;136(4):54S. doi:10.1378/chest.136.4_MeetingAbstracts.54S-e.
- Stites SW, Nelson ME, Wesselius LJ. Transferrin concentrations in serum and lower respiratory tract fluid of mechanically ventilated patients with COPD or ARDS. Chest 1995;107(6):1681–1685. doi:10.1378/chest.107.6.1681.
- Gocmen H, Ediger D, Uzaslan E, et al. The relationships of serum prealbumin levels with parameters that indicate severity of disease and emphysema pattern in patients with stable chronic obstructive pulmonary disease. Eurasian J Med. 2010;42(3):105–110. doi:10.5152/eajm.2010.31.
- Mohan A, Arora S, Uniyal A, et al. Evaluation of plasma leptin, tumor necrosis factor-α, and prealbumin as prognostic biomarkers during clinical recovery from acute exacerbations of chronic obstructive pulmonary disease. Lung India. 2017;34(1):3. doi:10.4103/0970-2113.197101.
