Abstract
Chronic obstructive pulmonary disease (COPD) is the 4th leading cause of death in the United States. Due to the substantial public health burden of COPD, there has been a lot of interest in developing new drug therapies, directed at improving the symptomatology and quality of life in COPD patients. Revefenacin is the first once daily nebulized long acting muscarinic antagonist for COPD treatment. It offers an advantage over other nebulized bronchodilators, as once daily administration may improve patient compliance. Revefenacin has a rapid onset of action, is long acting and significantly improves lung function (FEV1) in patients with COPD. It can play a major role in the management of COPD, especially in patients who have difficulty mastering inhaler techniques and those with low baseline FEV1 who may have difficulty generating flow with an inhaler. This manuscript is a review on revefenacin and outlines the pharmacologic profile and the clinical trials which have evaluated it’s the efficacy and safety. The authors also discuss their own perspective on the potential role of revefenacin in COPD management.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disorder characterized by airway obstruction caused by exposure to cigarette smoke, particulates, or other noxious fumes. It is currently the 4th leading cause of death in the United States and is projected to become the 3rd leading cause of death worldwide by 2030 [Citation1,Citation2]. There is considerable worldwide variation in the prevalence of COPD based on the geographic region studied. One study found that the reported prevalence of COPD ranged from 0.2% in Japan to 37% in the USA [Citation3]. Moreover, the reported prevalence of COPD in different parts of the world varied based on the age group studied and the criteria used to define COPD. Although COPD incidence has increased over the last 20 years, within the last 10 years, there has been found to be an overall decrease in COPD incidence. Overall, the incidence of COPD has been found to be higher in men as compared to women [Citation4] and is also higher in older individuals, especially those >75 years of age [Citation5].
Given the substantial public health burden associated with COPD, there has been a lot of interest in developing new drugs, directed at improving the symptomatology and quality of life in COPD patients. These medications are largely targeted toward improving bronchodilation, as bronchoconstriction is central to the symptoms in COPD.
Commonly used bronchodilators (BDs) in COPD include β-2 agonists and muscarinic antagonists. Both of these drug classes have short acting and long acting forms for rescue therapy and maintenance treatment in COPD, respectively. Long acting β-2 agonists (LABAs) and long acting muscarinic antagonists (LAMAs) form the cornerstone of maintenance treatment in COPD and hence recent drug development has focused on newer drugs in these classes. LABAs and LAMAs work via their action on the β2 adrenoreceptors and muscarinic receptors, respectively [Citation6]. LABAs such as Salmeterol are defined by a drug half-life that allows a twice a day dosing interval. Ultra-LABAs are ultra-long acting and are dosed once a day (depending on dose) such as indacaterol [Citation7], olodaterol [Citation8] and vilanterol [Citation9]. LAMAs such as tiotropium are long acting drugs and are dosed once a day. Besides improvement in bronchodilation, LABAs and LAMAs, have been shown to reduce the frequency of exacerbations in COPD [Citation10]. This may be due to the fact that LABAs have benefits in COPD which extend beyond their bronchodilatory effects. These include increased mucociliary clearance, anti-inflammatory effects on the airway smooth muscle, and effects on airway remodeling [Citation11]. Evidence is emerging that LABA/LAMA combinations have a synergistic rather than just an additive effect [Citation12]. This has led to the development of several LABA/LAMA combinations for COPD treatment. Treatment with inhaled corticosteroids (ICS) has also been shown to decrease the risk of exacerbations in COPD [Citation13] but is associated with increased risk of pneumonia [Citation14].
LAMAs have a significant therapeutic role in the management of COPD. The most recent Global Initiative for Obstructive Lung Disease (GOLD) recommendations [Citation15] continue to use the ABCD classification for grades of COPD severity, based on exacerbation frequency and symptom burden. The degree of airflow limitation is no longer used to guide treatment, unlike in the 2017 GOLD recommendations, thus recognizing that forced expiratory volume in 1 second (FEV1) alone is a poor marker for disease severity in COPD [Citation16]. For group A COPD patients, a trial of short-acting bronchodilator for intermittent symptoms and long-acting bronchodilator for low-grade persistent symptoms has been recommended, although the need for ongoing therapy/changing therapy should be reassessed based on treatment response. For group B patients, long-acting bronchodilator monotherapy with escalation to dual therapy has been recommended for persistent symptoms. LAMAs can be used for long acting bronchodilator therapy in groups A and B COPD patients. For group C patients, or frequent exacerbators with a low symptom burden, LAMA monotherapy is preferred which can be escalated to LABA/LAMA therapy, as the preferred option over inhaled corticosteroids (ICS)/LABA, given the higher risk of pneumonia reported with ICS in some studies [Citation17]. For group D patients, who have frequent exacerbations and high symptom burden, baseline therapy might include LAMA, LAMA/LABA or ICS/LABA, with escalation to triple therapy with ICS/LABA/LAMA if indicated.
BDs can be administered by several mechanisms such as nebulizers, metered dose inhalers, soft mist inhalers, breath actuated devices and dry powder inhalers [Citation18]. lists out the various long acting BDs (both LABAs and LAMAs) which are currently available in the US market as of fall 2019.
Table 1. Long acting bronchodilators currently available in the US market.
While there are multiple LAMA’s available as dry powder or soft mist inhalers, revefenacin is the first once daily nebulized LAMA for COPD treatment. The goal of this article is to highlight the key clinical trials and other relevant data which led to the approval of revefenacin and discuss the role of this nebulized LAMA in the management of COPD. Keywords such as ‘COPD’, ‘GOLD’, ‘bronchodilators’, ‘LAMA’, ‘Revefenacin’ and databases including PubMed, and MEDLINE were used in searches, which were limited to English language. Information on completed and ongoing studies was also retrieved from clinicaltrials.gov.
Pharmacologic profile
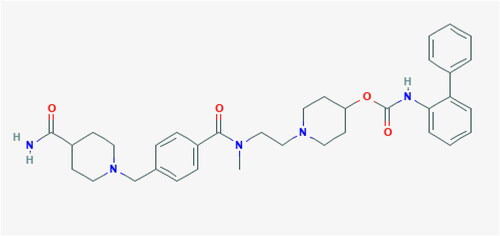
The chemical structure of revefenacin is shown in (downloaded from the National Center for Biotechnology Information. PubChem Database. Revefenacin, CID = 11,753,673, https://pubchem.ncbi.nlm.nih.gov/compound/Revefenacin, accessed on July 2, 2019).
Early animal studies showed that revefenacin produced potent protection against the bronchoconstrictor effect of cholinergic agents such as acetylcholine and methacholine in rats and dogs, which lasted for ≥24 hours and was comparable in duration to tiotropium [Citation19]. Another study found revefenacin to be a high affinity competitive antagonist at human recombinant muscarinic cholinergic receptor (mAChRs) with selectivity for M3 over M2 receptors [Citation20]. This study evaluated the action of revefenacin at human recombinant mAChRs and in airway tissues from rats, guinea pigs, and humans. Revefenacin was found to dissociate at a significantly slower rate from the human M3 receptors (t1/2 = 82 minutes) as compared to the human M2 receptors (t1/2 = 6.9 minutes) at 37 °C, thus making it kinetically more selective for the M3 subtype. In isolated tracheal tissues from rat and guinea pig and isolated bronchial tissues from humans, revefenacin was a potent antagonist of mAChR‐mediated contractile responses. This antagonist effect of revefenacin in rat, guinea pig, and human airway tissues was slowly reversible (t1/2 of 13.3, >16, and >10 hours, respectively). The potency of revefenacin at human mAChRs was found to be similar to that of glycopyrrolate but less than that of tiotropium in this study.
The benefit of lung selectivity is that systemic anti-muscarinic side effects are minimized, while having a potent and long acting bronchodilator effect. These early pre-clinical studies set the stage for clinical trials of revefenacin in humans.
Clinical efficacy
Two phase 2 clinical trials (Study 0059 and Study 0091) [Citation21] evaluated the pharmacodynamics (bronchodilator activity), pharmacokinetics (PK) and safety of single and multiple-dose administrations of revefenacin (TD-4208) in patients with moderate to severe COPD. In Study 0059, 32 patients were randomized to receive a single dose of revefenacin (350 or 700 μg), ipratropium (500 μg) or placebo inhalation solution administered via standard jet nebulizer in a double-blind, crossover manner. In Study 0091, 59 patients were randomized to receive once-daily inhalations of revefenacin (22, 44, 88, 175, 350 or 700 μg) or placebo for 7 days in a double-blind, five-way crossover design. The primary endpoint studied was change from baseline in peak (0–6 hours) FEV1 in Study 0059, and trough FEV1 after the final dose (Day 7) in Study 0091. In both studies, secondary endpoints included area under the FEV1-time curve (FEV1 AUC) values from time 12–24 hours post dose and FEV1 AUC values from time zero to 24 hours post dose. In Study 0059, mean peak FEV1 was significantly higher (p < 0.001) for revefenacin and ipratropium as compared to placebo; differences seen were 176.8 mL for 350 μg revefenacin, 162.2 mL for 700 μg revefenacin and 190.6 mL for ipratropium. In Study 0091, mean trough FEV1 on Day 7 was significantly higher (p < 0.006) for all revefenacin doses as compared to placebo, with differences ranging from 53.5 mL (22 μg dose) to 114.2 mL (175 μg dose). Revefenacin was seen to have long lasting (≥24 hours) bronchodilator effect following nebulized administration, unlike ipratropium which showed no evidence of bronchodilation from 12 to 24 hours post dose. The peak bronchodilator effect achieved with revefenacin was similar to that achieved with ipratropium in study 0059. It was found to be rapidly absorbed and extensively metabolized, with minimal accumulation after repeated dosing. In both studies, no significant adverse events were seen and there was no indication of significant systemic anti-cholinergic activity at any dose.
In a dose finding study of revefenacin (NCT02040792) [Citation22], 355 COPD patients (mean age 62 years, mean forced expiratory volume in 1 second [FEV1] 44% of predicted) were randomized in a double-blind, placebo-controlled parallel group study. Inhaled corticosteroids and short-acting bronchodilators were permitted. Once-daily treatments (44, 88, 175 or 350 μg revefenacin or matching placebo) were administered via a standard jet nebulizer, for 28 days. The primary endpoint was change from baseline in D28 trough FEV1, while the secondary endpoints included weighted mean (wm) FEV1 over 0 to 24 hours and rescue medication (albuterol) use. Wm FEV1 is a statistical measure derived by calculating the area under the curve, and then dividing the value by the relevant time interval. Safety evaluations were also done to assess the safety and adverse effect profile of revefenacin. Revefenacin (88, 175 and 350 μg) significantly improved D28 trough FEV1 over placebo (187.4, 166.6 and 170.6 mL, respectively, all p < 0.001); the 44 μg dose produced a sub-therapeutic response. For doses ≥88 μg, D28 24 hours wm differences from placebo for FEV1 were numerically similar to respective trough FEV1 values, thus indicating a sustained bronchodilator effect of revefenacin for 24 hours post dose. Doses ≥88 μg reduced the average number of albuterol puffs/day by more than one puff/day. The 350 μg dose did not demonstrate additional efficacy over that seen with the 175 μg revefenacin. Revefenacin was generally well tolerated, with minimal systemic anti-cholinergic effects. The authors concluded that 88 and 175 μg revefenacin are appropriate doses for use in longer-term safety and efficacy trials.
In two replicate phase III, randomized, double-blind, placebo-controlled clinical trials of revefenacin (NCT02459080 and NCT02512510) which evaluated patients aged >40 years with moderate to very severe COPD, 12 weeks of revefenacin treatment was found to significantly improve FEV1 [Citation23,Citation24]. At entry, 38.1% of patients were taking concomitant LABA or LABA/ICS therapy and 34.5% had very severe COPD (classified as GOLD D) in the 2 replicate trials [Citation25]. Pooled data showed that, compared with placebo (n = 428), patients treated with once daily revefenacin 88 μg (n = 425) or 175 μg (n = 402) had significantly (p ≤ 0.001) greater improvements in the 24 hours change from baseline in trough FEV1 on day 85 [between-group differences (BGDs) 118 mL (95% CI 86–150 mL) and 145 mL (95% CI 113–178 mL)] (primary endpoint) and the overall treatment effect in trough FEV1 over 12 weeks (88 μg: BGD 112 mL, 95% CI 106–118 mL; 175 μg: BGD 139 mL, 95% CI 133–145 mL) [Citation23,Citation25].
According to a 24 hours serial spirometry sub-study (n = 264) on day 84, revefenacin 88 or 175 μg were associated with significant (p < 0.01) improvements in trough FEV1 at all time points as compared to placebo (BGDs 111–185 and 154–269 mL) [Citation26], thus indicating that the bronchodilator effect of revefenacin was sustained for at least 24 hours post-dose. Both dosages of revefenacin were associated with a small reduction in the annualized rate of COPD exacerbations which was not statistically significant as compared to the placebo group [Citation27]. Significant (p ≤ 0.0203) improvements from baseline in health-related quality of life indicators [assessed by St. George’s Respiratory Questionnaire (SGRQ) and COPD Assessment Test (CAT)] with either revefenacin dose versus placebo were seen in only one (NCT02459080) of the two trials. Despite improvements from baseline in the revefenacin 175 μg group, statistically significant differences in the second trial (NCT02512510) were not seen, as the placebo group showed a greater than expected response [Citation28].
In a randomized, double-blind phase IIIb study, patients with moderate to very severe COPD (n = 207) and a PIFR (peak inspiratory flow rate, which is the fastest flow rate noted during inspiration) of <60 L/min (NCT03095456), were randomized to receive nebulized revefenacin 175 μg once daily or tiotropium for 28 days [Citation29]. On day 29, both the revefenacin and tiotropium groups showed improvements in trough FEV1 and FVC from baseline, with improvements favoring revefenacin over tiotropium; however, the differences were not statistically significant (FEV1: p = 0.481; FVC: p = 0.104). In a pre-specified subgroup analysis, revefenacin significantly (p ≤ 0.0407) improved trough FEV1 and FVC from baseline as compared to tiotropium in patients with severe to very severe COPD (approximately 80% of enrolled patients) [Citation29].
In a randomized, parallel-group, 52-week trial (NCT02518139), 1055 subjects with moderate to very severe COPD received revefenacin 175 μg or 88 μg in a double-blind manner, or open-label active control tiotropium [Citation30]. Subjects on a stable dose of LABA or LABA/ICS for ≥30 days at the time of screening (50.1% of the study group) were permitted to continue in the study. Over 52-weeks, both doses of revefenacin, as well as tiotropium, produced significant improvements (all p < 0.0003) from baseline in trough FEV1. The trough FEV1 profile (least squares mean change from baseline) for revefenacin 175 μg ranged from 52.3 to 124.3 mL and the trough FEV1 profile for tiotropium ranged from 79.7 to 112.8 mL. This established the long-term efficacy of once daily nebulized revefenacin as a bronchodilator in treatment of COPD.
Safety and tolerability
Revefenacin has been found to be safe and well tolerated with a low incidence of adverse events (AE) and few serious adverse events (SAE) noted in clinical trials. In the Pudi et al.’s study [Citation22], 12 subjects of the 355 enrolled (3.4%) had an AE that led to treatment discontinuation. This was seen more frequently in the two highest-dose revefenacin treatment groups (7.0% [n = 71] and 5.4% [n = 74] in the 175 μg and 350 μg groups, respectively) than in the other treatment groups (1.4% [n = 71], 1.5% [n = 68] and 1.4% [n = 71] in the placebo and revefenacin 44 μg and 88 μg groups, respectively). All AEs were self-resolving except one event of respiratory distress which occurred in a placebo patient. Commonest AEs noted were headache (ranging from 1.5% in revefenacin 44 μg group to 6.8% in revefenacin 350 μg group), shortness of breath (ranging from 0 to 4.2% in the various revefenacin groups) and cough (ranging from 0 to 4.2% in the various revefenacin groups). Worsening of COPD (4 exacerbations and one worsening of symptoms) was seen in 5 out of the 355 subjects. A small proportion of study subjects reported back pain (1.1%) and oropharyngeal pain (1.1%). Four SAEs were reported during the study; one in the 44 μg group (supraventricular tachycardia), two in the 175 μg group (unstable angina and hypertension) and one in the 350 μg group (intestinal obstruction), all of which resolved and were not felt to be related to revefenacin. Few anti-muscarinic side effects were noted; these included two incidents of dry mouth. No significant EKG changes were noted on Holter monitoring.
Another Phase 3 safety study of revefenacin was conducted (NCT02518139; n = 1055); this was a randomized, active-controlled parallel group trial designed to evaluate the safety and tolerability of nebulized revefenacin (88 μg or 175 μg, inhaled once daily) over 52 weeks, as compared to the standard of care [Citation31]. Tiotropium (Spiriva®), administered via a handheld device, served as the active comparator standard of care treatment arm. 50% of patients in this study were using other COPD therapies, including LABA or LABA/ICS. Low rates of AEs and SAEs for both doses of revefenacin were seen, and were comparable to tiotropium. Rates of SAEs were 15.9% in the 88 μg revefenacin group, 12.8% in the 175 μg revefenacin group vs 16.3% in the tiotropium group. All-cause mortality rates were low (0.27% in the 88 μg revefenacin group, 0.30% in the 175 μg revefenacin group vs. 0.28% in the tiotropium group). These were deemed by the investigators as not related to study treatment. The most commonly reported adverse events were COPD exacerbations, nasopharyngitis, upper respiratory tract infections, and cough. COPD exacerbation or worsening was the most frequent AE in all groups (n = 280 [26.5%]), and occurred at a lower proportion in the revefenacin 175 μg group (97 events in 73 [21.8%] patients) than in the other two treatment groups (revefenacin 88 μg, 145 events in 107 [29.4%] patients; tiotropium, 137 events in 100 [28.1%] patients) [Citation31]. The numerical frequency of anti-muscarinic side effects such as dry mouth and constipation was lowest in the revefenacin arms.
In a study designed to evaluate the safety of revefenacin in subjects with hepatic impairment [Citation32], revefenacin Cmax (maximum observed plasma revefenacin concentration) and AUCinf (area under the concentration–time curve from time 0 to infinity) were 1.03- and 1.18-fold higher, respectively, in subjects with moderate hepatic impairment as compared to those with normal hepatic function. The corresponding changes in THRX-195518 (the major metabolite of revefenacin), Cmax and AUCinf were 1.5- and 2.8-fold higher, respectively. The increase in plasma exposure to THRX-195518 in subjects with moderate hepatic impairment is unlikely to be clinically significant given its low antimuscarinic potency, and low systemic levels after inhaled revefenacin administration.
Thus overall, the safety profile of revefenacin is consistent with a superior lung selectivity index (ratio of anti-sialagogue to bronchoprotective potency) with inhaled revefenacin compared with either glycopyrronium or tiotropium as seen in preclinical studies [Citation19]. A low incidence of anti-muscarinic side effects and high bronchodilator efficacy positions it as an effective therapeutic agent for COPD. The clinical trials evaluating Revefenacin are outlined in .
Table 2. Clinical trials evaluating revefenacin.
Regulatory affairs
Theravance and Mylan announced in November 2018 that revefenacin had been approved by the FDA as maintenance treatment for COPD [Citation33]. It is marketed under the brand name Yupelri ™ in the United States.
Role of revefenacin in COPD management: authors’ perspective
LAMAs are a mainstay of COPD treatment and can be used in all groups of COPD patients in the ABCD classification system [Citation15]. They provide robust bronchodilation and decrease the risk of COPD exacerbations [Citation34]. LAMAs have also been shown to be effective in combination with LABAs [Citation35] and as triple therapy with LABA/LAMA/ICS [Citation36] in the management of COPD patients with significant symptom burden and frequent exacerbations. However, it has taken several years for the pharmaceutical industry to introduce a nebulized LAMA on the market.
BDs can be administered by several mechanisms such as nebulizers, metered dose inhalers, soft mist inhalers, breath actuated devices and dry powder inhalers [Citation18]. Drug deposition into the lungs is the result of a complex interaction between the device, patient technique and the aerosol formulation [Citation37]. Pressurized metered dose inhalers (pMDIs) include the drug as a suspension which must be shaken prior to inhalation or as a solution. Coordination between inhalation and device actuation is required with pMDIs for proper drug delivery into the lungs. Breath-activated pMDIs which release the drug with inhalation have been devised to avoid the errors associated with lack of coordination. Respimat is a soft mist inhaler which atomizes the drug and produces a fine mist, thus optimizing lung deposition [Citation38]. There are also dry powder inhalers (DPIs) which include single dose (Breezhaler, HandiHaler and Aerosolizer), multiple dose (Diskus) and reservoir (Turbuhaler) inhaler devices. DPIs deliver the drug by inhalation and hence coordinated activation is not required. However, DPIs require higher peak inspiratory flows for optimal drug delivery into the lungs than pMDIs [Citation39]. Thus all these delivery devices come with specific instructions on proper use and patient training is required for optimal use of each of these. Moreover, some devices maybe more suited for use in certain patient populations than others, for example, lack of coordination may cause a higher risk of errors with pMDI use in the elderly.
Errors in the use of an inhalation device are common [Citation40] and can increase risk of worsening COPD symptoms and COPD exacerbations [Citation41]. Factors which increase the probability of poor inhaler technique include older age, multiple medications and lack of education on inhaler technique [Citation42]. In addition, low inspiratory flow rates [Citation43], cognitive deficits especially in elderly patients and poor hand-mouth coordination can make it hard to achieve optimal drug delivery in the lungs with inhalation devices. Thus in certain patient populations, administration of BDs via nebulizer offers substantial benefit over dry powder and metered dose inhalers as stated in the GOLD recommendations [Citation15].
While nebulized LABA options, such as formoterol fumarate marketed as Perforomist® and arformoterol tartrate marketed as Brovana®, have been available for some time, nebulized LAMA options are only recently being developed. These include glycopyrrolate marketed as Lonhala Magnair, and most recently revefenacin marketed as Yupelri™.
Revefenacin can be administered via any standard jet nebulizer with a mouthpiece connected to an air compressor, per the package insert (https://dailymednlmnihgov/dailymed/fda/fdaDrugXslcfm?type=display&setid=6dfebf04-7c90-436a-9b16-750d3c1ee0a6). Specifically, the safety and efficacy of revefenacin was established in clinical trials when administered using the PARI LC® Sprint nebulizer with a mouthpiece and the PARI Trek® S compressor. The safety of revefenacin has not been evaluated in anything other than a standard jet nebulizer (no data has been collected using ultrasonic or vibrating mesh nebulizers). The mean nebulization time for revefenacin in clinical trials using the PARI LC® Sprint nebulizer with a mouthpiece and the PARI Trek® S compressor was 8 minutes. However, nebulization time may differ slightly among the many different standard jet nebulizers available on the market. Variations in nebulization time may also be related to the volume of the medication solution used and patient factors. In contrast to revefenacin, nebulized glycopyrrolate, which is the other nebulized LAMA currently available, is administered via the eFlow® Closed System nebulizer [Citation44], a drug delivery system that provides short nebulization times of around 2–3 minutes. The eFlow CS uses a vibrating membrane to generate a gentle, soft aerosol mist of the drug solution that allows efficient drug uptake, with up to 88% of the nominal drug dose delivered using tidal breathing.
In a Phase 3 b, 42-day, randomized, double-blind, placebo-controlled, parallel group study (NCT03573817; https://www.pulmonologyadvisor.com/home/meetings/chest-2019/chest-2019-in-depth-copd/chronic-obstructive-pulmonary-disease-combo-therapy-revefenacin-and-formoterol/), 122 patients with moderate to very severe COPD were randomized to receive 175 μg revefenacin (n = 63) or placebo (n = 59) followed by 20 μg formoterol via standard jet nebulizer (sequential delivery) each morning and evening for 21 days. Participants then continued another 21 days of treatments, but in this phase of therapy, revefenacin or placebo and formoterol were administered as combined solutions in the morning, and formoterol was given as a single treatment in the evening. In the revefenacin-formoterol sequential groups, the least squares mean in trough FEV1 vs baseline over each 21-day treatment period was 157.1 mL, compared with 53.3 mL in the placebo-formoterol sequential group, 115.6 mL in the revefenacin-formoterol combined group, and 35.0 mL in the placebo-formoterol combined group. No SAEs were reported in any group. Thus the authors concluded that sequential or combined nebulized LAMA and LABA therapy via a standard jet nebulizer could be a treatment option in COPD.
Revefenacin is the first once daily nebulized LAMA for COPD treatment, Lonhala Magnair being administered twice a day. Revefenacin may offer an advantage over other nebulized BDs, as it can improve patient compliance. Dosing frequency has a major impact on medication adherence in chronic diseases [Citation45]. Twice-daily dosing frequency is associated with a lower adherence rate than once-daily dosing, with regimen adherence reduced by 13.1% and timing adherence reduced by 26.7% compared to once daily [Citation45]. Medication non-adherence can increase the risk of worsening COPD symptoms and a higher risk of COPD exacerbations. Revefenacin has a rapid onset of action, is long acting and significantly improves lung function (FEV1) in patients with COPD [Citation46]. Thus, Revefenacin will likely play a major role in maintenance treatment of COPD.
Conclusion
Revefenacin is a once daily nebulized LAMA treatment, and may help to improve patient compliance with medications, thus decreasing the risk of COPD exacerbations. It may be also be useful in patients who have difficulty mastering inhaler techniques and those with reduced pulmonary function.
Declaration of interest
Dr. Lal has received grant support from Jazz pharmaceuticals and Invado pharmaceuticals and is a consultant for Jazz, Cipla pharmaceuticals and Suven Life Sciences. Dr. Khan reports grants from Glaxo Smith Kline, grants from United Theraputics, grants from Reata Pharmaceuticals, grants from Actelion Pharmaceuticals, grants from Lung LLC.
References
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi:10.1016/S0140-6736(96)07492-2.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO.
- Rycroft CE, Heyes A, Lanza L, et al. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–494. doi:10.2147/COPD.S32330.
- Garcia Rodriguez LA, Wallander MA, Tolosa LB, et al. Chronic obstructive pulmonary disease in UK primary care: incidence and risk factors. COPD. 2009;6(5):369–379. doi:10.1080/15412550903156325.
- Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance-United States, 1971-2000. Respir Care. 2002;47(10):1184–1199.
- Cazzola M, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. doi:10.1124/pr.111.004580.
- Baur F, Beattie D, Beer D, et al. The identification of indacaterol as an ultralong-acting inhaled beta2-adrenoceptor agonist. J Med Chem. 2010;53(9):3675–3684. doi:10.1021/jm100068m.
- Bouyssou T, Hoenke C, Rudolf K, et al. Discovery of olodaterol, a novel inhaled beta2-adrenoceptor agonist with a 24 h bronchodilatory efficacy. Bioorg Med Chem Lett. 2010;20(4):1410–1414. doi:10.1016/j.bmcl.2009.12.087.
- Slack RJ, Barrett VJ, Morrison VS, et al. In vitro pharmacological characterization of vilanterol, a novel long-acting beta2-adrenoceptor agonist with 24-hour duration of action. J Pharmacol Exp Ther. 2013;344(1):218–230. doi:10.1124/jpet.112.198481.
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800.
- Santus P, Radovanovic D, Paggiaro P, et al. Why use long acting bronchodilators in chronic obstructive lung diseases? An extensive review on formoterol and salmeterol. Eur J Intern Med. 2015;26(6):379–384. doi:10.1016/j.ejim.2015.05.001.
- Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res. 2013;14(1):49. doi:10.1186/1465-9921-14-49.
- Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi:10.1136/bmj.320.7245.1297.
- Sibila O, Soto-Gomez N, Restrepo MI. The risk and outcomes of pneumonia in patients on inhaled corticosteroids. Pulm Pharmacol Ther. 2015;32:130–136. doi:10.1016/j.pupt.2015.04.001.
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5). doi:10.1183/13993003.00164-2019.
- Mirza S, Clay RD, Koslow MA, et al. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502. doi:10.1016/j.mayocp.2018.05.026.
- Bourbeau J, Aaron SD, Barnes NC, et al. Evaluating the risk of pneumonia with inhaled corticosteroids in COPD: Retrospective database studies have their limitations SA. Respir Med. 2017;123:94–97. doi:10.1016/j.rmed.2016.12.015.
- Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331. doi:10.1183/09031936.00166410.
- Pulido-Rios MT, McNamara A, Obedencio GP, et al. In vivo pharmacological characterization of TD-4208, a novel lung-selective inhaled muscarinic antagonist with sustained bronchoprotective effect in experimental animal models. J Pharmacol Exp Ther. 2013;346(2):241–250. doi:10.1124/jpet.113.203554.
- Hegde SS, Pulido-Rios MT, Luttmann MA, et al. Pharmacological properties of revefenacin (TD-4208), a novel, nebulized long-acting, and lung selective muscarinic antagonist, at human recombinant muscarinic receptors and in rat, guinea pig, and human isolated airway tissues. Pharmacol Res Perspect. 2018;6(3):e00400. doi:10.1002/prp2.400.
- Quinn D, Barnes CN, Yates W, et al. Pharmacodynamics, pharmacokinetics and safety of revefenacin (TD-4208), a long-acting muscarinic antagonist, in patients with chronic obstructive pulmonary disease (COPD): Results of two randomized, double-blind, phase 2 studies. Pulm Pharmacol Ther. 2018;48:71–79. doi:10.1016/j.pupt.2017.10.003.
- Pudi KK, Barnes CN, Moran EJ, et al. A 28-day, randomized, double-blind, placebo-controlled, parallel group study of nebulized revefenacin in patients with chronic obstructive pulmonary disease. Respir Res. 2017;18(1):182. doi:10.1186/s12931-017-0647-1.
- Ferguson GT, Pudi K, Pendyala S, et al. Efficacy of revefenacin, a novel once-daily nebulized long-acting muscarinic antagonist: results of two randomized, double-blind, placebo-controlled, parallel-group phase 3 trials in participants with moderate to very severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:A5474.
- Ferguson GT, Feldman G, Pudi KK, et al. Improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019;6(2):154–165.
- Biopharma T. Revefenacin (TD-4208) phase 3 efficacy results [media release]. 13 Nov 2016.
- Donohue J, Pendyala S, Barnes C, et al. The 24-hour profile of FEV1 after 12-weeks treatment with revefenacin, a once daily long-acting muscarinic receptor antagonists for nebulization: a spirometry substudy [abstract]. Chest 2017;152(4):A770. doi:10.1016/j.chest.2017.08.801.
- Donahue JF, Sethi S, Barnes CN, et al. COPD exacerbations in the phase 3 revefenacin clinical trial program [abstract]. Eur Resp J. 2018;52(Suppl. 62):OA1655.
- Donohue J, Pendyala S, Barnes C, et al. Improvements in health status with revefenacin, a once-daily long-acting muscarinic antagonist for nebulization: changes in St George’s respiratory questionnaire and COPD assessment test in replicate 3-month studies [abstract]. Chest 2017;152(4):A819. doi:10.1016/j.chest.2017.08.850.
- Mahler DA, Ohar JA, Barnes CN, et al. Nebulized versus dry powder long-acting muscarinic antagonist bronchodilators in patients with COPD and suboptimal peak inspiratory flow rate. Chronic Obstr Pulm Dis. 2019;6(4). doi:10.15326/jcopdf.6.4.2019.0137.
- Donohue JF, Kerwin E, Sethi S, et al. Maintained therapeutic effect of revefenacin over 52 weeks in moderate to very severe Chronic Obstructive Pulmonary Disease (COPD). Respir Res. 2019;20(1):241. doi:10.1186/s12931-019-1187-7.
- Donohue JF, Kerwin E, Sethi S, et al. Revefenacin, a once-daily, lung-selective, long-acting muscarinic antagonist for nebulized therapy: safety and tolerability results of a 52-week phase 3 trial in moderate to very severe chronic obstructive pulmonary disease. Respir Med. 2019; 153:38–43. doi:10.1016/j.rmed.2019.05.010.
- Borin MT, Lo A, Barnes CN, et al. Pharmacokinetics and safety of revefenacin in subjects with impaired renal or hepatic function. COPD. 2019;14:2305–2318. doi:10.2147/COPD.S203709.
- Heo YA. Revefenacin: first global approval. Drugs. 2019;79(1):85–91. doi:10.1007/s40265-018-1036-x.
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;21(7):CD009285. doi:10.1002/14651858.CD009285.pub3.
- Calzetta L, Rogliani P, Ora J, et al. LABA/LAMA combination in COPD: a meta-analysis on the duration of treatment. Eur Respir Rev. 2017;26(143):160043. doi:10.1183/16000617.0043-2016.
- Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5.
- Scichilone N, Benfante A, Bocchino M, et al. Which factors affect the choice of the inhaler in chronic obstructive respiratory diseases? Pulm Pharmacol Ther. 2015;31:63–67. doi:10.1016/j.pupt.2015.02.006.
- Dalby R, Spallek M, Voshaar T. A review of the development of Respimat Soft Mist Inhaler. Int J Pharm. 2004;283(1–2):1–9. doi:10.1016/j.ijpharm.2004.06.018.
- Tarsin WY, Pearson SB, Assi KH, et al. Emitted dose estimates from Seretide Diskus and Symbicort Turbuhaler following inhalation by severe asthmatics. Int J Pharm. 2006;316(1–2):131–137. doi:10.1016/j.ijpharm.2006.02.040.
- Souza ML, Meneghini AC, Ferraz E, et al. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35(9):824–831.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. doi:10.1016/j.rmed.2011.01.005.
- van der Palen J, Klein JJ, Kerkhoff AH, et al. Evaluation of the effectiveness of four different inhalers in patients with chronic obstructive pulmonary disease. Thorax. 1995;50(11):1183–1187. doi:10.1136/thx.50.11.1183.
- Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(10):1333–1343. doi:10.1164/rccm.201604-0733OC.
- Kerwin E, Ferguson GT. An overview of glycopyrrolate/eFlow(R) CS in COPD. Expert Rev Respir Med. 2018;12(6):447–459. doi:10.1080/17476348.2018.1476853.
- Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. JMCP. 2012;18(7):527–539. doi:10.18553/jmcp.2012.18.7.527.
- Li F, Yang J. Revefenacin for the treatment of chronic obstructive pulmonary disease. Expert Rev Clin Pharmacol. 2019;12(4):293–298. doi:10.1080/17512433.2019.1587292.