Abstract
Our main objective was to demonstrate that, in smoker patients hospitalised for Chronic Obstructive Pulmonary Disease (COPD) exacerbation, early initiation of varenicline during 12 weeks, combined with an intensive counselling, is associated with a higher continuous abstainers rate (CAR) at one year as compared to intensive counselling alone. In this multicenter, prospective, double-blind, randomised study, 81 smoking COPD patients hospitalised for an acute exacerbation for at least 24 h were allocated to receive either varenicline (n = 42) or placebo (n = 39) for 12 weeks, in association with an intensive counselling in the 2 groups, and followed up for 40 weeks. The primary outcome was CAR at week 52. Secondary outcomes included CAR at week 12 and 26, partial abstinence rate (PAR) at week 12, 26 and 52, nicotinic substitute consumption and adverse events. At week 52, CAR was not different in placebo and varenicline groups (25.6%). At week 12, CAR was significantly higher in the varenicline group (50%) as compared to placebo group (27%) (p = 0.041). Nicotine consumption was significantly higher at week 52 in the placebo group (55.3%) as compared to the varenicline group (24.4%) (p = 0.005). There was no significant difference in PAR at week 12, 26 and 52; the frequency of adverse events was similar between the two groups. Among active smoker COPD patients with exacerbation, 12-week varenicline associated with intensive counselling for smoking cessation increased the rate of continuous abstainers as compared to placebo. However, benefit was not maintained after varenicline discontinuation.
Clinical Trials Registration: URL: http://www.controlled-trials.com. Unique identifier: NCT01694732
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is projected to be the third leading cause of death worldwide by 2020 [Citation1] and the main risk factor of COPD is tobacco smoking [Citation2]. Among available COPD treatment options, including bronchodilators and respiratory rehabilitation [Citation3], achievement of sustained smoking cessation is the most effective therapeutic strategy for reducing lung function decline rate and mortality [Citation3–6]. Smoking cessation is associated with a reduction of both cardiovascular and tobacco-related cancers deaths [Citation7]. Unfortunately, studies showed that the smoking cessation process in COPD patients is hampered by both a high level of tobacco dependence and a high frequency of relapses [Citation8]. As a consequence, quitting smoking is more difficult in smokers with COPD than in smokers without COPD [Citation9,Citation10].
Whereas many smoking cessation studies have been conducted in the general population, only a few have focussed on COPD patients: a meta-analysis including 8 randomised studies showed that intensive counselling associated with nicotinic substitutes is the most effective strategy, as compared to intensive counselling alone [Citation11]. Data on varenicline, a α4β2 neuronal nicotinic acetylcholine receptors partial agonist [Citation12–16], efficacy and safety profiles in COPD smokers are scarce: in one randomised double-blind controlled clinical trial comparing varenicline versus placebo during 12 weeks in 504 stable COPD patients, the rates of continuous abstainers in the varenicline group at 12 weeks and at 12 months were 8-fold and 4-fold higher than in the placebo group, respectively [Citation17]. Although most smoking cessation programmes have been initiated in stable patients, one study has suggested that initiation following admission to hospital due to a serious smoking related illness might also be efficient [Citation18].
We therefore conducted a multicentre, randomised, double blind, clinical trial involving smoker patients hospitalised for COPD exacerbation. The main objective of the study was to demonstrate that, in smoker patients hospitalised for a COPD exacerbation, early initiation of varenicline during 12 weeks, combined with an intensive counselling, is associated with a higher continuous long-term abstainer rate at one year as compared to placebo. Secondary objectives were to assess continuous abstainer rate during the study treatment period and safety issues during entire study period.
Methods
Ethical review and study organisation
The study has been conducted in accordance with the ethical principles stated in the Declaration of Helsinki, the good clinical practice guidelines and the relevant French regulations regarding ethics and data protection. A written informed consent was obtained from all patients before randomisation. An independent ethics committee approved the protocol. Protocol was registered with the number NCT01694732 on ClinicalTrials.gov.
Participants
Current smokers presenting a COPD exacerbation in accordance with ATS/ERS criteria [Citation19] that justified an hospitalisation at least for 24 h in chest medicine or in intensive care units were eligible. Participants were smokers (≥10 cigarettes per day during the last year) and motivated to quit smoking. Patients who used concomitant treatment for smoking cessation at the time of inclusion or/with a past history of severe depression requiring therapy drugs within 5 years or/with 2 or more episodes of severe depression requiring medication and an attempted suicide were not included. Other inclusion and exclusion criteria are listed in supplement (eTable 1). All patients had been enrolled in 11 French hospitals from August 22Th, 2012 to November 19Th, 2014. The last visit took place on November 10Th, 2015.
Randomisation and intervention
At inclusion, participants were randomised to receive placebo or varenicline in a 1:1 ratio. Investigators obtained patient randomisation numbers and treatment group assignments through a central computerised internet-based system. Participants received varenicline (1 mg) or placebo for 12 weeks (titrated during the first week) followed by 40 weeks of follow up after study treatment discontinuation. Appearance, taste and packaging of placebo and varenicline tablets were identical. Active treatment and placebo were available in two assays: 0.5 mg for the first week (0.5 mg once a day for three days, then 0.5 mg twice a day for 4 days) and 1 mg (twice a day) for the following eleven weeks. In case of treatment intolerance, medication dose was stepped down to 1 mg per day.
Intensive counselling consisted in a more than 10 min interview based on COPD patient counselling adapted methods [Citation20–24]. Investigators from each centre were free to choose the kind of motivational and/or behavioural approaches during interviews, taking into account psychological profile, dependence status and patient’s preference. In case of withdrawal from study treatment, intensive counselling was continued and other methods of smoking cessation were proposed.
Assessments and procedures are listed in supplement (eTable 2). Intensive counselling was provided at clinic visits (weeks 1, 4, 8, 12, 26 and 52) and by phone (weeks 2, 18, 34 and 42) for all participants. At each contact (clinic visit or phone call) throughout the study, participants completed Hospital anxiety and depression scale (HAD) and Patient Health Questionnaire 9 (PHQ-9) auto-questionnaires and exhaled Carbone monoxide (CO) level was measured during each follow-up visit. BODE index, spirometry and 6-minute walking distance (6MWD) test were performed on weeks 4, 12, 26 and 52. Saint-George respiratory quality of life questionnaire was performed on weeks 4, 12, 26 and 52.
Outcomes measures
The primary outcome was the continuous abstainers rate (CAR) at week 52, defined by the rate of patients who presented the all following criteria: smoking cessation for weeks 8–12 (last weeks of treatment); exhaled CO level ≤10 ppm at each clinic visit; less than 6 cigarettes to week 12 at 52; complete smoking cessation during the seven previous days before week 52. Secondary outcomes included: CAT at week 12 and 26, punctual abstainer rate (PAR) defined as patient’s rate who did not smoke within 7 days prior to follow-up visit with exhaled CO level of less than 10 ppm at week 12, 26 and 52, adverse events, nicotine substitute consumption. Other secondary outcomes are detailed in supplement (eTable 3).
Sample size and statistical analyses
Sample size
Based on a single study comparing varenicline to a placebo in COPD patients without exacerbation, the expected proportion of patients with complete smoking cessation was 19% in the varenicline group and 6% in the placebo group. Thus, a sample size of 131 patients per group was required to provide 90% power with a 2-sided significance level of 0.05. We anticipated a 5% lost to follow-up rate and therefore aimed to recruit a total of 276 patients. However, the study was prematurely interrupted due to difficulties in recruiting patients and to suspension of industrial founding (co-financed by an institutional French grant), inducing a lack of included patients.
Statistical analyses
The primary outcome was compared in both arms with chi-2 test in non adjusted analysis. Relative risk (RR) was calculated with a 95% confidence interval (CI). The adjusted analysis used a logistic regression, adjusting on confounding factors identified in univariate analysis (selecting variables at p value under 0.15) and on clinically relevant variables. For secondary outcomes, we used the same analyses for CAR at 3 and 6 months and PAR at 3, 6 and 12 months. COPD exacerbation recurrence rates were estimated by Kaplan-Meier method in univariate analysis and between-group comparisons were performed using log-rank test; the multivariate analysis was performed using Cox proportional hazard regression model. Hazard ratios and corresponding 95% confidence intervals were calculated. Clinically relevant variables or selected variables at p-value <0.1 were included in the multivariate analysis. Clinical scales and functional parameters (forced expiratory volume in one second (FEV1), 6MWD, BODE index) were compared using Student or Wilcoxon tests. Statistical analyses were performed with SAS Version 9.4 software (SAS Institute, Cary, NC, USA).
Results
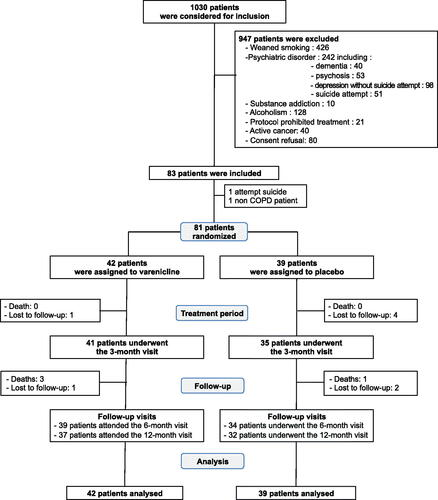
Between August 22Th, 2012 to November 19Th, 2014, 1030 patients were admitted to emergency room from 10 French hospitals for COPD exacerbation. Among these, 947 were not included for the followings: previous smoking cessation (n = 426), psychiatric disorders (n = 242), drug or alcohol abuse (n = 138), active cancer (n = 40), concomitant varenicline-contraindicated treatments (n = 21), and patient refusal to participate (n = 80). Finally, 83 patients were included in the study.
Treatment
Two patients were not randomised because they did not fulfil all inclusion criteria: the first patient revealed just after inclusion that he had an attempted of suicide past history and the second one had no COPD. Thus, the intention-to-treat analysis was performed on 81 patients. Forty-two and 39 patients were randomised into varenicline and placebo treatment groups, respectively (). One patient in the placebo group withdrew his consent and did not receive allocated treatment. In the varenicline group, 5 participants were lost to follow-up (one patient at 3 months, two patients at 6 months and two patients at one year). In the placebo group, 7 patients were lost to follow-up (four patients at 3 months, one patient at 6 months and two patients at one year). Consequently, the analysis of the primary outcome did not include 5 patients in the varenicline group and 7 in the placebo group. Median entire study period was 366 days (IQR 355–381) in the varenicline-treated group and 356 days (IQR 349–367) in the placebo group. Median study treatment period was 83 days (IQR 58–85) in the varenicline treatment group and 77 days (IQR 39–83) in the placebo group. Double-blind treatment was permanently discontinued in 13 varenicline-treated patients (reasons: 9 for study treatment intolerance, 3 for patient’s decision and one was lost to follow-up), and in 18 placebo-treated patients (reasons: 6 for study treatment intolerance, 8 for patient’s decision, 2 were lost to follow-up, one for smoking relapse and one for study protocol violation).
Baseline characteristics
Participants, of mean age 56.8 years, were mostly male (60.5%), smoked an average of 23.1 cigarettes a day and had a mean Fagerström test for nicotine dependence score of 5.2 and a mean FEV1 of 1.6 L in the past year prior inclusion. Sixty-four (79%) of the whole population had previous smoking cessation attempt. Clinicians identified bronchial infection as the main cause of exacerbation and 37% of them were hospitalised in intensive care unit at enrolment. Patient’s characteristics were comparable between groups ().
Table 1. Baseline characteristics.
Table 2. Non-adjusted and adjusted analyses for primary and secondary outcomes.
Primary outcome
At week 52 of follow-up, CAR was achieved in 10 patients (25.6%) in the varenicline-treated group and in 10 patients (25.6%) in the placebo-treated patients, yielding a non statistically significant RR of 1.00 (95%CI, 0.60–1.66). This result was observed both in non-adjusted and in adjusted analysis ().
Table 3. Use of other forms of nicotine consumption at Week 12, 26 and 52.
Secondary outcomes
At week 12, 19 patients (50%) and 10 patients (27%) were abstainers in varenicline group and in placebo group, respectively: week 12 CAR was significantly higher in varenicline group in non-adjusted (RR, 1.59, 95%CI, 1.03–2.45) and in adjusted analysis (RR, 3.57, 95%CI, 1.17–10.89) (). Non-adjusted or adjusted analyses did not show any difference on CAR at week 26, on PAR at weeks 12, 26 or 52, or on 50RR ().
At week 52, there was no difference between the two treatment groups on functional efficacy criteria in non-adjusted analysis. At week 12, recurrent bronchial exacerbation events appeared higher in varenicline group as compared to placebo group (23 versus 19 respectively) but this difference was not detected both in non-adjusted (RR, 1.02, 95%CI, 0.56–1.88), or in adjusted analysis (RR, 1.18, 95%CI, 0.55–2.52). Continuing smokers at Week 52 in placebo group were more likely to consume nicotine in other forms than continuing smokers in Varenicline group (64% vs 32%, p = 0.016) ().
Adverse events
Four patients died during follow-up after study treatment period, three in the varenicline group and one in the placebo group, all unrelated to study treatment. Forty hundred and thirty-five adverse events have been registered during follow-up (see supplement eTable 4 and 5): psychiatric disorders (26%), respiratory disorders (19%), gastro-intestinal disorders (11%) and neurological disorders (7%) were the main causes. Sleep disorder events (abnormal dreams, insomnia, nightmares, poor sleep quality, sleep disorders, abnormal phase of sleep cycle) were more frequent in the varenicline group as compared to those in the placebo group (24 versus 14 patients, respectively). One case of suicidal ideation in the varenicline group and two cases in the placebo group have been reported. Anxio-depressive episodes were slightly more frequent in the placebo group (n = 18) than in the varenicline group (n = 12).
Discussion
In smoker COPD patients admitted for exacerbation, we found that early varenicline initiation over 12 weeks combined with intensive counselling improved smoking cessation. However, this benefit was not maintained after varenicline discontinuation. In addition, given an unexpected high frequency of psychiatric disorders in COPD patients admitted for exacerbation, only a minority of these later might beneficiate of this treatment for smoking cessation.
Concerning our observed treatment effect, our results are similar to that of other randomised trials performed in stable COPD patients [Citation17]. In a multicenter, randomised study, comparing varenicline or placebo for 12 weeks on 504 stable mild or moderate COPD patients, Tashkin and colleagues showed that CAR at weeks 9 to12 was eight-fold higher for patients in the varenicline group (42.3%) than those in placebo group (8.8%) [Citation17]. At the end of the 40-week follow-up period after study treatment discontinuation, if varenicline remained more effective than placebo on CAR 9-52, however, approximately one-half of patients from the varenicline group had relapsed (CAR for weeks 9–52 decreased to 18.6%) whereas CAR for weeks 9–52 in the placebo group remained almost similar to CAR 9–12. These findings in stable COPD patients are similar to general smoker population based studies [Citation12,Citation13,Citation25]. In two similarly designed randomised controlled trials, each one including “healthy” smokers, varenicline was compared to placebo and bupropion given for 12 weeks and combined with smoking cessation counselling. During the study treatment period, CAR 9–12 was four-fold higher in the varenicline group than in the placebo group; after study treatment discontinuation, varenicline remained two-fold more effective than placebo on CAR 9–52 however, in the varenicline group, more than 50% had relapsed [Citation12,Citation13]. Lastly, such observations have been reported in smokers population with cardiovascular disease [Citation26]. These results and ours raise the question of varenicline treatment duration. In that instance, a recent study included 30 heavy COPD smokers receiving varenicline long time enough to achieve smoking cessation [Citation27]: a 18-month CAR of 71% was observed and the median time of treatment was 6 and 2 months for abstainers and non-abstainers, respectively. Interest for varenicline maintenance for more than 12 weeks to further increase cessation rate in COPD population remains to be evaluated.
Smoking cessation optimal initiation time in COPD patients remains unclear. To our knowledge, one open randomised trial has already been published including 392 hospitalised patients who were allocated to receive varenicline plus counselling or counselling alone [Citation18]. Participants were recruited following hospital admission for serious smoking related illness, within the disciplines of respiratory, cardiology, neurology and vascular medicine. COPD-patients proportion was not mentioned. The 12-week CAR was higher in the varenicline group as compared to the control group (48.5% versus 36.2%) and 12-month CAR remained significantly higher in the varenicline group as compared to the control group (31.1% versus 21.4%, RR of 1.45, 95%CI, 1.03–2.04). Thus, the benefit of varenicline on CAR at week 12 lost by about 40% at week 52, showing that smoking cessation benefit decreased when varenicline was discontinued. These results are in line with results reported from outpatient studies. Interestingly, the proportion of patients without relapse at 12 month was 31% in the varenicline group and 21% in the control group, suggesting that hospitalisation time may offer an opportunity for smoking cessation initiation.
If our study is in line with others concerning the treatment effect of varenicline, however, we had to face to an unexpected high rate of non-inclusion criteria in our targeted COPD population, mainly due to a very high frequency of psychiatric disorders and chronic alcoholism. On the 521 COPD patients who were not included in the study for reasons other than previous smoking, 242 (46%) had psychiatric disorders (of which 51 had a previous history of suicide attempt) and 128 (25%) had chronic alcoholism. In a recent cohort study on 1334 patients with chronic respiratory illness including COPD, 65% of COPD patients had depressive and anxiety symptoms of which only 31% were receiving treatment for depression and/or anxiety [Citation28]. Depression in COPD patients has been also found to be associated with an increase in mortality [Citation29]. One could argue that such patients should have been included in our trial, especially considering the reassuring results of the EAGLE randomised controlled trial [Citation30]. However, at the time where the study was performed, these results were not available and the French drug agency and Ethic boards contra-indicated varenicline administration in patients with previous or ongoing psychiatric disorders or alcoholism. In addition, assessing the potential benefit and safety of varenicline in COPD patients with concurrent psychiatric disorders and/or other addiction such as alcoholism would have considerably complexified the analyses to decipher the possible respective influences of these conditions on the results, such that a much greater sample size would have been required [Citation30,Citation31].
The main limitation of our study is the small sample size due to premature interruption in relation with the premature interruption of pharmaceutical funding (the study was co-financed by an institutional French grant and pharmaceutical grant). Therefore, we cannot conclude concerning the primary objective, which was to demonstrate superiority of varenicline over placebo at 12 months on smoking cessation. Nevertheless, primary outcome was similar in the two groups without any trends towards superiority. The strengths of our study include the followings: (i) a prospective enrolment of patients with a documented COPD that was assessment based in accordance with international recommendations; (ii) a double blinded randomised design; (iii) low rate of patients lost to follow-up; and (iiii) similar population characteristics with those observed in previous studies.
Conclusion
Among smoking COPD patients admitted at hospital for an acute exacerbation, early initiation of 12-week varenicline associated with intensive counselling for smoking cessation increased continuous abstainers rate as compared to placebo at 3 months. However, benefit was not sustained after varenicline discontinuation. No difference in safety issues was observed, particularly concerning psychiatric complications. These findings suggest that further trials in COPD patients are warranted (i) to assess the interest of extended treatment period in order to reduce relapse rate after 12 weeks conventional treatment (ii) to compare different times of initiation of smoking cessation.
Declaration of Interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Couturaud reports having received research grant support from Pfizer and fees for board memberships or symposia from Bayer and Astra Zeneca and having received travel support from Bayer, Daiichi Sankyo, Leo Pharma, Intermune and Actelion. Dr. Le Mao declares he has no conflict of interest related to this research. Dr. Tromeur declares she has no conflict of interest related to this research. Dr. Paleiron declares he has no conflict of interest related to this research. Dr. Sanchez reports having received research grant support from Bayer, Daiichi Sankyo and Portola Pharmaceuticals, and fees or non-financial support for consultancy activities from Actelion, GlaxoSmithKline, Boehringer Ingelheim and Chiesi. Dr Gagnadoux declares he has no conflict of interest related to this research. Dr Jouneau reports grants from AIRB, Boehringer Ingelheim, LVL, Novartis and Roche, and personal fees from Actelion, AIRB, AstraZeneca, BMS, Boehringer Ingelheim, Chiesi, GSK, LVL, Mundipharma, Novartis, Pfizer and Roche. Dr A. Magnan reports personal fees and non-financial support from GlaxoSmithKline, Novartis, Boehringer Ingelheim, AstraZeneca, Stallerg nes, ALK, MundiPharma, Teva, Menarini and Meda Pharma, during the past 5 years. Dr Hayem-Vannimenus declares she has no conflict of interest related to this research. Dr Dansou declares she has no conflict of interest related to this research. Dr Proust reports fees for consulting from Novartis and personal fees or nonfinancial support from AstraZeneca, Boehringer, Chiesi, Mundifarma, Glaxo‐Smith‐Klein, Novartis, Pearl, Portola, Roche, Sanofi, and Teva. Ms Dion declares he has no conflict of interest related to this research. Dr Larhantec declares he has no conflict of interest related to this research. Ms Le Brestec declares she has no conflict of interest related to this research. Dr. Dewitte declares he has no conflict of interest related to this research. Dr. Roche declares he has no conflict of interest related to this research. Dr Leroyer reports having received research grant support from Pfizer and fees for board memberships or symposia from Bayer and Astra Zeneca and having received travel support from Bayer, Daiichi Sankyo, Leo Pharma, Intermune and Actelion.
Author contributions
Dr Le Mao had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: F. Couturaud, C. Leroyer, R. Le Mao, C. Tromeur. Acquisition of data: A. Le Brestec. Statistical analysis: A. Dion. Analysis and interpretation of data: All. Drafting of the manuscript: F. Couturaud, R. Le Mao, C. Tromeur, N. Paleiron, C. Leroyer. Critical revision of the manuscript for important intellectual content: All. Final approval of the manuscript: All. Obtaining funding: F. Couturaud. Administrative, technical, or material support: F. Couturaud, C. Leroyer. Study supervision: F. Couturaud.
Role of the funding source
The funding source was not involved in designing or conducting the study, collecting, managing, analysing or interpreting the data, preparing, reviewing or approving the manuscript, or deciding to submit this for publication. An academic steering committee assumed overall responsibility for all these steps. Dr Couturaud takes responsibility for data access and integrity of the data.
Supplemental Material
Download PDF (427.5 KB)Acknowledgment
No member of the SAVE Study Group received compensation for his/her role in the study.
Additional information
Funding
References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370(9589):765–773. doi:10.1016/S0140-6736(07)61380-4.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2009. Available from: http://www.goldcopd.org/
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675–679. doi:10.1164/rccm.2112096.
- Godtfredsen NS, Lam TH, Hansel TT, et al. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J. 2008;32(4):844–853. doi:10.1183/09031936.00160007.
- Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi:10.7326/0003-4819-142-4-200502150-00005.
- Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497–1505. doi:10.1001/jama.1994.03520190043033.
- Jimenez-Ruiz CA, Masa F, Miravitlles M, et al. Smoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPD. Chest. 2001;119(5):1365–1370. doi:10.1378/chest.119.5.1365.
- Tashkin D, Kanner R, Bailey W, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet 2001;357(9268):1571–1575. doi:10.1016/S0140-6736(00)04724-3.
- Willemse B, Lesman-Leegte I, Timens W, et al. High cessation rates of cigarette smoking in subjects with and without COPD. Chest. 2005;128(5):3685–3687. doi:10.1378/chest.128.5.3685.
- Strassmann R, Bausch B, Spaar A, et al. Smoking cessation interventions in COPD: a network meta-analysis of randomised trials. Eur Respir J. 2009;34(3):634–640. doi:10.1183/09031936.00167708.
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi:10.1001/jama.296.1.47.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi:10.1001/jama.296.1.56.
- Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183(12):1359–1366. doi:10.1503/cmaj.110218.
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117(1):2–10. Discussion 4-20. doi:10.1007/BF02245088.
- Tonstad S, Davies S, Flammer M, et al. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf. 2010;33(4):289–301. doi:10.2165/11319180-000000000-00000.
- Tashkin DP, Rennard S, Hays JT, et al. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139(3):591–599. doi:10.1378/chest.10-0865.
- Smith BJ, Carson KV, Brinn MP, et al. Smoking Termination Opportunity for inPatients (STOP): superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: a 12-month randomised controlled trial for inpatients. Thorax. 2013;68(5):485–486. doi:10.1136/thoraxjnl-2012-202484.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi:10.1183/09031936.04.00014304.
- Rollnick S, Mason P, Butler C. Health behavior change: a guide for practitioners. New York: Churchill Livingstone; 1999.
- Fiore MC. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care. 2000;45(10):1196–1199.
- Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;(4):Cd000165.
- Miller W, Rollnick S. Motivationnal interviewing: preparing people for change. 2nd ed. New York (NY): Guilford Press; 2002. p. 428.
- Tonnesen P, Mikkelsen K, Bremann L. Nurse-conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest. 2006;130(2):334–342. doi:10.1378/chest.130.2.334.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011;(2):Cd006103.
- Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–229. doi:10.1161/CIRCULATIONAHA.109.869008.
- Sansores RH, Ramirez-Venegas A, Arellano-Rocha R, et al. Use of varenicline for more than 12 months for smoking cessation in heavy chronic obstructive pulmonary disease smokers unmotivated to quit: a pilot study. Ther Adv Respir. 2016;10(5):383–390. doi:10.1177/1753465816654823.
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205–1211. doi:10.1016/S0012-3692(15)34468-8.
- de Voogd JN, Wempe JB, Koëter GH, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest. 2009;135(3):619–625. doi:10.1378/chest.08-0078.
- Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520.
- O’Malley SS, Zweben A, Fucito LM, et al. Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):129–138. doi:10.1001/jamapsychiatry.2017.3544.
Appendix
Members of the SAVE Study Group (all in France) were as follows: Steering Committee - F. Couturaud, MD (Chair), Centre Hospitalo-Universitaire de Brest, Brest, C. Leroyer, MD, Centre Hospitalo-Universitaire de Brest, Brest, O. Sanchez, MD, Hôpital Européen Georges Pompidou, Paris, N. Roche, MD, Hôpital Cochin, Paris, F. Gagnadoux, MD, Centre Hospitalo-Universitaire d’Angers, S. Jouneau, MD, Centre Hospitalo-Universitaire de Rennes, N. Paleiron, MD, Hôpital d’Instruction Militaire Saint Anne, Toulon; Coordinating Committee - F. Couturaud, MD, (Chair), A. Le Brestec, MS, M. Guégan, MS, S. Mélac, MS, S. Barillot, MS, E. Poulhazan, MS, G. Marhic, MS, A. Le Hir, RN, Centre Hospitalo-Universitaire de Brest, Brest; Data Safety Monitoring Board – J.D. Dewite, MD, (Chair), Centre Hospitalo-Universitaire de Brest, Brest, B. Lodde, MD, Centre Hospitalo-Universitaire de Brest, Brest, G. Héno, MD, Centre Hospitalier de Vannes, Vannes; Statistical Analysis: A. Dion, MS, Centre Hospitalo-Universitaire de Brest, Brest; Data Management (ClinInfo, Lyon) – E. Poulhazan, Centre Hospitalo-Universitaire de Brest, Brest; Operation team (Brest University Hospital) – E. Dauphou, Centre Hospitalo-Universitaire de Brest, Brest; Central Pharmacy (study drug conditioning, distribution) - G. Lahrantec, PharmaD, V. Cogulet, PharmaD, Centre Hospitalo-Universitaire de Brest, Brest; Investigators (by city and in order of the number of patients enrolled) - Brest (51 patients): F. Couturaud, MD, C. Leroyer, MD, C. Tromeur, MD, R. Le Mao, MD, A. Barnier, MD, Centre Hospitalo-Universitaire de Brest, Brest; HIA Clermont-Tonnerre Brest (9 patients): N. Paleiron, MD, HIA Toulon, Toulon; Paris (7 patients): O. Sanchez, MD, Hôpital Européen Georges Pompidou, Paris; Angers (6 patients) F. Gagnadoux, MD, Centre Hospitalo-Universitaire d’Angers, Angers; Rennes (3 patients): S. Jouneaux, MD, Centre Hospitalo-Universitaire de Rennes, Rennes; Lille (2 patients) C. Hayem-Vannimenus, MD, Centre Hospitalo-Universitaire de Lille, Lille; Tours (2 patients): A. Dansou, MD, Centre Hospitalo-Universitaire de Tours, Tours; Nantes (2 patients): A. Magnan, MD, Centre Hospitalo-Universitaire de Nantes, Nantes; Nîmes (1 patient): A. Proust, MD, Centre Hospitalo-Universitaire de Nîmes, Nîmes.