Abstract
Several nutrients have been suggested to protect against airway destruction via antioxidant activity. The present study aimed to evaluate the association between disease severity and dietary nutrient intake in chronic obstructive pulmonary disease (COPD) patients using the Korea National Health and Nutrition Examination Survey. Of the 22,948 participants, 702 patients (418 men and 284 women) with COPD, who were defined as the fifth percentile from a reference population were selected. The severity of airflow limitation was measured by the predicted percentage of forced expiratory volume in 1 second (FEV1%). The Jonckheere–Terpstra test was used to evaluate the dose-dependent association between nutrient intake and disease severity. Multivariate linear regression analysis was used to evaluate the relationship between dietary nutrient intake and predicted FEV1%. Vitamin A intake showed a positive association with FEV1% in men in a model adjusted for covariates. Carbohydrate, protein, fiber, thiamin, riboflavin, niacin, and vitamin C intake were significantly associated with decreased disease severity in elderly men (aged ≥60 years). On the contrary, statistical significance was not observed for all the nutrients in women. In conclusion, intake of carbohydrate, protein, fiber, thiamin, riboflavin, niacin, and vitamin C was associated with decreased severity of airway impairment in elderly men with COPD. Our results are in line with those of previous studies into the importance of nutritional status in airway disease. A longitudinal study is required to clarify the mechanisms underlying the association between dietary nutrient intake and COPD severity.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex and heterogeneous condition with many different mechanisms and contributing factors [Citation1]. Both systemic and airway inflammation continuously occur in COPD, and several inflammatory markers such as acute-phase reactant plasma fibrinogen are elevated [Citation2]. Over time, this inflammation results in either chronic bronchitis or emphysema, further increasing the risk of cardiovascular disease [Citation3]. Airway inflammation is thought to be responsible for structural damage and tissue injury which collectively result in airway remodeling [Citation4]. Several techniques and parameters have been developed to evaluate the degree of airway impairment and emphysema. However, observer bias, reproducibility, and the lack of sophisticated software are all limitations associated with these techniques [Citation5]. Indeed, spirometry performed by a trained technician remains important in the diagnosis of airway diseases.
Recently, a number of studies have reported that the anti-inflammatory effect of some micro-nutrients may protect against the worsening of airway disease [Citation6, Citation7]. Vitamin C is an antioxidant which has been reported to protect against airway inflammation [Citation8]. Furthermore, low dietary intake of fruit, vitamin E, and beta-carotene has been associated with poor lung function [Citation9]. Although these studies provide evidence that some dietary nutrients play an important role in protecting against airway inflammation in the general population, only a few studies have focused on COPD patients.
Therefore, the present study aimed to evaluate the dose-dependent relationship between dietary nutrient intake and airflow limitation in COPD patients using data from the 6th Korea National Health and Nutrition Examination Survey (KNHANES).
Methods
Study participants
Data from the 6th KNHANES (2013–2015) were used in this study. The KNHANES is a population-based, cross-sectional, nationwide survey conducted annually by the Korea Centers for Disease Control and Prevention. The survey is designed to evaluate the health and nutritional status of nationally representative non-institutionalized Korean citizens. This survey is composed of a health interview, health examination, and nutritional survey to obtain information on the socio-demographic characteristics, health behaviors, dietary intake, and anthropometric profile of each participant. Detailed survey protocols and data resource profiles have been presented in a previous study [Citation10]. All raw data are freely accessible to the public (https://knhanes.cdc.go.kr).
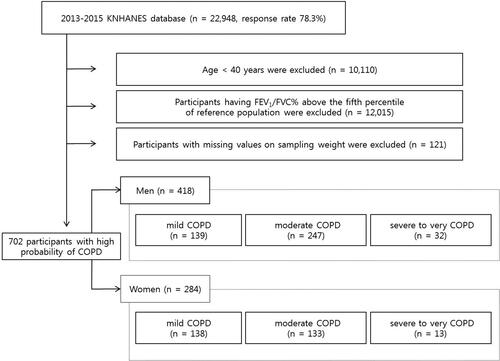
The study flow diagram is presented in . The 2013–2015 KNHANES assessed 29,321 individuals—the response rate was 78.3% and 22,948 individuals ultimately participated in the survey. The participants were selected by stratified two-stage cluster sampling through a two-step process of probability proportional to size sampling and systematic sampling. Because only participants aged over 40 underwent pulmonary function tests (PFT), data for 12,838 adults were available. Participants with obstructive spirometry patterns were selected (n = 1239). However, because an obstructive pattern in the absence of symptoms does not justify a diagnosis of COPD and the KNHANES did not evaluate subjective respiratory symptoms, we classified COPD as the lower limit of normal (LLN), defined as the fifth percentile derived from a reference population (n = 823) [Citation11]. Participants with missing data on factors that may affect lung function, namely age, sex, residential area, education level, and household income level, were excluded (n = 60). Because the present study evaluated the effect of nutrition on lung function in COPD patients, participants with missing data on nutritional status (carbohydrate, fat, protein, vitamin A, beta-carotene, retinol, thiamin, riboflavin, niacin, and fiber intake) were also excluded (n = 61). Finally, 702 participants (418 men and 284 women) with COPD were included in the analysis.
Pulmonary function test
Spirometry was performed by highly trained medical personnel in the mobile examination center (MEC) using dry rolling seal spirometers (Model 2130; Sensor Medics, Yorba Linda, CA, USA). All equipment was calibrated periodically, and quality control and standardization were conducted according to the manual of the American Thoracic Society and European Respiratory Society [Citation12]. Participants with the value of forced expiratory volume in 1 second (FEV1) divided by forced vital capacity (FVC) below LLN were considered as having COPD [Citation11]. The LLN of FEV1/FVC was calculated using an equation defined by a previous study of Korean population [Citation13]. Subsequently, the participants were divided into four groups according to the degree of airflow limitation based on the classification of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Citation14]: mild (GOLD stage 1, predicted FEV1 ≥ 80%), moderate (GOLD stage 2, 50% ≤ predicted FEV1 < 80%), severe (GOLD stage 3, 30% ≤ predicted FEV1 < 50%), and very severe (GOLD stage 4, predicted FEV1 < 30%). However, because the very severe COPD group comprised only 4 males and 0 females, we combined GOLD stages 3 and 4 to form a severe to very severe group.
Smoking status
Based on the National Health Interview Survey, a current smoker was defined as an adult who has smoked more than 100 cigarettes in their lifetime and who smokes currently. A former smoker was defined as an adult who has smoked at least 100 cigarettes in their lifetime but who had quit smoking at the time of the survey. A never smoker was defined as an adult who has never smoked, or who has smoked less than 100 cigarettes in their lifetime [Citation15].
Anthropometric indices
Anthropometric measurements were performed by trained assistants in the MEC. Height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.1 kg. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2). Based on the Korean Society for the Study of Obesity guidelines [Citation16], participants were divided into four groups according to BMI: underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), pre-obese (23–24.9 kg/m2), and obese (≥25 kg/m2).
Socio-economic variables
Socio-economic variables included sex, age, residential area, educational level, and household income. Age was divided into four groups: 40–49 years, 50–59 years, 60–69 years, and ≥70 years. Household income was categorized into four quartiles according to age and sex. Household income was calculated by dividing the mean monthly income by the square root of household size. Educational level was divided into three groups (middle school or lower, high school, and college or above). Residential area was divided into rural and urban based on data sourced from the KNHANES.
Nutrient intake
Nutrient intake was determined from the sum of the nutrient content of all foods that an individual participant consumed during the day. Nutritional surveys were carried out by a team of specialists using a computer-assisted personal interview system. Although it has several limitations, the 24-hour dietary recall method can be a useful tool in large-scale population studies, and the validity of this method has previously been evaluated [Citation17, Citation18]. The measured nutrients included carbohydrate, fat, protein, fiber, vitamin A, beta-carotene, retinol, thiamin, riboflavin, niacin, and vitamin C. The intake of each nutrient was expressed as a continuous variable and referred to the total intake of that nutrient over the previous 24 hours.
Statistical analysis
Because KNHANES data were obtained using complex sampling, sample weights and stratification variables were constructed to represent the Korean population. All statistical analyses were performed using complex sample analyses. Categorical variables were compared using the Chi-squared test and continuous variables were compared using analysis of variance (ANOVA). The Jonckheere–Terpstra test was used to evaluate the dose-dependent association between nutrient intake and COPD severity.
Variables previously reported to have an impact on lung function (age, sex, height, smoking status, residential area, educational level, and household income) were included in the multivariate analysis as covariates. Before these variables were included in the analysis, we tested for multicollinearity to identify any inter-associations among the independent variables. Because the distribution of each nutrient intake was skewed to the left and violated the assumption of normality, the amount of nutrient intake was log-transformed. Linear regression analysis was performed to evaluate the association between spirometry results, the intake of each nutrient, age, sex, smoking status, residential area, educational level, and household income. Categorical variables in the linear regression model were dummy coded. Sensitivity analysis was performed in elderly participants (≥60 years old, 254 men and 133 women), because aged patients are more prone to malnutrition.
All statistical analyses were performed using SPSS software (version 24 for Windows, IBM Corp, Armonk, NY, USA). For all analyses, a P-value <0.05 was considered statistically significant.
Results
The general characteristics of the participants are summarized in . Significant differences in age, height, education, and spirometry results were noted in men among the three groups. Participants with severe to very severe COPD were older than those with mild COPD. Participants with severe to very severe COPD were shorter than those with mild or moderate COPD. Participants with severe to very severe COPD were less educated than those with mild or moderate COPD. None of the other characteristics showed significant differences between the three groups. Statistically significant differences were noted in age, height, education, and spirometry result in women with COPD. The pattern of significance was similar with the male participants, but the educational level differed only between the mild and severe to very severe groups.
Table 1. General characteristics of study participants.
shows the dietary macro-nutrient intake of the participants according to COPD severity. Disease severity tended to increase, as the intake of macro-nutrients decreased, for both sexes. However, only the association between carbohydrate intake and COPD severity was statistically significant.
Table 2. Dietary macro-nutrient intake of the participants according to disease severity of COPD.
shows the dietary micro-nutrient intake of the participants according to COPD severity. Disease severity increased, as the intake of micro-nutrients decreased in men. However, statistical significance was observed with vitamin A, beta-carotene, retinol, thiamin, and riboflavin intake. There was no significant trend for disease severity with respect to dietary micro-nutrients intake in women.
Table 3. Dietary micro-nutrient intake of the participants according to disease severity of COPD.
presents the relationships between the intake of each nutrient and predicted FEV1% in men with COPD. In the crude model, carbohydrate, protein, fiber, vitamin A, beta-carotene, thiamin, riboflavin, and vitamin C intake showed a positive correlation with predicted FEV1%. In model 1, which was adjusted for age and height, carbohydrate, fiber, vitamin A, beta-carotene, riboflavin, and vitamin C intake were associated with decreased disease severity. In model 2, which was adjusted for age, height, smoking status, household income, educational level, and residential area, only vitamin A intake was positively associated with predicted FEV1%.
Table 4. Association between predicted FEV1% and nutrient intake in men (n = 418).
presents the relationships between the intake of each nutrient and predicted FEV1% in women with COPD. In the crude model, carbohydrate and niacin intake showed a positive correlation with predicted FEV1%. However, statistical significance disappeared on the application of the confounding factors.
Table 5. Association between predicted FEV1% and nutrient intake in women (n = 284).
The sensitivity analyses of elderly men and women with COPD are shown in and Citation7, respectively. In elderly men, carbohydrate, protein, fiber, vitamin C, and vitamin B-complex (i.e. thiamin, riboflavin, niacin) intake were associated with decreased disease severity. No significant association was observed between dietary nutrient intake and predicted FEV1% in elderly women.
Table 6. Association between predicted FEV1% and nutrient intake in elderly men (n = 254).
Table 7. Association between predicted FEV1% and nutrient intake in elderly women (n = 133).
Discussion
In the present study, data from the nationally representative KNHANES (2013–2015) were used to evaluate the dose-dependent relationship between COPD severity and dietary micro/macro-nutrient intake. Our study found that increased vitamin A intake was associated with decreased disease severity in men with COPD. Although sufficient nutrient intake showed a tendency for decreased disease severity in men with COPD, statistical significance and strength of regression were more prominent in elderly patients. Carbohydrate, protein, fiber, vitamin B1 (thiamin), B2 (riboflavin), B3 (niacin), and vitamin C intake showed significant association with decreased disease severity in elderly male patients. In addition to the confounders considered in this study, other factors may have contributed to the correlations observed. In a previous study, polymorphisms of the genes encoding glutamate-cysteine ligase, an enzyme responsible for glutathione biosynthesis, were significantly associated with lower lung function, especially in patients in the lowest tertile of vitamin C intake [Citation19].
Before the correlation between lung function and nutrient intake can be applied to all COPD patients, it should be considered that Korea differs from other countries with respect to diet and socio-economic status. First, the characteristics of the Korean diet should be considered. The traditional Korean diet consists of “bab” (cooked rice), which is high in carbohydrates and low in protein and fat [Citation20], “kuk” (soup), “kimchi” (fermented food), and “banchan” (side dishes). Thus, this diet significantly differs from the diets of Western and other Asian countries [Citation21]. Since the strength of regression in the relationship between lung function and nutrient intake was observed with only a few nutrients, the applicability of the results of this study to the diets of other countries is limited. Second, the socio-economic status of Korea should be considered. Income levels and socio-economic status differ globally [Citation22]. Because South Korea is a high-income country and COPD prevalence and mortality differ compared to low- and middle-income countries, it is difficult to apply the findings of the current study to COPD patients from other countries.
Nutrition is an important issue for COPD patients. Although the nutrients required by COPD patients are different from those required by other disease patients, there are no specific nutritional guidelines for COPD patients regarding the recommended daily intake of micro- and macro-nutrients. Guidelines by the European Society for Parenteral and Enteral Nutrition state that nutrient composition should not be manipulated, but rather nutritional supplements should be given in small frequent doses to avoid postprandial heaviness [Citation23]. However, there is mounting evidence that malnutrition is associated with poorer prognosis—it reduces respiratory muscle strength and leads to cachexia, sarcopenia, and disease exacerbation [Citation24–27].
Because chronic airway inflammation can result in airway remodeling, thickening, and irreversible airway obstruction [Citation28], it is important that this inflammation is minimized. Several previous studies have reported that some dietary nutrients are protective against airway inflammation [Citation29]. However, there remain inconsistencies with respect to the effects of dietary nutrients on airway inflammation and airway disease severity.
Vitamin A is essential for normal functioning of the organs, and is important for vision and skin health [Citation30]. In a mouse model of asthma, vitamin A deficiency decreased airway inflammation and hyper-responsiveness. In contrast, high dietary vitamin A intake promoted the expression of Th2 cytokines, evoking eosinophilic airway inflammation [Citation31]. In another mouse model, however, vitamin A regulated bronchial responsiveness by reducing the function of muscarinic M2 receptors, which are responsible for airway constriction [Citation32]. In a randomized controlled trial in patients with asthma, the high anti-oxidant diet group had higher plasma levels of carotenoids, the precursors of vitamin A, and exhibited clinical improvements [Citation33]. In the general population, high vitamin A levels clinically improved FEV1 by 22–31.2 ml [Citation34].
Vitamin C has also been widely studied as an antioxidant. As oxidative stress is thought to be a major factor in the pathogenesis of COPD, the antioxidant effects of dietary vitamin C have gained attention. An experimental study into lung repair after tobacco-induced emphysema investigated the effect of vitamin C supplementation in mice. Compared to the control group, the vitamin C supplemented group exhibited lower catalase, superoxide dismutase, and matrix metalloproteinase activity and lower levels of tumor necrosis factor alpha, suggestive of reduced pulmonary tissue destruction [Citation35]. In one nationwide cross-sectional study in South Korea which involved 512 participants with COPD and 2771 participants with normal spirometry results, low vitamin C intake was an independent risk factor for COPD [Citation36]. A double-blind randomized study which evaluated the effect of vitamin C on nitrogen dioxide-induced airway hyper-responsiveness in normal subjects showed that pretreatment with vitamin C prevented hyper-responsiveness [Citation37].
Recent findings have suggested that dietary fiber intake has a positive effect on airway inflammation. Dietary fiber influences gut microbiota, balances Th1/Th2 immunity, and alleviates airway allergic inflammation [Citation38]. Two large cohort studies, involving 111,580 men and women, examined the effect of dietary fiber intake on the prevalence of COPD. Particularly in women, there was an independent and significant association between fiber intake and the risk of COPD, even after adjustment for many confounders (relative risk: 0.60, 95% confidence interval (CI): 0.40–0.94) [Citation39]. Another cohort study showed that long term dietary fiber intake reduced the incidence of COPD by 30% (95% CI: 17–41%). This study indicated that increasing dietary fiber intake may decrease the risk of airflow limitation in both current and previous smokers [Citation40]. A randomized trial conducted by Keranis et al. further supports the beneficial effect of dietary fiber in COPD patients. COPD patients with a diet rich in fruit and vegetables had a significantly higher predicted FEV1% than patients without a fruit and vegetable-rich diet (P = 0.03) [Citation41].
The role of carbohydrates in patients with COPD has been debated. Initially, there were concerns that a high carbohydrate diet increased the production of carbon dioxide and had detrimental effects on ventilation [Citation42]. However, a randomized, double-blind, crossover study conducted by Vermeeren et al. showed that an energy load up to 2092 kJ had no adverse effects on metabolism or energy capacity [Citation43]. In patients with stable COPD, exercise rehabilitation improves survival outcome, so adequate carbohydrate support should be prioritized. Early muscle fatigue and exercise intolerance can occur in COPD patients due to increased glucose production [Citation44].
Interestingly, in elderly men with COPD patients, vitamin B-complex intake (thiamin, riboflavin, and niacin) showed a strengthened association with predicted FEV1%. The strength of regression was more prominent in thiamin than with riboflavin and niacin. Thiamin is an essential water-soluble vitamin which is an essential cofactor for carbohydrate metabolism [Citation45]. Thiamin is phosphorylated in the human body, forming thiamin monophosphate, thiamin triphosphate, and thiamin pyrophosphate. Thiamin pyrophosphate, also known as thiamin diphosphate, is an important cofactor for energy metabolism [Citation46]. Elderly male COPD patients have been shown to develop increased energy expenditure and consequently exhibit hypermetabolism. Therefore, this could provide a mechanistic link between thiamin intake and COPD severity and performance status [Citation47].
The study has some limitations. First, because the KNHANES is a cross-sectional study and does not take into account temporality, the causal relationship between dietary nutrient intake and pulmonary function is unclear. Inverse causality cannot be excluded in this analysis. Second, the post-bronchodilator response test was not performed in the KNHANES, so only pre-bronchodilator data were provided via spirometry. The GOLD classification of COPD, however, is based on post-bronchodilator values. In addition, FEV1/FVC < 70% may be found in up to 20% of healthy subjects aged over 60 [Citation48]. Hence, the prevalence of COPD may have been over-estimated in this study. Indeed, based on the KNHANES, the prevalence of COPD in Korea in 2015 was reported to be 13.4% [Citation49], This is more prevalent than the estimated global burden of 10% [Citation50]. To overcome over-estimation, we selected patients with a high probability of COPD based on the frequency distribution of a reference population with a cutoff value of 5%. Third, we used the 24-hour dietary recall method, which depends on the subject’s memory. Although this method is known to measure food intake relatively accurately [Citation51, Citation52], it does have a number of limitations. In particular, most of the study participants were older adults, who may have had difficulty in accurately recalling their diet the previous day. Fourth, given that the KNHANES is a complex sample survey, selection bias should be considered. In a complex sample survey, unequal selection and biased population estimates can occur. Because of the inherent nature of the complex survey design, the KNHANES provides guidelines for data analysis using both SAS and SPSS (https://knhanes.cdc.go.kr/knhanes/sub03/sub03_06_02.do). These guidelines warn that ignoring the complex design or sampling weights leads to biased estimates and overstated significance levels [Citation53]. Therefore, to ensure appropriate estimates and standard errors, all statistical analyses in this study were conducted using complex samples analyses in SPSS.
Despite these aforementioned limitations, our study demonstrated the importance of dietary nutrient intake in COPD patients. The importance of nutritional status was more significant in elderly patients. Although a previous study investigated the effect of nutritional status on COPD severity in Koreans, only 41 patients were included [Citation54]. Therefore, this is the first population-based large-scale study to evaluate the association between nutritional status and disease severity in COPD patients. A notable advantage of the present study is that it adjusted for socio-economic status, which is known to have an effect on diet.
Conclusions
Carbohydrate, protein, fiber, vitamin A, thiamin, riboflavin, niacin, and vitamin C intake were associated with decreased airway impairment severity in COPD patients, and this association was more prominent in elderly patients. Our findings are in line with those of previous studies emphasizing the importance of appropriate nutrient intake in relieving airway inflammation and supporting energy production. Adequate nutritional support in patients with COPD should not be ignored. A longitudinal study is required to clarify the mechanisms underlying the association between dietary nutrient intake and COPD severity and to elucidate the causal relationship.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Singh D, Roche N, Halpin D. Current controversies in the pharmacological treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(5):541–549. doi:10.1164/rccm.201606-1179PP.
- Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128(4):1995–2004. doi:10.1378/chest.128.4.1995.
- King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Trans Med. 2015;4(1):26. doi:10.1186/s40169-015-0068-z.
- Homer RJ, Elias JA. Consequences of long-term inflammation: airway remodeling. Clin Chest Med. 2000;21(2):331–343. doi:10.1016/S0272-5231(05)70270-7.
- Mair G, Maclay J, Miller JJ, et al. Airway dimensions in COPD: relationships with clinical variables. Respir Med. 2010;104(11):1683–1690. doi:10.1016/j.rmed.2010.04.021.
- Berthon BS, Macdonald‐Wicks LK, Gibson PG, et al. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology. 2013;18(3):447–454. doi:10.1111/resp.12015.
- Kelly FJ. Vitamins and respiratory disease: antioxidant micronutrients in pulmonary health and disease. Proc Nutr Soc. 2005;64(4):510–526. doi:10.1079/PNS2005457.
- Kirschvink N, Fievez L, Bougnet V, et al. Effect of nutritional antioxidant supplementation on systemic and pulmonary antioxidant status, airway inflammation and lung function in heaves‐affected horses. Equine Vet J. 2010;34(7):705–712. doi:10.2746/042516402776250298.
- Burns JS, Dockery DW, Neas LM, et al. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132(1):238–245. doi:10.1378/chest.07-0038.
- Kweon S, Kim Y, Jang M-J, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES). Int J Epidemiol. 2014;43(1):69–77. doi:10.1093/ije/dyt228.
- Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–1051. doi:10.1136/thx.2008.098483.
- Miller M. ATS/ERS task force: standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Hwang YI, Kim CH, Kang H-R, et al. Comparison of the prevalence of chronic obstructive pulmonary disease diagnosed by lower limit of normal and fixed ratio criteria. J Korean Med Sci. 2009;24(4):621–626. doi:10.3346/jkms.2009.24.4.621.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP.
- Barbeau EM, Krieger N, Soobader M-J. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94(2):269–278. doi:10.2105/AJPH.94.2.269.
- Seo MH, Lee W-Y, Kim SS, et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J Obes Metab Syndr. 2019;28(1):40. doi:10.7570/jomes.2019.28.1.40.
- Carter RL, Sharbaugh CO, Stapell CA. Reliability and validity of the 24-hour recall. J Am Diet Assoc. 1981;79(5):542–547.
- Karvetti R, Knuts LR. Validity of the 24-hour dietary recall. J Am Diet Assoc. 1985;85(11):1437–1442.
- Siedlinski M, Postma DS, van Diemen CC, et al. Lung function loss, smoking, vitamin C intake, and polymorphisms of the glutamate-cysteine ligase genes. Am J Respir Crit Care Med. 2008;178(1):13–19. doi:10.1164/rccm.200711-1749OC.
- Moon H-K, Kong J-E. Assessment of nutrient intake for middle aged with and without metabolic syndrome using 2005 and 2007 Korean National Health and Nutrition Survey. Korean J Nutr. 2010;43(1):69–78. doi:10.4163/kjn.2010.43.1.69.
- Kim SH, Kim MS, Lee MS, et al. Korean diet: characteristics and historical background. J Ethn Foods. 2016;3(1):26–31. doi:10.1016/j.jef.2016.03.002.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi:10.1016/S0140-6736(07)61380-4.
- Anker S, John M, Pedersen P, et al. ESPEN guidelines on enteral nutrition: cardiology and pulmonology. Clin Nutr. 2006;25(2):311–318. doi:10.1016/j.clnu.2006.01.017.
- Ferreira IM, Brooks D, White J, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi:10.1002/14651858.CD000998.pub3.
- Morley JE, Thomas DR, Wilson M-M. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr.2006;83(4):735–743. doi:10.1093/ajcn/83.4.735.
- Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26(4):389–399. doi:10.1016/j.clnu.2007.03.008.
- von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1(1):1–5. doi:10.1007/s13539-010-0002-6.
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi:10.1056/NEJMoa032158.
- Heffner JE, Repine JE. Pulmonary strategies of antioxidant defense 1. Am Rev Respir Dis. 1989;140(2):531–554. doi:10.1164/ajrccm/140.2.531.
- Dao DQ, Ngo TC, Thong NM, et al. Is vitamin A an antioxidant or a pro-oxidant? J Phys Chem B. 2017;121(40):9348–9357. Oct 12doi:10.1021/acs.jpcb.7b07065.
- Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol. 2008;180(3):1834–1842. doi:10.4049/jimmunol.180.3.1834.
- McGowan SE, Smith J, Holmes AJ, et al. Vitamin A deficiency promotes bronchial hyperreactivity in rats by altering muscarinic M2 receptor function. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L1031–L1039. doi:10.1152/ajplung.00319.2001.
- Wood LG, Garg ML, Smart JM, et al. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96(3):534–543. doi:10.3945/ajcn.111.032623.
- Kelly Y, Sacker A, Marmot M. Nutrition and respiratory health in adults: findings from the health survey for Scotland. Eur Respir J. 2003;21(4):664–671. doi:10.1183/09031936.03.00055702.
- Valenca SS, Bezerra FS, Romana-Souza B, et al. Supplementation with vitamins C and E improves mouse lung repair. J Nutr Biochem. 2008;19(9):604–611. doi:10.1016/j.jnutbio.2007.08.004.
- Park HJ, Byun MK, Kim HJ, et al. Dietary vitamin C intake protects against COPD: the Korea National Health and Nutrition Examination Survey in 2012. Int J Chronic Obstr Pulm Dis. 2016;11:2721. doi:10.2147/COPD.S119448.
- Mohsenin V. Effect of vitamin C on NO2-induced airway hyperresponsiveness in normal subjects. Am Rev Respir Dis. 1987;136(6):1408–1411. doi:10.1164/ajrccm/136.6.1408.
- Zhang Z, Shi L, Pang W, et al. Dietary fiber intake regulates intestinal microflora and inhibits ovalbumin-induced allergic airway inflammation in a mouse model. PLoS ONE. 2016;11(2):e0147778. doi:10.1371/journal.pone.0147778.
- Varraso R, Willett WC, Camargo CA. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. Am J Epidemiol. 2010;171(7):776–784. doi:10.1093/aje/kwp455.
- Szmidt MK, Kaluza J, Harris HR. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: a prospective cohort study of women. Eur J Nutr. 2019. doi:10.1007/s00394-019-02038-w.
- Keranis E, Makris D, Rodopoulou P, et al. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36(4):774–780. doi:10.1183/09031936.00113809.
- Kuo C-D, Shiao G-M, Lee J-D. The effects of high-fat and high-carbohydrate diet loads on gas exchange and ventilation in COPD patients and normal subjects. Chest. 1993;104(1):189–196. doi:10.1378/chest.104.1.189.
- Vermeeren MA, Wouters EF, Nelissen LH, et al. Acute effects of different nutritional supplements on symptoms and functional capacity in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2001;73(2):295–301. doi:10.1093/ajcn/73.2.295.
- Franssen FM, Sauerwein HP, Ackermans MT, et al. Increased postabsorptive and exercise-induced whole-body glucose production in patients with chronic obstructive pulmonary disease. Metabolism. 2011;60(7):957–964. doi:10.1016/j.metabol.2010.09.004.
- Fattal-Valevski A. Thiamine (vitamin B1). J Evid Based Complement Altern Med. 2011;16(1):12–20. doi:10.1177/1533210110392941.
- Bettendorff L, Wins P. Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS J. 2009;276(11):2917–2925. doi:10.1111/j.1742-4658.2009.07019.x.
- Sergi G, Coin A, Marin S, et al. Body composition and resting energy expenditure in elderly male patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(11):1918–1924. doi:10.1016/j.rmed.2006.03.008.
- Quanjer PH, Hall GL, Stanojevic S, et al. Age-and height-based prediction bias in spirometry reference equations. Eur Respir J. 2012;40(1):190–197. doi:10.1183/09031936.00161011.
- Hwang YI, Park YB, Yoo KH. Recent trends in the prevalence of chronic obstructive pulmonary disease in Korea. Tuberc Respir Dis. 2017;80(3):226–229. doi:10.4046/trd.2017.80.3.226.
- Halbert R, Natoli J, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi:10.1183/09031936.06.00124605.
- Kipnis V, Subar AF, Midthune D, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158(1):14–21. doi:10.1093/aje/kwg091.
- Resnicow K, Odom E, Wang T, et al. Validation of three food frequency questionnaires and 24-hour recalls with serum carotenoid levels in a sample of African-American adults. Am J Epidemiol. 2000;152(11):1072–1080. doi:10.1093/aje/152.11.1072.
- Kim Y, Park S, Kim N-S, et al. Inappropriate survey design analysis of the Korean National Health and Nutrition Examination Survey may produce biased results. J Prev Med Public Health. 2013;46(2):96. doi:10.3961/jpmph.2013.46.2.96.
- Park Y-M, Yoon H-I, Sohn C-M, et al. Nutritional status of chronic obstructive pulmonary disease patients according to the severity of disease. J Nutr Health. 2008;41(4):307–316.