Abstract
Pulmonary function testing (PFT) is required to diagnose chronic obstructive pulmonary disease (COPD) but is completed for only 30-50% of patients with the disease. We determined patient factors associated with decreased likelihood for PFT acquisition (i.e. underutilization) in the United States Veterans Affairs (VA) health care system.
We performed a retrospective analysis of Veterans who survived a VA-based COPD hospitalization between 2012 and 2015. COPD was identified using International Classification of Disease (ICD)-9 codes. Our primary outcome was PFT acquisition, using Current Procedural Terminology (CPT) codes any time prior to the index hospitalization. We compared patients with and without PFTs and used logistic regression to identify associations with PFT underutilization.
Of the 48,888 Veterans included, 78% underwent PFTs prior to hospitalization. Patients without PFTs were younger and more likely to be: women (4.2% vs. 3.6%; p = 0.01), nonwhite (22% vs. 19%; p < 0.0001), and current smokers (66% vs 61%; p < 0.0001). PFT acquisition was less likely in Veterans with alcohol and drug use disorders. Using logistic regression, Veterans who were women (Odds Ratio (OR) = 1.17 [95% confidence limit 1.03–1.32]), nonwhite (OR 1.12 [1.06–1.20]), and with a history of alcohol (OR = 1.07 [1.00–1.14]) or drug use disorders (OR = 1.15 [1.06–1.24]) were less likely to undergo PFTs.
Though most Veterans hospitalized for COPD had PFTs prior to admission, PFTs are underutilized in Veterans who are: women, younger, nonwhite, and have alcohol or drug use disorders. These groups may be “at-risk” for delayed diagnosis or substandard COPD quality care.
Introduction
Chronic lower respiratory tract disease is the fourth leading cause of death in the United States (U.S.), and chronic obstructive pulmonary disease (COPD) is the largest contributor to this category [Citation1]. In the U.S., COPD is frequently due to cigarette smoking [Citation2] and affects more than 11 million Americans [Citation3]. COPD exacerbations are a significant contributor to hospitalizations and readmissions [Citation4]. Pulmonary function testing (PFT) is required to diagnose COPD [Citation2]. To reduce inpatient healthcare utilization due to COPD, ensuring accurate diagnosis of COPD and determining disease severity are crucial initial steps.
Multiple studies have shown that PFTs are underutilized in patients suspected of having COPD, with only 30–50% of patients with a diagnosis of COPD undergoing testing. Several factors have been shown to influence PFT acquisition, including patient age [Citation5–8], sex [Citation6, Citation7], and comorbidity as well as age and specialty of the treating physician [Citation8]. In addition to assuring an accurate diagnosis, PFT acquisition has been associated with improved clinical outcomes in COPD. For example, in patients with diagnosed COPD, Gershon and colleagues reported lower risk of COPD-related hospitalization and death in patients with PFT-confirmed COPD (compared to patients who did not undergo PFTs) [Citation9]. These findings may be explained by more guideline-concordant care in patients with an accurate diagnosis of COPD. Overall, the published data suggest that PFTs are underutilized, and underutilization of PFTs in COPD may contribute to increased risk of exacerbation and hospitalization due to delayed or inaccurate treatment.
The Veterans Health Administration is the largest healthcare provider in the U.S., providing healthcare to over 9 million Veterans [Citation10]. Based on administrative data and clinical patient cohorts, approximately 5–10% of all Veterans are living with COPD [Citation11–13]. Since accurate and timely diagnosis of COPD is related to better clinical outcomes, the quality of COPD care in Veterans with COPD may be improved by identifying factors associated with not undergoing PFTs (i.e. underutilization). Therefore, we sought to determine patient factors associated with decreased likelihood of PFT acquisition in Veterans prior to a hospitalization for COPD. We hypothesized that patient demographics (including age, sex, and race), tobacco smoking status, and comorbidities would be associated with underutilization of PFTs.
Methods
Patients
We evaluated men and women Veterans receiving primary care in Veterans Affairs (VA) facilities who survived a hospitalization for COPD [Citation14]. Hospitalizations for COPD were identified using principal International Classification of Diseases (ICD)-9 codes at discharge (491.2, 492.8, 493.2, and 496.xx) between fiscal years 2012–2015 [Citation14]. Demographic and clinical data from the electronic medical record (EMR) on Veterans hospitalized with COPD were linked to VA administrative and clinical data within the Corporate Data Warehouse (CDW).
Outcomes
Our primary outcome was PFT acquisition any time prior to COPD hospitalization. We identified PFT acquisition using Current Procedural Terminology (CPT) codes (Supplementary material Table 1) [Citation15].
Covariates
Covariates included demographics (age at hospitalization, sex, and race), body mass index (BMI), and smoking status (current, former, or never from VA Health Factors) [Citation16]. We also included the diagnosis of dyspnea, medical comorbidities, and psychiatric comorbidities using ICD-9 codes: asthma, sleep disorders, hypertension, diabetes, congestive heart failure (CHF), anxiety, post-traumatic stress disorder (PTSD), major depressive disorder (MDD), and alcohol and drug use/dependence. Comorbidities were chosen based on known associations with COPD and prevalence in this cohort from prior work [Citation14, Citation17]. We also determined pulmonary-related medication prescriptions up to 1 year prior to index hospitalization, including inhaled medications (i.e. beta-agonists, anti-muscarinics, corticosteroids, and combinations) and nicotine replacement therapy (NRT).
Statistical analysis
We used descriptive statistics to compare categorical and continuous variables between patients with and without PFTs. Categorical variables were compared using Chi-squared tests, and continuous variables were compared via t-testing. In patients with complete demographic and smoking data, we used logistic regression with 95% confidence interval (95% CI) to identify associations with underutilization of PFTs. All analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina, USA), and statistical significance was defined as p < 0.05.
Results
Among 48,888 Veterans hospitalized for COPD during the study period, 10,554 (22%) did not have PFTs prior to their hospitalization (). In those with and without PFTs, most Veterans were men (>95%) and white race/ethnicity (>70%). Comparing Veterans without vs. with PFTs, mean age (standard deviation, SD) was 68.9 (11.5) and 69.5 (9.7) years, respectively; p < 0.0001. Veterans without PFTs were more likely to be women (4.2% vs 3.6%; p = 0.01) and nonwhite (22% vs 19%; p < 0.0001). Mean BMI was similar between the two groups (28.2 vs 28.3; p = 0.6). Compared to patients with PFTs, those without were more likely to be current smokers (66.0% vs. 61.2%) but less likely to be former smokers (23.8% vs. 28.5%; p < 0.0001 for both).
Table 1. Demographics and Comorbidities of Veterans admitted for COPD exacerbation by PFT acquisition prior to hospitalization.
Underutilization of PFTs varied by symptoms, comorbidities, and pulmonary-related prescriptions. Veterans without PFTs had higher rates of alcohol and drug use disorders. However, dyspnea, all medical comorbidities included in analyses, and several psychiatric comorbidities (anxiety, post-traumatic stress disorder, and depression) were more common amongst those with PFTs (; all p < 0.05). Veterans who completed PFTs were also more likely to be treated with inhalers and NRT (all p < 0.05).
In patients with complete demographic data (n = 45,347), multi-variable logistic regression models were adjusted for age, race, smoking history, asthma, diabetes, CHF, anxiety, MDD, PTSD, antimuscarinic inhaler prescription, and NRT prescription (). We chose to only include 1 inhaled medication in the model. Since our cohort was defined by a COPD hospitalization, we chose to include the medication class most likely to reduce risk of hospitalization (i.e. antimuscarinics) [Citation2]. Female sex was associated with 17% risk for underutilization of PFTs (odds ratio (OR) = 1.17, 95% confidence interval (95% CI) [1.03–1.32]; ). Nonwhite race was associated with a 12% risk for underutilization of PFTs (OR 1.12 [1.06–1.20]. Alcohol and drug use were also associated with underutilization of PFTs (alcohol OR = 1.07 [1.00–1.14]; drug use OR = 1.15 [1.06–1.24]). Conversely, older age, former smoking history, medical comorbidities, several psychiatric comorbidities (i.e. MDD, PTSD and anxiety), and treatment with antimuscarinic inhalers and NRT were associated with decreased odds for underutilization of PFTs (). Antimuscarinic inhaler prescription was associated with 77% decreased odds for not having PFTs (OR = 0.23 [0.22–0.24]).
Figure 1. Forest plot demonstrating logistic regression model for underutilization of PFTs (Odds Ratio Estimates)*.
*Model adjusted for age, smoking history, asthma, diabetes, congestive heart failure, anxiety, major depressive disorder, post-traumatic stress disorder, treatment with antimuscarinic inhaler, and treatment with nicotine replacement therapy.
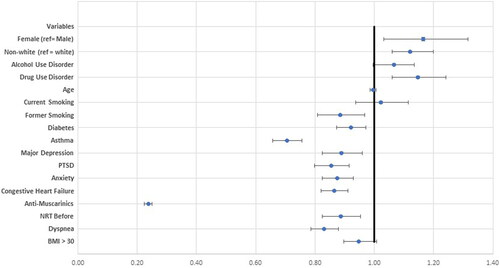
Table 2. Logistic regression model for not obtaining PFTs.
Discussion
This study shows that PFT acquisition prior to a COPD hospitalization is influenced by multiple patient-level factors. We identified 4 important trends. First, female Veterans who might not “fit” the historical stereotype for COPD (i.e. older, male, current or former smokers, multi-morbidity) were less likely to be tested. Second, nonwhite Veterans were also less likely to be tested. Third, comorbidities affected the likelihood of PFT acquisition, with PFT acquisition less likely in Veterans with certain mental health conditions (i.e. alcohol and drug use). Finally, patients undergoing PFTs were much more likely to receive medical treatments for COPD, suggesting that guideline-concordant care is best-achieved with accurate ascertainment of COPD. Since PFT acquisition in COPD is associated with improved outcomes, ensuring appropriate testing and identifying groups “at risk” for sub-standard evaluation can guide future efforts to optimize COPD care. We will discuss our trends sequentially.
We will start by addressing the role of sex and age in PFT acquisition. Historically, COPD has been considered a disease of older men [Citation18, Citation19], and this stereotype has influenced evaluation and treatment strategies. Specifically, COPD may not be considered as a cause of dyspnea or cough in younger women, despite comparable smoking histories. Chapman and colleagues demonstrated that primary care physicians (PCPs) were more likely to overlook COPD in women [Citation20]. The authors surveyed 192 North American PCPs and described hypothetical patients who were smokers presenting with cough and dyspnea. Varying only the age and sex of the patients, the PCPs were asked to state the most likely diagnosis and recommended testing. Despite the same presentation, COPD was diagnosed more frequently in men compared to women [Citation20]. Introduction of spirometry results reduced, but did not eliminate, misdiagnosis for women compared with men. Since female sex was associated with underutilization of PFTs in this cohort, our results suggest that a sex bias for COPD persists. Due to a slower decline in smoking prevalence for women compared to men [Citation19, Citation21] and increased susceptibility of women to tobacco smoke [Citation22, Citation23], the burden of COPD in women is currently higher than in men [Citation4, Citation24]. With a growing proportion of women serving in the military, the burden of COPD in the VA medical system is almost certain to rise. Therefore, it may be argued that it is more urgent to obtain PFTs in women Veterans with chronic respiratory symptoms compared to men.
Interestingly, trends of PFT acquisition by sex and age have varied in the literature. In a non-Veteran cohort of n = 5,039 patients with newly-diagnosed COPD, Han and colleagues reported that PFT acquisition was more likely in women and younger patients [Citation7]. In a cohort of 197,878 Veterans with diagnoses of COPD based on outpatient visits, Lee and colleagues reported PFT acquisition was more likely in younger Veterans, with Veterans <50 years being most likely to undergo testing and Veterans ≥80 years the least likely to undergo testing [Citation5]. In Ontario, Canada, Gershon and colleagues reported PFT acquisition was more likely in between ages 50–80 years [Citation8]. In a cohort of Swedish patients with COPD (n = 1,114), Arne and colleagues reported PFT acquisition was also most likely in patients under age 50 [Citation6]. By contrast, Herrick and colleagues reported PFTs were less likely in younger patients (ages 18–44 years) [Citation25], and Mowls and colleagues and reported that likelihood of PFT acquisition in COPD increased with age [Citation26]. Since prior analyses have focused on out-patient diagnosis of COPD, comparison of our findings to other studies is difficult. Further, several analyses were on non-US populations, only Lee and colleagues focused on a Veteran population, and our cohort of Veterans had a higher percentage of women than the cohort from Lee and colleagues. Though our findings suggest that PFT acquisition may be overlooked for Veterans not fitting the stereotype for COPD, the discrepant findings of PFT acquisition by sex and age in different studies suggests more work is needed on this topic.
In addition to sex-based disparities in PFT acquisition, we also identified a potential race disparity, with underutilization of PFTs more common in nonwhite Veterans. Findings from several other studies suggest that racial bias may be present in COPD and associated diagnostic testing. In an analysis of the COPD Genetic Epidemiology (COPDGene®) study that included patients with a ≥ 10 pack-year smoking history, Mamary and colleagues found that, compared to non-Hispanic whites, African-Americans were less likely to have a prior COPD diagnosis for all severities of obstruction [Citation27]. Racial bias is also present in the diagnosis and treatment of other smoking-related lung conditions (e.g. lung cancer and lung cancer screening) [Citation28–30]. Therefore, it seems likely that racial bias may also be present in testing for COPD. Unfortunately, little data is available on racial and ethnic factors influencing likelihood of PFT acquisition. At least 2 recent studies have analyzed data from the Behavioral Risk Factor Surveillance System (BRFSS) that collects state level data via a telephone-based survey [Citation31]. In a cross-sectional analysis of patients with COPD, Mowls and colleagues reported that spirometry was less likely in Hispanic patients [Citation26]. By contrast, in a cohort of self-reported COPD patients in North Carolina, Herrick and colleagues found no differences in PFT acquisition by race or education level [Citation25]. Though our cohort is unique in that the study population is U.S. Veterans, our results support a possible racial bias to testing for COPD. More work is clearly needed to better understand patterns of PFT acquisition by race and any potential barriers to testing in nonwhite patients.
Veterans in this cohort who completed PFTs were much more likely to be treated with a COPD-regimen, including inhalers and NRT. Since the rate of PFT acquisition in this cohort was quite high, it seems likely that clinicians had confirmed COPD (or had a sufficiently high suspicion) and initiated treatment prior to the index hospitalization. Gershon and colleagues also reported that PFT acquisition in patients with COPD was associated with increased prescription of long-acting bronchodilators as well as reduced risk of hospitalization [Citation9]. The close association between accurate diagnosis, appropriate treatment, and improved clinical outcomes re-iterates the importance of clinicians having a low threshold for PFTs in symptomatic patients. Clinicians should be particularly vigilant in those patients where the disease might not be suspected or could go overlooked.
Turning to comorbidities, underutilization of PFTs was more common in Veterans with COPD and concurrent alcohol or drug use disorders. Since patients with other comorbidities were more likely to undergo PFTs, alcohol and drug use disorders may have a distinct influence on medical evaluations, and these conditions may represent a group of Veterans who are at risk for under-evaluation or under-treatment of COPD. It is unclear if lower PFT acquisition in Veterans with these comorbidities is due to (1) patients with these conditions not having their respiratory comorbidities recognized; (2) patients not obtaining outpatient evaluations or recommended testing, even when symptoms are recognized; (3) patients have these tests ordered but are unable to attend testing; or (4) or some combination of these factors. Prospective studies of PFT acquisition in patients with alcohol and drug use disorders would clarify the reasons for lower PFT acquisition. Since likelihood of PFT acquisition appears to be biased by sex, age, race, and comorbidities, PFT acquisition guided by objective criteria (rather than clinical intuition alone), may overcome disparities in COPD care.
Overall, PFT acquisition in this cohort was high (78%). Compared to prior estimates of 30–50% PFT acquisition in patients carrying a COPD diagnosis [Citation5–7, Citation32], our data suggests that clinicians are frequently recognizing COPD in Veterans nearing a hospitalization. The high rate of PFT acquisition could be explained, in part, by our approach that included PFT acquisition any time prior to hospitalization. Prior work by Lee and colleagues evaluated PFT acquisition in outpatient Veterans with COPD during a 1-year interval [Citation5]. In general, PFT acquisition was more likely in “sicker” patients. That is, patients with medical and several psychiatric comorbidities (i.e. anxiety, PTSD, and depression) are more likely to have PFTs. Since comorbidities in COPD predict likelihood of COPD-related readmission in Veterans and non-Veterans [Citation14, Citation33], it is reassuring that most of these “sicker” patients are undergoing appropriate testing and treatment.
Since removing barriers to PFT acquisition may facilitate earlier disease recognition and treatment, our study may identify several patient groups that could benefit from in-office or handheld spirometry measurements rather than consultation of a pulmonary function testing laboratory. Since the accuracy and reliability of in-office spirometers are low in some studies, such testing is not currently mainstream [Citation34]. However, in patients with known asthma or COPD, Bambra and colleagues noted significant correlation between laboratory-obtained and office spirometry [Citation35]. Even more, the National Lung Health Education Program recommends use of office spirometry for detection and management of COPD [Citation36]. Though the role of in-office testing remains unclear, from a clinical perspective, clinicians caring for Veterans with drug and alcohol use disorders should have a low threshold for requesting PFTs in patients have risk factors or symptoms suggestive of COPD.
The study has several strengths. To our knowledge this cohort is largest group of Veterans hospitalized with COPD. Further, few studies have addressed patterns of PFT acquisition in patients with COPD [Citation6–8]. Only one of these was in a Veteran cohort [Citation5], and no studies have focused on patients after a hospitalization for COPD. Finally, since the VA is a single payer system and utilizes a uniform medical record, the likelihood of missing data is less.
There are several limitations to this study. Since the approach was retrospective, we can identify associations between underutilization of PFTs and patient factors, though we cannot determine the directionality of these associations. Also, since our patient population was identified by a hospitalization, we cannot extrapolate our results to outpatients with COPD. Third, due to the minority of patients with quantifiable PFTs (34%), COPD diagnosis by ICD-9 codes could not be spirometrically-confirmed for most patients. Finally, since some Veterans utilize both VA and private physicians, it is possible that PFTs were obtained outside the VA system and could not be included in our analysis. Evaluation of PFT acquisition in a prospective cohort of patients with risk factors for COPD would further establish clinical patterns of PFT acquisition and confirm groups “at risk” for undiagnosed or under-treated COPD.
Conclusions
Our study emphasizes the importance of PFT acquisition in Veterans with COPD. PFTs were acquired at lower rates in younger, nonwhite, women, and Veterans with comorbid alcohol and drug use disorders. Though the high rate of PFT acquisition in Veterans is encouraging, the significance of this study is the identification of patient groups who are at risk for inaccurate or delayed diagnosis of COPD and potentially under-treatment. Since this study focused on hospitalizations, we speculate that younger out-patients with fewer comorbidities (i.e. “less sick”) would be even more likely to be under-diagnosed. Ideally, all patients with COPD would have the disease confirmed by PFTs and therapy initiated prior to hospitalization. Since PFT acquisition is associated with much higher COPD-directed treatment and better clinical outcomes, clinicians should consider earlier testing in these “at risk” groups when chronic cough, wheezing, or dyspnea are present. To optimize COPD care in Veterans, future work should evaluate for barriers to PFT acquisition in these “at risk” groups and consider the role of in-office spirometry in select patients and facilities.
Declaration of interest
The authors report no conflicts of interest related to the submitted work.
Dr. Bade reports salary support outside the submitted work from Boehringer-Ingelheim for a research fellowship from November 2017-November 2018.
Dr. Brandt reports salary support outside the submitted work from the VA and Yale University as well as grant support outside the submitted work from the National Institutes of Health.
Authors’ notes
Views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US Government.
Part of this manuscript has been previously-presented in the form of a poster presentation at the ATS International Conference in 2019.
Author contributions
BCB Study design, data analysis, data interpretation, and drafting/revising the work.
ECD Study design, data acquisition, data analysis, data interpretation, and revising the work.
MS Study design, data acquisition, data interpretation, and revising the work.
KC Study design, data interpretation, and revising the work.
SH Study design, data interpretation, and revising the work.
BBM Study design, data interpretation, and revising the work.
HCC Study design, data interpretation, and revising the work.
CB Study design, data acquisition, data interpretation, and revising the work.
LAB Study design, data interpretation, and revising the work.
KMA Study design, data acquisition, data analysis, data interpretation, and drafting/revising the work.
All authors approve of this final version of the work and agree to be accountable for its accuracy and integrity.
Supplemental Material
Download PDF (280 KB)Acknowledgements
Supported by VA Central Office Operations Funds. This work was completed as an operations project at VA Connecticut and VA Greater Los Angeles for the Department of Veterans Affairs through a specific request from VA Women’s Health Services within the Office of Patient Care Services. It was also approved by the local Institutional Review Board at VA Connecticut and determined to be operations work.
References
- Centers for Disease Control and Prevention. Mortality in the United States, 2017. 2017; https://www.cdc.gov/nchs/products/databriefs/db328.htm.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP.
- American Lung Association. How Serious is COPD. 2018. https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/copd/learn-about-copd/how-serious-is-copd.html.
- Ford ES, Croft JB, Mannino DM, et al. COPD surveillance–United States, 1999-2011. Chest. 2013;144(1):284–305. doi:10.1378/chest.13-0809.
- Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest. 2006;129(6):1509–1515. doi:10.1378/chest.129.6.1509.
- Arne M, Lisspers K, Stallberg B, et al. How often is diagnosis of COPD confirmed with spirometry?. Respir Med. 2010;104(4):550–556. doi:10.1016/j.rmed.2009.10.023.
- Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up?. Chest. 2007;132(2):403–409. doi:10.1378/chest.06-2846.
- Gershon AS, Hwee J, Croxford R, et al. Patient and physician factors associated with pulmonary function testing for COPD: a population study. Chest. 2014;145(2):272–281. doi:10.1378/chest.13-0790.
- Gershon A, Mecredy G, Croxford R, et al. Outcomes of patients with chronic obstructive pulmonary disease diagnosed with or without pulmonary function testing. CMAJ. 2017;189(14):E530–E538. doi:10.1503/cmaj.151420.
- Veterans Health Administration. About VHA. 2018. https://www.va.gov/health/aboutvha.asp.
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696–1703. doi:10.1097/j.pain.0000000000000567.
- Higgins DM, Fenton BT, Driscoll MA, et al. Gender differences in demographic and Clinical correlates among Veterans with musculoskeletal disorders. Womens Health Issues. 2017;27(4):463–470. doi:10.1016/j.whi.2017.01.008.
- Thompson WH, St-Hilaire S. Prevalence of chronic obstructive pulmonary disease and tobacco use in veterans at Boise Veterans Affairs Medical Center. Respir Care. 2010;55(5):555–560.
- Bade BC, DeRycke EC, Ramsey C, et al. Sex differences in Veterans admitted to the hospital for COPD exacerbation. Ann Am Thorac Soc. 2019;16(6):707–714.
- MacIntyre NR, Foss CM. Pulmonary function testing: coding and billing issues. Respir Care. 2003;48(8):786–790.
- McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. doi:10.1093/ntr/ntr206.
- Rinne ST, Elwy AR, Liu CF, et al. Implementation of guideline-based therapy for chronic obstructive pulmonary disease: Differences between men and women veterans. Chron Respir Dis. 2017;14(4):385–391. doi:10.1177/1479972317702141.
- Aryal S, Diaz-Guzman E, Mannino DM. COPD and gender differences: an update. Transl Res. 2013;162(4):208–218. doi:10.1016/j.trsl.2013.04.003.
- Camp PG, Goring SM. Gender and the diagnosis, management, and surveillance of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(8):686–691. doi:10.1513/pats.200706-081SD.
- Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119(6):1691–1695. doi:10.1378/chest.119.6.1691.
- Varkey AB. Chronic obstructive pulmonary disease in women: exploring gender differences. Curr Opin Pulm Med. 2004;10(2):98–103. doi:10.1097/00063198-200403000-00003.
- Sorheim IC, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men?. Thorax. 2010;65(6):480–485. doi:10.1136/thx.2009.122002.
- Prescott E, Bjerg AM, Andersen PK, et al. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: results from a Danish longitudinal population study. Eur Respir J. 1997;10(4):822–827.
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Trends and patterns of differences in chronic respiratory disease mortality among US counties, 1980-2014. JAMA 2017;318(12):1136–1149. doi:10.1001/jama.2017.11747.
- Herrick H, Pleasants R, Wheaton AG, et al. Chronic obstructive pulmonary disease and associated health-care resource use-North Carolina, 2007 and 2009 (Reprinted from MMWR, vol 61, pg 143-146, 2012. JAMA J Am Med Assoc. 2012;307(18):1905–1908.
- Mowls DS, Cheruvu VK, Schilz R, et al. COPD in a nationally representative sample: sociodemographic factors and co-morbidity, diagnosis method, and healthcare utilization. COPD. 2015;12(1):96–103. doi:10.3109/15412555.2014.922065.
- Mamary AJ, Stewart JI, Kinney GL, et al. Race and gender disparities are evident in COPD underdiagnoses across all severities of measured airflow obstruction. Chronic Obstr Pulm Dis. 2018;5(3):177–184. doi:10.15326/jcopdf.5.3.2017.0145.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551.
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–1205. doi:10.1056/NEJM199910143411606.
- Tanner NT, Gebregziabher M, Hughes Halbert C, et al. Racial differences in outcomes within the National Lung Screening trial. Implications for widespread implementation. Am J Respir Crit Care Med. 2015;192(2):200–208. doi:10.1164/rccm.201502-0259OC.
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. 2019. https://www.cdc.gov/brfss/index.html.
- Gershon AS, Macdonald EM, Luo J, et al. Concomitant pulmonologist and primary care for chronic obstructive pulmonary disease: a population study. Fam Pract. 2017;34(6):708–716. doi:10.1093/fampra/cmx058.
- Jacobs DM, Noyes K, Zhao J, et al. Early hospital readmissions following an acute exacerbation of COPD in the nationwide readmissions database. Ann Am Thorac Soc. 2018;15:837–845.
- Hegewald MJ, Gallo HM, Wilson EL. Accuracy and quality of spirometry in primary care offices. Annals ATS. 2016;13(12):2119–2124. doi:10.1513/AnnalsATS.201605-418OC.
- Bambra G, Jalota L, Kapoor C, et al. Office spirometry correlates with laboratory spirometry in patients with symptomatic asthma and COPD. Clin Respir J. 2017;11(6):805–811. doi:10.1111/crj.12419.
- Ruppel GL, Carlin BW, Hart M, et al. Office spirometry in primary care for the diagnosis and management of COPD: National Lung Health Education Program Update. Respir Care. 2018;63(2):242–252. doi:10.4187/respcare.05710.
