Abstract
The efficacy and feasibility of high flow nasal therapy (HFNT) use in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and bronchiectasis is unknown. We performed a single-center, single-arm prospective observational study in patients with AECOPD, documented bronchiectasis, pH ≥ 7.35, respiratory rate (RR) ≥ 26 breaths/minute despite receiving maximal medical treatment and oxygen via face mask up to 10 L/m. Patients received HFNT (Airvo 2, Fisher & Paykel) at a gas flow of 50 L/min and FIO2 adjusted to maintain SpO2 ≥92%. Dyspnea, rated by Borg scale, RR, arterial blood gases and mucus production (ranging from 1 to 3) were collected before and 1 h after starting HFNT and then every 24 h for 3 days. Tolerance was measured using a visual analogic scale (VAS). Fifteen patients were enrolled. After 24 h, patients showed a significant improvement in dyspnea score [Borg scale from 6.7 ± 1.4 to 4.1 ± 1.3 (p<.001)]; RR decreased from 29.6 ± 2.7 breaths/min to 23.2 ± 2.9 breaths/min (p<.001); pCO2 significantly decreased after 24 h [58.4 ± 13 vs. 51.7 ± 8.2 (p=.003)] while quantity of mucus production increased [(1.1 ± 0,6 vs. 2.4 ± 0.7, p<.001)]. No patient received invasive or noninvasive mechanical ventilation. Overall VAS score for HFNT tolerance was 6.5. HFNT was effective in improving dyspnea score, decreasing RR, improving gas exchange, and increasing mucus production in patients with AECOPD and coexisting bronchiectasis. Moreover, no safety concerns on its use were detected. Nevertheless, due to the single-arm design, the effect of HFNT could not be isolated from standard pharmacological treatment due to the study design.
Introduction
Bronchiectasis is a frequent comorbidity in patients with chronic airway diseases [Citation1], determining a more severe phenotype in both asthma [Citation2] and chronic obstructive pulmonary disease (COPD) [Citation3]. Moreover, COPD-related bronchiectasis has an independent impact on disease course and outcomes, [Citation4] leading to a worse prognosis [Citation5], increased severity of exacerbation [Citation6] and mortality rate [Citation7]. Indeed, patients with bronchiectasis during acute exacerbations very often have an increased secretion viscosity, worsening sputum retention and consequently an increase in respiratory impedance and work of breathing [Citation8]. Therefore, COPD severity, during acute exacerbation of COPD (AECOPD) may be influenced by coexisting bronchiectasis [Citation9].
High-flow nasal therapy (HFNT) has become an increasingly used modality for the management of patients with type 1 and type 2 acute respiratory failure in different clinical settings [Citation10–12]. HFNT delivers high flow gas (up to 60 L/min), warmed to body temperature, saturated for reaching optimal humidification (37 degrees, 44 mg H20/L) and eventually oxygen-enriched to achieve an inspiratory oxygen fraction up to 100% [Citation13,Citation14]. From a physiological point of view, the delivery of warmed humidified gas by HFNT preserves mucociliary transport and promotes mobilization of secretions [Citation15], preventing mucus plugging that may obstruct the airway and decrease ventilation.
Recently, some studies showed the beneficial effects of heated humidification therapy on mucociliary clearance and ventilation in patients with bronchiectasis [Citation16,Citation17] and COPD [Citation18–20], both muco-obstructive diseases characterized by airway inflammation, mucus hypersecretion and impaired mucociliary transport [Citation21]. Nevertheless, few data exist about the use of HFNT for the treatment of bronchiectasis and bronchiectasis-COPD exacerbations.
We hypothesized that HFNT may be effective and well tolerated in patients with AECOPD and concomitant bronchiectasis in terms of gas exchange, respiratory rate, and mucus expectoration; therefore we performed a prospective observational study to evaluate the feasibility of a randomized controlled trial (RCT).
Methods
Patients and setting
We performed a prospective single-arm, observational study from September 2018 to October 2019 in patients admitted to the Respiratory Medicine Unit of AOU Policlinico Vittorio Emanuele di Catania for AECOPD and concomitant bronchiectasis.
All the patients considered for this observational feasibility study were treated according to routine care and not assigned with a specific treatment by investigators’ ad hoc for the study.
The inclusion criteria were as follows: acute respiratory failure (ARF) define as PaO2/FIO2<300 mmHg and arterial blood pH ≥ 7.35); respiratory rate (RR) ≥ 26 breaths/min despite receiving maximal medical treatment [Citation22] and after 1 h of oxygen via Venturi mask (O2 max 10 L/min); COPD GOLD Class ≥ 2 (based on GOLD classification of severity of airflow limitation) [Citation22]; MRC (Medical Research Council) dyspnea score ≥ 2; history of documented bronchiectasis [confirmed by a recent (< 1 year) chest computed tomography].
The presence of bronchiectasis was detected by an independent radiologist who performed the patient’s chest CT scan and then confirmed by a respiratory physician (RC) who was unaware of the patient’s clinical condition. Bronchiectasis was defined as present if the broncho-arterial ratio was equal to or greater than 1 [Citation23].
We excluded patients with: life-threatening hypoxemia (PaO2/FIO2 < 100 mm Hg) requiring noninvasive ventilation (NIV) or intubation, acidosis (pH < 7.35) requiring NIV or intubation, tracheostomy, receiving NIV in the emergency department, domiciliary long-term NIV, malignant co-morbidities, hemodynamic instability (systolic arterial pressure < 90 mm Hg or mean arterial pressure < 60 mm Hg, or use of vasoactive agents), severe heart failure (New York Heart Association stage IV), unstable angina/myocardial infarction or severe arrhythmias [Citation24], pulmonary embolism, pulmonary infiltrates of new origin suggesting pneumonia, abnormalities of the thorax or lung diseases other than COPD/bronchiectasis, refusal of consent. All enrolled patients signed written informed consent.
The study was approved by the Ethics Committee “Catania 1” of AOU Policlinico-Vittorio Emanuele di Catania (N°176/2018/PO).
Study design
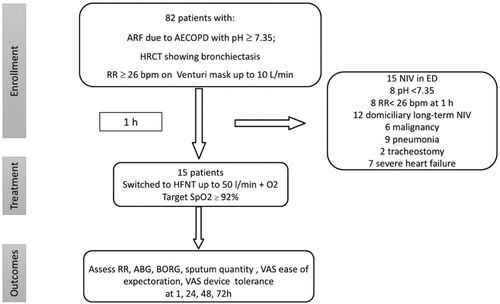
The study design is shown in . In brief, patients with AECOPD and bronchiectasis who remained tachypneic despite maximal medical treatment according to GOLD strategy [Citation22] [antibiotics (fluoroquinolones), intravenous glucocorticoid (methylprednisolone), inhaled beta-agonist and anticholinergic agents via a nebulizer or a metered-dose inhaler (MDI)] and after 1 h on oxygen, started HFNT continuously for 72 h, as an escalation of care for their acute respiratory failure.
Figure 1. Study design flow-chart and patients selection.
ARF: acute respiratory failure; AECOPD: acute exacerbation chronic obstructive pulmonary disease; HRCT: high resolution computed tomography; RR: respiratory rate; NIV: noninvasive ventilation; ED: emergency department; HFNT: high flow nasal cannula; ABG: arterial blood gas analysis; VAS: visual analogic scale.
Outcomes and timepoints
Dyspnea, rated by Borg scale [Citation25], RR, SpO2, arterial blood gases (pH, PaO2, pCO2), sputum production and self-reported ease of expectoration were collected while the subject was breathing through the high-FiO2 face mask and 1 h, 24 h, 48 h, and 72 h after initiation of HFNT. Daily entire expectorated sputum for 3 consecutive days was collected to assess the average daily sputum production. Mucus quantity was reported as ranging from 1 to 3 (1 = 1 teaspoon, 2 = 1-3 tablespoons, 3 = 1 cup). Patients rated their ease of sputum expectoration before and after treatment using a 10-cm visual analog scale (VAS, 0 = extremely easy; 10 = extremely difficult) [Citation26] which asked ‘how easy was it to cough and expectorate sputum?’. Patients’ tolerance to the HFNT device was assessed using a VAS.
HFNT settings
HFNT was delivered using a dedicated high flow system (Airvo 2, Fisher & Paykel Healthcare) initially set at a gas flow of 50 L/min, temperature was set at 37 °C, and FIO2 adjusted to maintain SpO2 ≥ 92%; HFNT interface was a nasal cannula (Optiflow; Fisher & Paykel Healthcare) and the size of interface was selected to occlude patient’s nostril of about 2/3 of their size. HFNT settings were titrated based on patients’ severity and tolerance, never falling below 35 L/min of flow rate.
Prespecified criteria for HFNT interruption
Severe hypoxemia (PaO2/FIO2 < 100 mm Hg) or development of respiratory acidosis (pH< 7.35) requiring escalation of therapy (NIV or intubation), or increase of dyspnea, RR >30 breaths/min were considered criteria for HFNT interruption.
Statistical analysis
The normality of data distribution was checked graphically and by the Shapiro-Wilk test. Data are expressed as mean ± standard deviation (SD) or as median and interquartile range (IQR) when appropriate. Sphericity was assessed by Mauchly’s test.
A one-way repeated measures ANOVA was conducted to determine whether there was a statistically significant difference in the variables of interest during HFNT treatment. Post-hoc analysis was performed with a Bonferroni adjustment. Epsilon (ε) was calculated according to Greenhouse and Geisser and was used to correct the one-way repeated measures.
Results
A convenience sample of 15 consecutively admitted patients were enrolled from 82 screened (reasons for screening failure are reported in ). summarizes the enrolled patients’ characteristics. shows the clinical and radiological characteristics of bronchiectasis. All enrolled patients completed the pre-planned follow-up to 72 h and there was no treatment interruption due to the pre-specified criteria. One patient had an interface displacement, between 48 and 72 h, which was prompt solved.
Table 1. Patients’ characteristics.
Table 2. Bronchiectasis details.
shows the assessment of study outcomes at different time points. Globally, there was a statistically significant change in RR, pCO2, pO2, Borg score, the quantity of mucus production and patients’ self-reported ease of expectoration over the course HFNT intervention ().
Figure 2. Changes of RR, BORG scale, pO2, pCO2 at baseline and at different time points during HFNT treatment. RR: respiratory rate; HFNT: high flow nasal therapy.
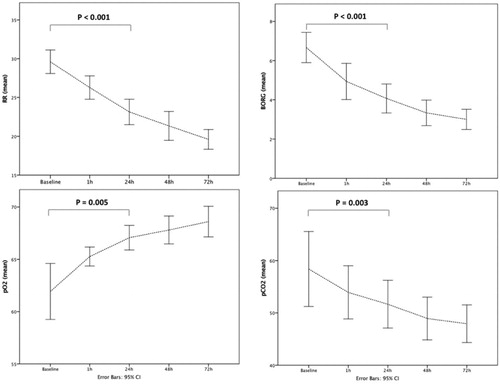
Table 3. Assessment of study outcomes at the different time-points.
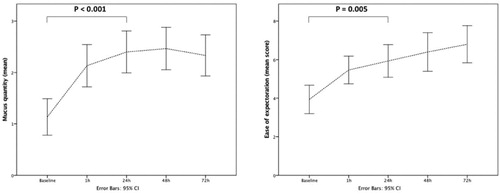
Post-hoc analyses showed that pCO2 decreased from baseline to 48 h while RR and Borg scale during the first 24 h. Mucus quantity was significantly increased at 24 h then remained stable, while pO2 was significantly increased at 72 h in comparison to baseline values (). Overall tolerance of the HFNT device evaluated trough the VAS scale was 6.5. The average patients’ length of stay was 9 ± 1.5 days; 30-days readmission rate was 6.6%.
Discussion
The main finding of our study was that HFNT might be effective to improve gas exchange, dyspnea, RR and mucus clearance in patients with AECOPD and bronchiectasis and its implementation did not showed any safety concerns.
COPD and bronchiectasis are both muco-obstructive diseases [Citation27] characterized by hyper-concentrated mucus with formation of adherent and thick mucus plaques and plugs [Citation28], therefore the rationale for using HFNT in these patients relies on the well-known beneficial effects of high humidified and warm flow in the improvement of mucociliary transport and, in the increasing mobilization of airways secretions that lead to mucus expectoration [Citation29].
An in vitro study [Citation15] showed that mucociliary beating and hence mucus clearance are increased at 37 °C and 100% relative humidity, which represents the optimal condition for preserving mucosa function [Citation30]; at these conditions, there is a reduction of mucus viscosity that helps expectoration [Citation15]. Furthermore, Hasani et al. [Citation16] showed that three hours per day of humidification treatment for seven days significantly increased mucociliary clearance in patients with bronchiectasis. Moreover, Rea et al. [Citation17] showed that in a group of mixed patients with COPD and bronchiectasis an average of 1–2 h a day of humidification treatment reduces the number of days of exacerbation and increases time on first exacerbation. Indeed, recently it has been shown the effectiveness of HFNT in the treatment of ARF due to AECOPD [Citation18–20,Citation31,Citation32].
Our data showed that HFNT was able to significantly reduce RR of patients with ARF due to AECOPD and coexisting bronchiectasis after 24 h, and this is a very important point since sustained high respiratory rate despite conventional oxygen therapy is indicative of a worsening prognosis in patients hospitalized for ARF and represents an early marker of potential health complications and need for ICU admission [Citation33]. Furthermore, dyspnea had significantly improved (BORG and VAS scale).
Of note, a statistically significant reduction of pCO2 was observed after HFNT treatment; this finding is probably due to the documented ability of HFNT of reducing dead space [Citation34] and eventually patient’s effort [Citation35]. We can speculate that the mechanism beyond pCO2 improvement is also related to the concomitant increase in sputum production and patients’ self-reported ease of expectoration at 24 h compared to baseline, since the volume of secretions may be linked to the humidification provided by HFNT ().
Figure 3. Changes of mucus production and patients’ self-reported ease of sputum expectoration at baseline and at different time points during HFNT.
None of the patients required an escalation of treatment with NIV [Citation36], highlighting the feasibility of using HFNT in these circumstances. Moreover, HFNT is a simple tool with an easy to wear nasal cannula that it has also the advantage of allowing patients to eat, drink and speak, and leaving them free to cough and clear their secretions, compared to oxygen facial masks or NIV interfaces. Indeed, HFNT, as opposed to NIV, does not require attention for leaks, minimizing the risk of patient-ventilator dyssynchrony [Citation37].
Although a better comfort during HFNT is still a matter of debate [Citation38], it could be speculated that better well-being during the time on HFNT may, in turn, lead to less need for constraints and sedation [Citation39].
Additionally, our data confirm previous findings [Citation40] on the benefits of HFNT for managing patients with ARF outside ICU settings.
Strengths and limitations
Our study has limitations, mainly related to the low sample size and the single-arm study design. Further studies with adequate sample size should test this intervention in this setting, considering stronger patient’s related outcomes.
Furthermore, due to the observational nature of the study and the single-arm design, we cannot isolate the effects of HFNT as an adjunct to standard pharmacological treatment (antibiotics, bronchodilators and, corticosteroids).
In this study, the time of assessment was set at 72 h. Although relatively short, it was enough to detect significant beneficial effects of HFNT on both gas exchange and mucous expectoration, therefore it might be enough for patients with AECOPD with bronchiectasis.
Moreover, the humidification treatment with HFNT lasted for 24 h per day for three days, unlike previous studies [Citation16,Citation17,Citation31]; nevertheless, this prolonged treatment duration might have had a high impact on the beneficial results.
Indeed, this is the first study exploring the quantity of mucus production and patients’ ease of sputum expectoration during HFNT.
Conclusions
HFNT may be effective in improving gas exchange, dyspnea and mucus clearance in patients with AECOPD and bronchiectasis. Adequately powered and controlled studied are needed to confirm these preliminary findings.
Declaration of interest
The authors declare no conflicts of interest associated with this study.
References
- Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52(3):1800328. doi:10.1183/13993003.00328-2018.
- Crimi C, Ferri S, Crimi N. Bronchiectasis and asthma: a dangerous liaison? Curr Opin Allergy Clin Immunol. 2019;19(1):46–52. doi:10.1097/ACI.0000000000000492.
- Ni Y, Shi G, Yu Y, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465–1475. [https://doi.org/26251586]
- Gatheral T, Kumar N, Sansom B, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD. 2014;11(6):605–614. doi:10.3109/15412555.2014.922174.
- Martinez-Garcia MA, de la Rosa Carrillo D, Soler-Cataluna JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. doi:10.1164/rccm.201208-1518OC.
- Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):400–407. doi:10.1164/rccm.200305-648OC.
- Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med. 2014;108(2):287–296. doi:10.1016/j.rmed.2013.12.015.
- Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. 2017;49(6):1700051. doi:10.1183/13993003.00051-2017.
- Kawamatawong T, Onnipa J, Suwatanapongched T. Relationship between the presence of bronchiectasis and acute exacerbation in Thai COPD patients. COPD. 2018;13:761–769. doi:10.2147/COPD.S139776.
- Helviz Y, Einav S. A systematic review of the high-flow nasal cannula for adult patients. Crit Care. 2018;22(1):71. doi:10.1186/s13054-018-1990-4.
- Cortegiani A, Crimi C, Sanfilippo F, et al. High flow nasal therapy in immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. J Crit Care. 2019;50:250–256. doi:10.1016/j.jcrc.2018.12.015.
- Pisani L, Astuto M, Prediletto I, et al. High flow through nasal cannula in exacerbated COPD patients: a systematic review. Pulmonology. 2019;25(6):348–354. doi:10.1016/j.pulmoe.2019.08.001.
- Spoletini G, Cortegiani A, Gregoretti C. Physiopathological rationale of using high-flow nasal therapy in the acute and chronic setting: a narrative review. Trends in Anaesthesia and Critical Care. 2019; 26-27:22–29. doi:10.1016/j.tacc.2019.02.001.
- Cortegiani A, Accurso G, Mercadante S, et al. High flow nasal therapy in perioperative medicine: from operating room to general ward. BMC Anesthesiol. 2018;18(1):166. doi:10.1186/s12871-018-0623-4.
- Kilgour E, Rankin N, Ryan S, et al. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. 2004;30(7):1491–1494. doi:10.1007/s00134-004-2235-3.
- Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5(2):81–86. doi:10.1177/1479972307087190.
- Rea H, McAuley S, Jayaram L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010;104(4):525–533. doi:10.1016/j.rmed.2009.12.016.
- Braunlich J, Kohler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1077–1085.
- Kim ES, Lee H, Kim SJ, et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018;10(2):882–888. doi:10.21037/jtd.2018.01.125.
- Fraser JF, Spooner AJ, Dunster KR, et al. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71(8):759–761. doi:10.1136/thoraxjnl-2015-207962.
- Hurst JR, Elborn JS, De Soyza A. COPD-bronchiectasis overlap syndrome. Eur Respir J. 2015;45(2):310–313. doi:10.1183/09031936.00170014.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2018 report; 2018.
- Naidich DP, McCauley DI, Khouri NF, et al. Computed tomography of bronchiectasis. J Comput Assist Tomogr. 1982;6(3):437–444. doi:10.1097/00004728-198206000-00001.
- Kohnlein T, Windisch W, Kohler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. doi:10.1016/S2213-2600(14)70153-5.
- Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078–1081.
- Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62(10):989–993.
- Boucher RC. Muco-Obstructive Lung Diseases. N Engl J Med. 2019;380(20):1941–1953. doi:10.1056/NEJMra1813799.
- Panchabhai TS, Mukhopadhyay S, Sehgal S, et al. Plugs of the air passages: a clinicopathologic review. Chest. 2016;150(5):1141–1157. doi:10.1016/j.chest.2016.07.003.
- Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1):253–261. doi:10.1378/chest.14-2871.
- Williams R, Rankin N, Smith T, et al. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med. 1996;24(11):1920–1929. doi:10.1097/00003246-199611000-00025.
- Pilcher J, Eastlake L, Richards M, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology. 2017;22(6):1149–1155. doi:10.1111/resp.13050.
- Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046–2056. doi:10.1111/crj.12772.
- Garrido D, Assioun JJ, Keshishyan A, et al. Respiratory rate variability as a prognostic factor in hospitalized patients transferred to the intensive care unit. Cureus. 2018;10(1):e2100.10.7759/cureus.2100. doi:10.7759/cureus.2100.
- Mundel T, Feng S, Tatkov S, et al. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol.2013;114(8):1058–1065. doi:10.1152/japplphysiol.01308.2012.
- Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–1215. doi:10.1164/rccm.201605-0916OC.
- Gregoretti C, Pisani L, Cortegiani A, et al. Noninvasive ventilation in critically ill patients. Crit Care Clin. 2015;31(3):435–457. doi:10.1016/j.ccc.2015.03.002.
- Carlucci A, Schreiber A, Mattei A, et al. The configuration of bi-level ventilator circuits may affect compensation for non-intentional leaks during volume-targeted ventilation. Intensive Care Med. 2013;39(1):59–65. doi:10.1007/s00134-012-2696-8.
- Cortegiani A, Crimi C, Noto A, et al. Effect of high-flow nasal therapy on dyspnea, comfort, and respiratory rate. Crit Care. 2019;23(1):201. doi:10.1186/s13054-019-2473-y.
- Mistraletti G, Pelosi P, Mantovani ES, et al. Delirium: clinical approach and prevention. Best Pract Res Clin Anaesthesiol. 2012;26(3):311–326. doi:10.1016/j.bpa.2012.07.001.
- Pirret AM, Takerei SF, Matheson CL, et al. Nasal high flow oxygen therapy in the ward setting: a prospective observational study. Intensive Crit Care Nurs. 2017;42:127–134. doi:10.1016/j.iccn.2017.04.001.
