Abstract
Nasal high flow (NHF) therapy has recently gained attention as a new respiratory support system and is increasingly being utilized in every day clinical practice. Recent studies suggest that it may also be effective in patients with hypercapnia and suggest NHF as a possible alternative for patients who cannot tolerate standard noninvasive ventilation. The present review discusses the mechanisms of action that make NHF potentially suitable for chronic obstructive pulmonary disease (COPD) patients and evaluates the current evidence of NHF use for treatment of stable hypercapnic COPD patients as well as acute hypercapnic exacerbation of COPD. An algorithm is also proposed for the clinical application of NHF in patients with acute hypercapnic exacerbation of COPD, based on current literature.
Introduction
Noninvasive ventilation (NIV) is universally recognized as the mainstay therapy for patients with acute hypercapnic exacerbation of chronic obstructive pulmonary disease (COPD) [Citation1,Citation2]. Recently, NIV was also suggested by the European Respiratory Society guidelines for chronic stable hypercapnic COPD patients, targeting a reduction or even normalization of PaCO2 level [Citation2,Citation3]. However, regardless of the indication of NIV application, tolerance is often poor due to a variety of side-effects, such as claustrophobia, eye irritation and pressure sores [Citation4]. To date there is no established alternative treatment for patients who cannot tolerate standard NIV.
Nasal high flow (NHF) therapy has recently gained attention as a new respiratory support system and is increasingly being utilized in every day clinical practice [Citation5,Citation6]. Delivery of a preheated and humidified air-oxygen gas mixture at high flow rates (up to 60 L · min−1) is administered through a transnasal application. Although its benefits have been demonstrated mainly for the treatment of patients with acute hypoxemic respiratory failure [Citation5–8], recent studies suggest that it may also be effective in patients with hypercapnia and suggest NHF as a possible alternative for patients who cannot tolerate standard NIV [Citation9].
This review discusses the mechanisms of action that make NHF potentially suitable for COPD patients who cannot tolerate NIV. In addition, we evaluate the current evidence of NHF for treatment in stable hypercapnic COPD patients with chronic hypercapnia as well as COPD patients with acute exacerbation and type II respiratory failure. Moreover, we propose an algorithm for the clinical application of NHF in patients with acute hypercapnic exacerbation of COPD, based on current literature.
Materials and methods
A computerized database search was performed to identify randomized and non-randomized controlled trials and case series studies where NHF therapy was used in COPD patients. We searched for published and articles ahead of print in bibliographic databases, including ISI Web of Science, PubMed, Science Direct Scopus, Wiley online library, Google Scholar and other international databases and websites. The literature search also involved a manual review of the bibliography of the identified papers and relevant information to ensure the objectives of this study were met. Keywords used in the search were: “High flow nasal cannula”, “Nasal high flow”, “COPD”, “hypercapnic respiratory failure”. The literature was limited to journal articles written in English (three articles were excluded). Two reviewers screened all potential references for inclusion. The last update of the search was performed in November 2019.
Mechanisms of action
NHF delivers flow rates up to 60 L · min−1 that can match or even exceed the patient’s inspiratory flow demand via nasal cannulas that are designed to avoid creating a jet directed at mucosal surfaces. These high flow rates are well tolerated because of the continuous warming and humidification of the applied gas that prevents airway dehydration [Citation10]. An air-oxygen blender delivers a fraction of inspired oxygen (FiO2) ranging from 0.21 to 1.0 via a single limb heated inspiratory circuit to avoid heat loss and condensation [Citation6]. An obvious advantage of a nasal cannula therapy application over a respiratory mask is the facilitation of daily activities, ability to communicate and eat easily, and higher levels of compliance.
Several physiologic benefits make NHF potentially suitable for hypercapnic COPD patients [Citation11]. NHF therapy generates a modest degree of positive end-expiratory pressure (PEEP) due to the imposed expiratory resistance of the patient’s exhalation against flow of incoming gas [Citation12,Citation13] that appears to counterbalance the intrinsic PEEP [Citation14] and recruit atelectatic areas [Citation15–17]. Moreover, the high velocity of incoming gas pressurizes the upper airway above atmospheric pressure during inspiration that markedly attenuates the inspiratory resistance associated with the nasopharynx [Citation18].
In addition, NHF therapy decreases dead space ventilation through a washout effect of the CO2 from the upper airway, which leads to reduced PaCO2 levels [Citation19,Citation20]. The decrease in PaCO2 is not pressure dependent but leakage dependent which is extremely important since it separates NHF from NIV [Citation21].
Furthermore, NHF enhances lung mucociliary clearance by heating the delivered gas at core temperature level (37 °C) and humidifying the respiratory system to saturation, especially in high flow rates [Citation10,Citation22]. The breathing of warm and humidified air inhibits the nasopulmonary bronchoconstrictor response, preventing any increase in airway resistance that could be triggered by cold and dry air [Citation23]. Moreover, since NHF supplies fully conditioned gas to the airway, the required metabolic cost of warming and humidifying the inspired gas is reduced [Citation24].
Another reason that makes NHF potentially suitable for COPD patients is the fact that NHF provides more stable FiO2 values than those of standard oxygen delivery systems, since high flow rates can match or even exceed the patient’s inspiratory flow demand, thus reducing the entrainment of room air and dilution of oxygen delivered [Citation25,Citation26].
As a result, NHF reduces work of breathing in COPD patients leading to a decrease in respiratory rate with concomitant improved ventilation [Citation7], a decrease in rapid shallow breathing index (RSBI) [Citation13] and an improvement in thoraco-abdominal synchrony [Citation27].
Nasal high flow in stable COPD with hypercapnia
Management of stable COPD patients in advanced stages is multifactorial. Pharmacological therapies, LTOT for resting chronic hypoxemia and NIV for patients with concomitant severe hypercapnia are used in order to control symptoms as well as reducing exacerbations and improving quality of life (QoL) and survival [Citation2]. NHF appears to play a substantial role in each of the abovementioned areas for COPD management ().
Table 1. Studies in stable COPD patients.
NHF significantly decreases PaCO2 level in a flow dependent manner in short and long term (≥6 weeks) studies compared to conventional oxygen treatment (COT) [Citation28–31]. It also reduces respiratory muscle load and the excess work of breathing, similarly to NIV, leading to a more efficient ventilation and breathing pattern and a change in the pressure-volume curve [Citation6,Citation12,Citation32,Citation33]. A concomitant significant increase in tidal volume and EELV accompanied by a decrease in breathing frequency and decrease in PaCO2 level have been described [Citation6,Citation7,Citation13,Citation27,Citation33] implying that patients are reenabled to ventilate more effectively [Citation6]. Furthermore, NHF seems to be well tolerated by most patients [Citation28,Citation34] and the comfort is significantly better in comparison to nCPAP and nasal BiPAP [Citation14].
NHF also seems to exert beneficial effects on exercise capacity of COPD patients. In a study by Chatila et al., COPD patients exercised longer on a cycle ergometer with less dyspnea, better breathing pattern and lower arterial pressure that was attributed to resistive unloading and improved lung mechanics, compared to COT [Citation35]. Additionally, oxygenation was higher due to a more consistent FiO2 with NHF. These results were further supported by Cirio et al. [Citation36], who reported a significant improvement in endurance time in severe COPD patients during high-intensity, constant-load exercise under NHF therapy. The effect was associated with an improvement in oxygen saturation, less dyspnea and less leg fatigue [Citation36].
NHF does not seem to reduce exacerbation frequency significantly, although it reduces exacerbation days and increases time to first exacerbation [Citation37]. Pulmonary function, as it is measured with FEV1 and FVC is improved and lung gas trapping is reduced. Both effects are attributed to better small airway function through better mucociliary clearance [Citation37].
The beneficial effects of NHF on respiratory mechanics, gas exchange, exercise capacity and exacerbations in the advanced stages of COPD is accompanied by an improvement in QoL measures, as shown in long term studies, with the benefits observed in all of the four components of SGRQ [Citation30,Citation31,Citation37].
Nasal high flow in COPD exacerbation
One of the most important technological advances in respiratory support is the introduction of NIV in the management of acute type II respiratory failure in COPD patients [Citation38]. NIV has been shown to improve acute respiratory acidosis, decrease respiratory rate, work of breathing, severity of breathlessness, rates of intubation and length of hospital stay [Citation2]. Most importantly, mortality has been shown to reduce by 50% [Citation1,Citation38]. However, NIV is not tolerated by almost 20% of patients frequently leading to intubation and invasive mechanical ventilation since there is no established alternative [Citation39].
In 2014, Millar et al. [Citation40], published a case in which NHF successfully managed acute hypercapnic respiratory failure in a COPD patient unable to tolerate conventional NIV. Two years later, Pavlov et al. [Citation41], reported that 4 patients contraindicated for NIV therapy with severe respiratory failure were successfully rescued using NHF with high flow rate (55 L · min−1) and FiO2 adjusted to maintain peripheral pulse oximetry ≥ 90%. Two of four patients achieved complete clinical and laboratory response in less than 4 h [Citation42]. These results were further confirmed by a larger study. Thirty-eight patients with severe to moderate acidotic and non-acidotic hypercapnic (pH <7.38 and PaCO2 >45 mmHg) acute exacerbation of COPD (AECOPD), who did not tolerate NIV showed significant improvement in pH and PaCO2 when NHF was used with a flow rate titrated to achieve the greatest tolerability [Citation42].
In a mixed population study of patients with acute respiratory failure type II (mainly pneumonia and AECOPD) and failure or intolerance of COT, including NIV, a significant improvement in PaCO2 after 1 h of NHF therapy (50 L · min−1) was reported [Citation43]. Similar findings were reported in another study in a mixed population of 30 patients with moderate acute hypercapnic respiratory failure that where treated with NHF. Respiratory rate was improved during the first 4 h of treatment (although not statistically significant) and pH was normalized within 24 h (7.28 vs 7.37, p = 0.02). Only 4 patients (13.3%) did not respond. One required NIV and the rest were intubated [Citation44]. A limitation of the above studies is that the results from mixed populations cannot be generalized in patients with AECOPD. Nevertheless, NHF seems to be effective in improving clinical and gas exchange parameters in patients with moderate hypercapnic respiratory failure, with an acceptable rate of non-responders who required additional ventilatory support.
As already stated, NHF has the potential to reduce PaCO2 in COPD patients by improving pulmonary mechanics and decreasing work of breathing [Citation45]. Another important factor that could independently result in a reduction in PaCO2 level is the more stable FiO2 delivered via NHF. However, in a cross-over study on COPD patients with acute exacerbation that focused on supplementing oxygen titrated to the patient’s baseline peripheral capillary oxygen saturation (SpO2), NHF (35 L/min) in comparison to standard nasal prongs (30 min trial) resulted in a significant reduction in PtCO2 although clinical relevance was questionable since the difference was only 1.4 mmHg [Citation46]. There was not any difference in FiO2 after 30 min which suggests that the difference in PtCO2 between treatments was due to the physiological effects of NHF, rather than alterations to oxygen delivery. A limitation of the study was that only 58% of the participants were hypercapnic, and a greater difference in PtCO2 may have resulted if all patients were hypercapnic. Additionally, a higher flow rate and use of NHF for a longer period of time may also have had a greater effect on PtCO2 [Citation46].
In an attempt to compare NHF with NIV, which is the gold standard for patients with hypercapnic AECOPD, Lee et al. [Citation47], conducted a prospective study in patients with severe AECOPD and moderate hypercapnic respiratory failure. The NHF group showed similar primary end point results compared with NIV group including intubation rate (25% and 27.3% respectively) and 30-day mortality (15.9% and 18.2% respectively). Other end points, such as pH, PaO2 and PaCO2 level as well as the median duration of therapy were not statistically different between groups.
Sonographic evaluation of the diaphragmatic function was performed in a cross-over study that included 30 COPD patients with hypercapnic acute respiratory failure receiving NIV for more than 24 h [Citation48]. Patients underwent five 30-min trials, the first, third, and fifth trial using NIV, and the second and fourth employed either standard oxygen or NHF therapy (50 L/min). Standard oxygen therapy resulted in a noticeable increase in diaphragm thickening fraction while on NHF therapy the diaphragm thickening fraction remained unchanged which implies less work of breathing. However, another study in 12 COPD patients suggested that setting NHF above 30 L/min may lead to an increase in inspiratory effort (estimated by a simplified esophageal pressure time product) in half cases, although it decreases respiratory rate [Citation49].
Another possible role of NHF highlighted by Longhini et al., is during NIV interruptions, in order for patients to rest, eat and communicate without any deterioration in pulmonary mechanics [Citation48]. Indeed, even in postextubated COPD patients, application of NHF in the highest flow rate tolerated by the patients, significantly decreased the neuroventilatory drive and work of breathing (estimated by the pressure time product) compared to COT [Citation50]. NHF has been also reported to be able to keep PaCO2 unmodified and decrease the work of breathing by a similar extend to NIV, making it ideal during NIV interruptions [Citation51].
summarizes the results from studies performed in patients with COPD and acute hypercapnic respiratory failure.
Table 2. Studies in acute hypercapnic exacerbation of COPD.
Aerosol therapy with nasal high flow
Bronchodilators constitute the first line treatment for chronic as well as acute exacerbation of COPD. Administrating them while the patient is on NIV is challenging, since discontinuing therapy could lead to rapid deterioration of the patient’s clinical condition. On the contrary, administering the inhalation therapy without discontinuation of NIV may result in inappropriate lung deposition depending on various factors such as nebulizer type, position of the nebulizer in the circuit, interface type, mode of ventilation and ventilation settings [Citation52]. Similar considerations about the optimal aerosol delivery technique apply for NHF therapy also. Many factors have to be taken into account: i) aerosol deposition within the circuit and upper airways may be favored by humidification and high flow rates through increasing the particle size and its deposition by impaction respectively, ii) nasal aerosol inhalation may promote local deposition, given the turbulent gas flow in the nose and rhino-pharynx and iii) continuous delivery of aerosolized particles (i.e. during both inspiration and expiration) within a NHF single-limb circuit would increase aerosol loss during expiration, especially with a low inspiration to expiration ratio as observed in COPD patients [Citation53,Citation54].
Studies on adult models of invasive and NIV suggest that the optimal drug delivery efficiency depends on the aerosol generator, the ventilator circuit, and the aerosol generator position [Citation55,Citation56]. Today, most research involves vibrating-mesh nebulizers mainly due to some obvious advantages in comparison to jet nebulizers, such as reduced residual volume, shorter nebulization duration and no alteration of FiO2 since they do not add gas flow to the NHF circuit [Citation55,Citation56]. Reminiac et al. [Citation57], in a bench study, tried to optimize the amount of aerosol emitted at the nasal cannula outlet according to position and different types of nebulizer (vibrating-mesh and jet nebulizer) in an adult NHF circuit. They found that the most relevant nebulizer position for both types of nebulizer was immediately upstream prior to the humidification chamber [Citation57]. A position close to the cannulas leads to the partial obstruction of the nasal cannulas due to accumulation of droplets resulting from large aerosol particle impaction and turbulences within the cannulas [Citation58]. Also, in the upstream position the respirable mass at the nasal cannula outlet was greater, without any significant differences between the types of nebulizers tested, since the aerosol filled tube can act similarly to a chamber, increasing delivery during inspiration. As expected, increasing flow rates and open-mouth breathing resulted in a decrease in the respirable mass.
As previously stated, flow rate is another significant factor that can influence lung deposition inversely, especially during quiet breathing. In a study using a breathing simulator, aerosol delivery was increased eight-fold at the cannula outlet by reducing the administered flow from 50 to 10 L · min−1 [Citation59]. Less aerosol loss is anticipated with lower flows due to reduced turbulence, inertial impaction and leaks from the nasal cannula into the atmosphere. However, for patients in respiratory distress, a flow rate much lower than their inspiratory flow rate will also decrease aerosol delivery due to circuit sedimentation and/or aerosol dilution with room air [Citation57]. In two bench studies [Citation57,Citation59], simulating patients’ inspiratory flow, reducing the administered NHF flow rate from 50–60 L · min−1 to 30–40 L · min−1 led to an increased amount of aerosol delivery although a further reduction to 10 L · min−1 resulted in a reduction in drug delivery [Citation59]. Thus, adequate matching between the patient’s inspiratory flow and the flow delivered by the NHF will increase the final delivered dose. It is suggested to use flow rates of 30–60 L · min−1 during distressed breathing for optimal aerosol delivery [Citation57,Citation59].
The extent to which flow rate in conjunction to the size of the cannula affect the aerosol delivery through NHF was further studied by Perry et al. [Citation60]. In this bench model study, the highest inspired dose was reported when the cannula size increased. However, the overall amount of the drug emerging from the device was very low (0–2.5%) which was attributed to the position of the nebulizer at the end of the heated delivery tube before the nasal cannula. In this position, gas flowing through the circuit travel into the nasal cannula which has a smaller diameter resulting in turbulent gas flow and therefore more drug accumulation in the adaptor and less to the patient [Citation57,Citation60]. Placement of a jet nebulizer above the nasal cannula even at low flow rates which is very common in clinical practice, has been proven ineffective [Citation61].
Apart from the above, there are only few clinical data regarding aerosolized medication administered in conjunction with NHF. In a study with 30 stable COPD patients, salbutamol and ipratropium bromide solution was delivered using a jet nebulizer either orally via a mouthpiece or through a NHF circuit connected immediately before the nasal cannula at a flow rate of 35 L · min−1 [Citation62]. The results were similar, without any post inhalation difference regarding pulmonary mechanics and function. Both significantly increased FEV1, and decreased airway resistance and hyperinflation. In this study, the nebulizer in the NHF group, was placed closed to the nasal prongs, a position that in the study by Reminiac et al. [Citation57], has been shown to result in a lesser respirable mass than the two other positions closed to humidification chamber. However, although this seems true in a closed circuit ventilation such as the circuit used in the bench study by Reminiac et al. [Citation57], in vivo the results may vary considerably, since the specific effects of NHF such as adding a small positive airway pressure, a tendency to increase tidal volumes in combination with a reduction in functional dead space, may enhance the efficiency of bronchodilators. Thus, if an appropriate adapter/connector for the nebulizer to humidification chamber is not available, placing the nebulizer immediately before the nasal cannula appears to be an effective alternative. In another study of 25 patients with reversible airflow obstruction, Reminiac et al. [Citation63], demonstrated that albuterol delivered by vibrating mesh nebulization through a NHF at 30 L · min−1 was non inferior to standard facial mask jet nebulization in terms of FEV1 increase. Both resulted in a significantly higher FEV1 than that of the control group (NHF without nebulization). Interestingly, a statistically significant increase in FEV1, albeit modest in magnitude (median increase of 50 mL and 3%, values below validated thresholds to define reversibility) [Citation64] was noticed also in the control group which supports the hypothesis of an NHF-induced bronchodilation. Changes in respiratory pattern induced by high flow rates with NHF can lead to higher tidal volume and eventually to deeper inspiration during spirometry maneuvers. Future studies are needed to investigate the specific underlying mechanisms of NHF-induced bronchodilation.
Practical application
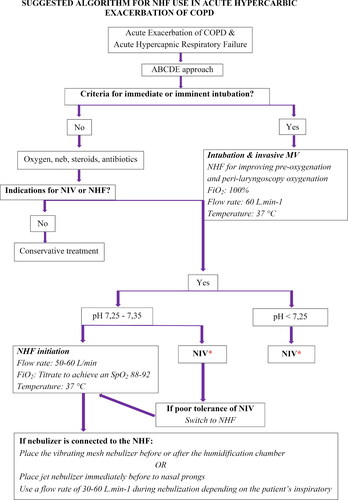
Summarizing the existing literature has led us to propose the following algorithm for NHF use in patients with AECOPD and hypercapnic respiratory failure when NHF is available and has been chosen as the initial ventilatory support device or in case of NIV intolerance ().
Figure 1. Suggested algorithm for NHF use in acute hypercapnic exacerbation of COPD. Abbreviations: NHF, Nasal high flow; COPD, Chronic obstructive pulmonary disease; ABCDE, Airway, breathing, circulation, disability, exposure; MV, Mechanical ventilation; NIV, Noninvasive ventilation; FiO2, Fraction of inspired oxygen, SpO2, Peripheral oxygen saturation; COT, conventional oxygen treatment. *Consider NHF use during NIV interruptions
For patient’s admitted with AECOPD and respiratory failure type II, it is necessary to check primarily if the criteria for immediate or imminent intubation and invasive mechanical ventilation are met, such as respiratory or cardiac arrest, severe hemodynamic instability, ventricular arrhythmias or heart rate < 50 bpm with loss of alertness and diminished consciousness or psychomotor agitation inadequately controlled by sedation [Citation2]. If yes, and there is time to setup NHF, NHF can be used for pre-oxygenation and apneic oxygenation during laryngoscopy using maximum device settings (flow rate: 60 L · min−1, FiO2: 100%) [Citation34]. Delivery of high flow oxygen during intubation extends the apneic window by maintaining oxygen saturation within safer values (>90%) for a longer period with slower rate of CO2 rise [Citation65]. It has been demonstrated that NHF minimizes intubation related adverse events such as rate and level of desaturation, arrhythmia and cardiac arrest [Citation66].
For patients not requiring immediate or imminent intubation and invasive mechanical ventilation, initial treatment with supplemental oxygen aiming an SpO2 of 88–92%, bronchodilators, steroids (orally or intravenously) and antibiotics when signs of bacterial infection are present should be provided [Citation2]. Within one hour of the initial management severe dyspnea with clinical signs of respiratory muscle fatigue and increased work of breathing (use of accessory respiratory muscles, paradoxical motion of the abdomen, retraction of the intercostal spaces) or impaired gas exchange (pH ≤ 7.35 with PaCO2 ≥ 45 mmHg) requiring respiratory support with NIV or NHF should be assessed.
It is recommended for patients with pH < 7.25, that NIV should be the first choice of treatment as there are no data regarding NHF efficiency in more severe AECOPD. For patients with hypercapnic respiratory failure and pH value between 7.25 and 7.35, NHF may be used either as a first choice or as an alternative therapy if the patient cannot tolerate NIV [Citation45]. Based on physiological studies [Citation17,Citation27,Citation67–69], NHF is shown to exert beneficial effects with maximal flow rates, so it is suggested that therapy is initiated adjusting this parameter first (flow rate at 50–60 L · min−1), and then if supplementary oxygen is still needed titrate FiO2 to maintain target oxygenation (SpO2: 88–92%) [Citation20,Citation21,Citation24]. It is important to remember that lower FiO2 values are often required with NHF due to a more consistent gas mixture delivery compared to COT. The temperature should be set to 37 °C. Other criteria, such as inability to tolerate NIV, life-threatening hypoxemia in patients unable to tolerate NIV and inability to remove secretions do not signify absolute indications for immediate intubation and invasive mechanical ventilation [Citation2], as those patients can have a trial of NHF therapy. Furthermore, it should be kept in mind that NHF can be used as complementary therapy during breaks off NIV [Citation48,Citation70]. Using NHF instead of COT has been proven to be more comfortable and even prevent the increase in respiratory rate, PaCO2 level and dyspnea seen with COT [Citation51,Citation71].
During NHF therapy bronchodilators can be given through a vibrating mesh nebulizer placed before or after the humidification chamber [Citation57,Citation63] or jet nebulizer placed closed to the nasal prongs if the appropriate connector for the humidification chamber is unavailable [Citation62]. It is suggested to use flow rates of 30–40 L · min−1 during aerosol delivery. Higher flows are needed for patients with distressed breathing and higher inspiratory flows [Citation57,Citation59]. If a jet nebulizer is used, the gas driving the nebulizer need to be set to 5–6 L · min−1 [Citation62].
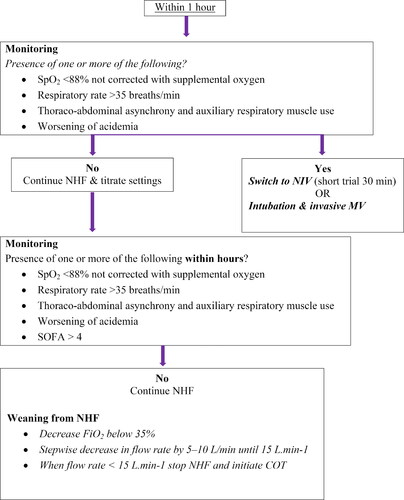
Within one hour of NHF initiation, appearance or persistence of parameters that have negative prognostic significance should be checked, such as SpO2 <88% not corrected with supplemental oxygen, respiratory rate >35 breaths/min, thoraco-abdominal asynchrony and auxiliary respiratory muscle use and concomitant worsening of hypercapnia indicating further respiratory muscle fatigue [Citation72]. The presence of one or more of these parameters identifies patients who do not respond to NHF. Direct intubation or a short trial of NIV (30 min) may be considered before intubating the patient. If none of the parameters are present, NHF can be continued and the initial settings should be titrated based on the patient’s respiratory rate (<25–30 breaths/min), SpO2 (>88–90%) and the patient’s comfort and tolerance. If the target SpO2, respiratory rate and work of breathing are not achieved, while the flow rate is <60 L · min−1, an increase in the flow rate by 5–10 L · min−1 is recommended, as higher flow rates unload respiratory muscles to a greater degree and improve pulmonary mechanics by further increasing both inspiratory and expiratory airway pressures.
It is necessary to closely monitor the patient on NHF therapy to avoid undesired respiratory and cardiac complications. The parameters that need regular monitoring and which have prognostic significance are those listed earlier. Apart from respiratory parameters, the presence of additional organ dysfunction as expressed by a sequential organ failure assessment score > 4, mainly hemodynamic failure, has also been significantly associated with NHF failure, indicating a more severe group of patients [Citation73,Citation74]. Once the patient’s clinical and gasometric parameters improve, if supplementary oxygen was given, it should be decreased first to FiO2 values < 35%, followed by a stepwise decrease in flow rate by 5–10 L · min−1 until 15 L · min−1. The intervals of these decrements may be longer or shorter based on the patient’s clinical and gasometric parameters. When the patient is stable for 1–2 h with flow <15 L · min−1, NHF should be stopped and a Venturi mask or nasal oxygen can be initiated.
Conclusion
NHF therapy is a user-friendly ventilatory support that can act as an intermediate form of respiratory support between traditional oxygen delivery systems and NIV. It may well also be used in place of NIV in the least tolerant and compliant patients, or in association with NIV to reduce mask-related side effects. Although NHF seems able to reduce the level of hypercapnia, larger interventional studies are needed to investigate the use of NHF oxygen therapy for acute and chronic hypercapnic respiratory failure in COPD patients. Furthermore, more work is needed to determine how to best select candidates and make adjustments (air flow, levels of heat and humidity) to optimize comfort. Currently, three major clinical trials (NCT03466385, NCT03214458, NCT03370666) are ongoing in an attempt to clarify the role of NHF in acute exacerbation of COPD. Until the safety of NHF can be established in different settings, we recommend that NHF use be limited to departments where patients can be closely monitored such as the respiratory or intensive care units of the hospital.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgments
Many thanks to Stylianos Sergios Chatziioannou and Maria Lahana medical students from the European University of Cyprus for their help in data collection.
References
- Osadnik CR, Tee VS, Carson-Chahhoud KV, et al. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:CD004104. doi:10.1002/14651858.CD004104.pub4.
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019.
- Ergan B, Oczkowski S, Rochwerg B, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J. 2019;54(3):1901003. doi:10.1183/13993003.01003-2019.
- Gay PC. Complications of noninvasive ventilation in acute care. Respir Care. 2009;54(2):246–257.
- Ischaki E, Pantazopoulos I, Zakynthinos S. Nasal high flow therapy: a novel treatment rather than a more expensive oxygen device. Eur Respir Rev. 2017;26(145):170028. doi:10.1183/16000617.0028-2017.
- Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest 2015;148(1):253–261. doi:10.1378/chest.14-2871.
- Ischaki E, Pantazopoulos I. Blow with the high flow” an updated algorithm. J Emerg Crit Care Med. 2019;3:61–61. doi:10.21037/jeccm.2019.10.03.
- Pantazopoulos I, Adamos G, Sotiriou A, et al. Nasal high flow application for perioperative support of respiratory system in adult patients. J Emerg Crit Care Med. 2019; doi:10.21037/jeccm.2019.11.02.
- Fraser JF, Spooner AJ, Dunster KR, et al. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax 2016;71(8):759–761. doi:10.1136/thoraxjnl-2015-207962.
- Chikata Y, Izawa M, Okuda N, et al. Humidification performance of two high-flow nasal cannula devices: a bench study. Respir Care. 2014;59(8):1186–1190. doi:10.4187/respcare.02932.
- Pisani L, Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. COPD 2017;14(3):346–350. doi:10.1080/15412555.2017.1315715.
- Okuda M, Tanaka N, Naito K, et al. Evaluation by various methods of the physiological mechanism of a high-flow nasal cannula (HFNC) in healthy volunteers. BMJ Open Resp Res. 2017;4(1):e000200. doi:10.1136/bmjresp-2017-000200.
- Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20(4):126–131. doi:10.1016/j.aucc.2007.08.001.
- Bräunlich J, Kohler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1077–1085. doi:10.2147/COPD.S104616.
- Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107(6):998–1004. doi:10.1093/bja/aer265.
- Parke RL, Bloch A, McGuinness SP. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. 2015;60(10):1397–1403. doi:10.4187/respcare.04028.
- Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–1215. doi:10.1164/rccm.201605-0916OC.
- Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56(8):1151–1155. doi:10.4187/respcare.01106.
- Moller W, Celik G, Feng S, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol.2015;118(12):1525–1532. doi:10.1152/japplphysiol.00934.2014.
- Moller W, Feng S, Domanski U, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122(1):191–197. doi:10.1152/japplphysiol.00584.2016.
- Bräunlich J, Mauersberger F, Wirtz H. Effectiveness of nasal high flow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm Med. 2018;18(1):14. doi:10.1186/s12890-018-0576-x.
- Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5(2):81–86. doi:10.1177/1479972307087190.
- Fontanari P, Burnet H, Zattara-Hartmann MC, et al. Changes in airway resistance induced by nasal inhalation of cold dry, dry, or moist air in normal individuals. J Appl Physiol.1996;81(4):1739–1743. doi:10.1152/jappl.1996.81.4.1739.
- Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400–1405. doi:10.1016/j.rmed.2009.04.007.
- Ritchie JE, Williams AB, Gerard C, et al. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography, and measurement of upper airway pressures. Anaesth Intens Care. 2011;39(6):1103–1110. doi:10.1177/0310057X1103900620.
- Chanques G, Riboulet F, Molinari N, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol. 2013;79(12):1344–1355.
- Itagaki T, Okuda N, Tsunano Y, et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients. Respir Care. 2014;59(1):70–74. doi:10.4187/respcare.02480.
- Vogelsinger H, Halank M, Braun S, et al. Efficacy and safety of nasal high-flow oxygen in COPD patients. BMC Pulm Med. 2017;17(1):143. doi:10.1186/s12890-017-0486-3.
- Bräunlich J, Seyfarth HJ, Wirtz H. Nasal high-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med. 2015;10(1):27. doi:10.1186/s40248-015-0019-y.
- Nagata K, Kikuchi T, Horie T, et al. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease. a multicenter randomized crossover trial. Ann ATS. 2018;15(4):432–439. doi:10.1513/AnnalsATS.201706-425OC.
- Bräunlich J, Dellweg D, Bastian A, et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD. COPD. 2019;14:1411–1421. doi:10.2147/COPD.S206111.
- O'Donoghue FJ, Catcheside PG, Jordan AS, et al. Effect of CPAP on intrinsic PEEP, inspiratory effort, and lung volume in severe stable COPD. Thorax 2002;57(6):533–539. doi:10.1136/thorax.57.6.533.
- Pisani L, Fasano L, Corcione N, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax 2017;72(4):373–375. doi:10.1136/thoraxjnl-2016-209673.
- Miguel-Montanes R, Hajage D, Messika J, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to moderate hypoxemia. Crit Care Med. 2015;43(3):574–583. doi:10.1097/CCM.0000000000000743.
- Chatila W, Nugent T, Vance G, et al. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest 2004;126(4):1108–1115. doi:10.1378/chest.126.4.1108.
- Cirio S, Piran M, Vitacca M, et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med. 2016;118:128–132. doi:10.1016/j.rmed.2016.08.004.
- Rea H, McAuley S, Jayaram L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010;104(4):525–533. doi:10.1016/j.rmed.2009.12.016.
- Ram FS, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004;3:CD004104.
- Liu J, Duan J, Bai L, et al. Noninvasive ventilation intolerance: characteristics, predictors, and outcomes. Respir Care. 2016;61(3):277–284. doi:10.4187/respcare.04220.
- Millar J, Lutton S, O’Connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Ther Adv Respir Dis. 2014;8(2):63–64. doi:10.1177/1753465814521890.
- Pavlov I, Plamondon P, Delisle S. Nasal high-flow therapy for type II respiratory failure in COPD: a report of four cases. Respir Med Case Rep. 2017;20:87–88. doi:10.1016/j.rmcr.2016.12.006.
- Bräunlich J, Wirtz H. Nasal high-flow in acute hypercapnic exacerbation of COPD. COPD. 2018;13:3895–3897. doi:10.2147/COPD.S185001.
- Kim ES, Lee H, Kim SJ, et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018;10(2):882–888. doi:10.21037/jtd.2018.01.125.
- Yuste ME, Moreno O, Narbona F, et al. Efficacy and safety of high-flow nasal cannula oxygen therapy in moderate acute hypercapnic respiratory failure. Rev Bras Ter Intensiva. 2019;31(2):156–163. doi:10.5935/0103-507X.20190026.
- Bruni A, Garofalo E, Cammarota G, et al. High flow through nasal cannula in stable and exacerbated chronic obstructive pulmonary disease patients. Rev Recent Clin Trials. 2019;14(4):247–260. doi:10.2174/1574887114666190710180540.
- Pilcher J, Eastlake L, Richards M, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology 2017;22(6):1149–1155. doi:10.1111/resp.13050.
- Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046–2056. doi:10.1111/crj.12772.
- Longhini F, Pisani L, Lungu R, et al. High-flow oxygen therapy after noninvasive ventilation interruption in patients recovering from hypercapnic acute respiratory failure: a physiological crossover trial. Crit Care Med. 2019;47(6):e506–e511. doi:10.1097/CCM.0000000000003740.
- Rittayamai N, Phuangchoei P, Tscheikuna J, et al. Effects of high-flow nasal cannula and non-invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: a preliminary study. Ann Intensive Care. 2019;9(1):122doi:10.1186/s13613-019-0597-5.
- Di Mussi R, Spadaro S, Stripoli T, et al. High-flow nasal cannula oxygen therapy decreases postextubation neuroventilatory drive and work of breathing in patients with chronic obstructive pulmonary disease. Crit Care. 2018;22(1):180. doi:10.1186/s13054-018-2107-9.
- Pisani L, Astuto M, Prediletto I, et al. High flow through nasal cannula in exacerbated COPD patients: a systematic review. Pulmonology 2019;25(6):348–354. doi:10.1016/j.pulmoe.2019.08.001.
- Rzepka-Wrona P, Skoczynski S, Wrona D, et al. Inhalation techniques used in patients with respiratory failure treated with noninvasive mechanical ventilation. Can Respir J. 2018;2018:1–8. doi:10.1155/2018/8959370.
- Zhou Y, Ahuja A, Irvin CM, et al. Evaluation of nebulizer performance under various humidity conditions. J Aerosol Med. 2005;18(3):283–293. doi:10.1089/jam.2005.18.283.
- Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172(12):1497–1504. doi:10.1164/rccm.200410-1414OC.
- Ari A, Areabi H, Fink JB. Evaluation of aerosol generator devices at 3 locations in humidified and nonhumidified circuits during adult mechanical ventilation. Respir Care 2010;55(7):837–844.
- Michotte JB, Jossen E, Roeseler J, et al. In vitro comparison of five nebulizers during noninvasive ventilation: analysis of inhaled and lost doses. J Aerosol Med Pulm Drug Deliv. 2014;27(6):430–440. doi:10.1089/jamp.2013.1070.
- Reminiac F, Vecellio L, Heuze ́-Vourc’h N, et al. Aerosol therapy in adults receiving high flow nasal cannula oxygen therapy. J Aerosol Med Pulm Drug Deliv. 2015;29((2):134–141. doi:10.1089/jamp.2015.1219.
- Dugernier J, Reychler G, Vecellio L, et al. Nasal high-flow nebulization for lung drug delivery: theoretical, experimental, and clinical application. J Aerosol Med Pulm Drug Deliv. 2019;32(6):341–351.
- Dailey PA, Harwood R, Walsh K, et al. Aerosol delivery through adult high flow nasal cannula with heliox and oxygen. Respir Care. 2017;62(9):1186–1192. doi:10.4187/respcare.05127.
- Perry SA, Kesser KC, Geller DE, et al. Influences of cannula size and flow rate on aerosol drug delivery through the vapotherm humidified high-flow nasal cannula system. Pediatr Crit Care Med. 2013;14(5):250–256.
- Reminiac F, Vecellio L, Loughlin RM, et al. Nasal high flow nebulization in infants and toddlers: an in vitro and in vivo scintigraphic study. Pediatr Pulmonol. 2017;52(3):337–344. doi:10.1002/ppul.23509.
- Bräunlich J, Wirtz H. Oral versus nasal high-flow bronchodilator inhalation in chronic obstructive pulmonary disease. J Aerosol Med Pulm Drug Deliv. 2018;31(4):248–254. doi:10.1089/jamp.2017.1432.
- Reminiac F, Vecellio L, Bodet-Contentin L, et al. Nasal high-flow bronchodilator nebulization: a randomized cross-over study. Ann Intensive Care. 2018;8(1):128. doi:10.1186/s13613-018-0473-8.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Patel A, Nouraei SA. Transnasal humidified rapid insufflation ventilatory exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015;70(3):323–329. doi:10.1111/anae.12923.
- Guitton C, Ehrmann S, Volteau C, et al. Nasal high-flow preoxygenantion for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive Care Med. 2019;45(4):447–458. doi:10.1007/s00134-019-05529-w.
- Shepard JW, Jr, Burger CD. Nasal and oral flow-volume loops in normal subjects and patients with obstructive sleep apnea. Am Rev Respir Dis. 1990;142(6_pt_1):1288e93–1281293. doi:10.1164/ajrccm/142.6_Pt_1.1288.
- Berg KM, Lang GR, Salciccioli JD, et al. The rapid shallow breathing index as a predictor of failure of noninvasive ventilation for patients with acute respiratory failure. Respir care. 2012;57(10):1548–1554. doi:10.4187/respcare.01597.
- Nilius G, Franke KJ, Domanski U, et al. Effects of nasal insufflation on arterial gas exchange and breathing pattern in patients with chronic obstructive pulmonary disease and hypercapnic respiratory failure. Adv Exp Med Biol. 2013;755:27–34. doi:10.1007/978-94-007-4546-9_4.
- Thille AW, Muller G, Gacouin A, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA 2019;322(15):1465. doi:10.1001/jama.2019.14901.
- Spoletini G, Mega C, Pisani L, et al. High-flow nasal therapy vs standard oxygen during breaks off noninvasive ventilation for acute respiratory failure: a pilot randomized controlled trial. J Crit Care. 2018;48:418–425. doi:10.1016/j.jcrc.2018.10.004.
- Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37(11):1780–1786. doi:10.1007/s00134-011-2354-6.
- Kim WY, Sung H, Hong SB, et al. Predictors of high flow nasal cannula failure in immunocompromised patients with acute respiratory failure due to non-HIV pneumocystis pneumonia. J Thorac Dis. 2017;9(9):3013–3022. doi:10.21037/jtd.2017.08.09.
- Messika J, Ben Ahmed K, Gaudry S, et al. Use of high flow nasal cannula oxygen therapy in subjects with ARDS: a 1-year observational study. Respir Care. 2015;60(2):162–169.