Abstract
The aim of this study was to develop a simplified screening questionnaire to detect the existence of severe obstructive sleep apnea (OSA) in chronic obstructive pulmonary disease (COPD) patients to reduce mortality and hospitalization rates. Seventy-seven stable Asian COPD patients aged 69.2 ± 11.5 years were retrospectively analyzed into the development group. The simplified screening questionnaire was developed from factors identified from sleep surveys and demographic data to predict severe OSA. Receiver operating characteristic (ROC) curve analysis was used to validate the simplified screening questionnaire. Data from another 78 stable COPD patients were used for validation. The apnea-hypopnea index was similar between the development and validation groups (26.3 ± 21.9 and 27.6 ± 21.1, respectively). After logistic regression analysis in the development group, snoring, body mass index ≥27.5 kg/m2, witnessed apnea and coronary artery disease were incorporated into the screening questionnaire to predict OSA. When this questionnaire was applied to the validation group, the results were similar. The simplified screening questionnaire developed is useful in identifying severe OSA in COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a serious health burden worldwide, and the mortality of COPD is increasing [Citation1, Citation2]. However, only a few factors influencing this mortality are known. While COPD patients coexist with obstructive sleep apnea (OSA), the use of continuous positive airway pressure (CPAP) therapy improves the overall survival in these patients [Citation3], particularly in those with severe OSA [Citation4]. In addition, CPAP therapy can improve the exercise capacity in COPD patients with OSA [Citation5]. Moreover, the prevalence of COPD patients with OSA is 1%∼3.6% and this value increases to 39% in those with moderate-to-severe COPD and increases even further to 65% in those with severe COPD [Citation6–8]. Therefore, detecting the presence of severe OSA in COPD patients is an important issue [Citation9]. Despite increasing awareness, the underdiagnosis of OSA in patients with COPD remains a problem [Citation10, Citation11]. A possible reason for this is the lack of a reliable and simplified screening questionnaire to detect the possible existence of severe OSA in COPD patients. Based on this need, we aimed to develop a simplified screening questionnaire.
An ideal screening questionnaire should only contain a few items (less than five) and be easy to interpret without specialized techniques or equipment [Citation12–16]. Simplified screening questionnaires (OSA50) have been designed to detect OSA in the general population based on self-reported waist circumference, snoring witnessed apneas and age. However, the clinical characteristics of patients with COPD and OSA may be different to those with OSA alone, and the existing screening questionnaires may not be suitable for COPD patients [Citation12, Citation13, Citation15]. Therefore, this study aimed to develop and validate a simplified screening questionnaire to identify the existence of severe OSA in COPD patients.
Materials and methods
Study population
This is a retrospective study was performed at one sleep center of Chang Gung Memorial Hospital from January 2009 to December 2017. Patients with COPD were retrospectively recruited from the sleep center. Those COPD patients were referred to the sleep center with complaints or symptoms prompting sleep testing. Patients were excluded if history of witnessed apnea and snoring were not available. None of the patients had a history of renal insufficiency, heart failure Functional class > IV, mood disorder, malignancy, chronic respiratory failure, or sleep disorders other than OSA. Those COPD patients were randomly divided into two groups as development and validation respectively. The Ethics Committee of Chang Gung Memorial Hospital approved the study (IRB 201701665B0C601) and waived the requirement for informed consent due to the retrospective nature of this study. The patient data accessed was de-identified. Each patient’s medical records were reviewed to collect the clinical characteristics and laboratory results. Information, including results of age, sex, BMI, pulmonary function and polysomnography were analyzed.
Definition
COPD was defined as patients had chronic and progressive dyspnea, cough and sputum production. In addition, FEV1/FVC ratio was less than lower limit of normality (LLN) [Citation17]. For those patients with FEV1/FVC ratio above LLN, airflow obstruction was confirmed according to dynamic hyperinflation on 6MWT, increased RV in total lung capacity or emphysema on HRCT. The methods to perform polysomnography were described in previous publication [Citation18]. According to the polysomnography results, the definition of OSA was an apnea/hypopnea index (AHI) > 5/h, of which ≥50% were obstructive type. Patients were defined as severe OSA if the apnea/hypopnea index (AHI) was ≥30 per hour, of which ≥50% were obstructive. AASM criteria was used to score sleep stages and arousals [Citation19]. Respiratory events such as hypopnea, obstructive apnea, central apnea, mixed type apnea, and Cheyne-Stokes respiration were scored based on the established criteria [Citation20]. The definition of apnea was oronasal flow cessation for more than 10 s. Also, the definition of hypopnea was 50% reduction of baseline oronasal flow for more than 10 s or a 30% reduction followed by more than 3% decrease in SaO2 or arousal.
Study design
For the design of the simple questionnaire, we retrospectively analyzed the data from the development group to identify the factors that were associated with the severity of OSA. According to Chai-Coetzer’s study [Citation13], age, body mass index (BMI), snoring and witnessed apneas were chosen to examine the associations. In addition, a recent study revealed that cardiovascular diseases (hypertension, coronary artery disease, atrial fibrillation, congestive heart failure and peripheral artery disease) are also significantly associated with OSA [Citation21]. Therefore, cardiovascular disease is also chosen into the examination. A simplified screening questionnaire was then developed based on the associated factors, which were weighted according to odds ratios (OR). For the validation of the simplified screening questionnaire, we retrospectively analyzed the data from validation group to exam the sensitivity and specificity of the simplified screening questionnaire.
Statistical analysis
Data were expressed as mean ± SD. The Student’s t test was chosen to compare continuous variables between those with and without OSA. For data with uneven distribution, the Mann-Whitney test was used to analyze. For categorical variables, we used Chi-square or Fisher’s exact tests. Univariate logistic regression was used to examine the correlations between variables and severe OSA (AHI ≥ 30). Multivariate logistic regression analysis was used to determine the independent factors associated with severe OSA (AHI ≥ 30). Receiver operating characteristic (ROC) curve analysis was used to assess the accuracy of the simple questionnaire against AHI ≥ 30. All analyses were performed using SPSS software v13.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics
There were 77 COPD patients were analyzed in the development group and 78 COPD patients in the validation group. The baseline characteristics of the development and validation groups are shown in . The mean ages of the development and validation groups were 69.2 and 69.4 years, respectively, indicating that that the patients represented an elderly population. Pulmonary function between the two groups was not significantly difference. The mean AHI values in the development and validation groups were 26.3 and 27.6, respectively, suggesting that the severity of OSA in both groups was mainly moderate to severe.
Table 1. Subjects demonstration.
Questionnaire in development group
The univariate analyses are summarized in . Witnessed apnea, snoring, BMI ≥ 27.5 kg/m2 and coronary artery disease were significantly correlated with an AHI ≥ 30 (OR 21.4, 5.37, 3.07 and 5.04, respectively). Age was not correlated with AHI ≥ 30. Therefore, witnessed apneas, snoring, BMI ≥ 27.5 kg/m2 and coronary artery disease were included into multivariate logistic regression (). According to the OR in multivariate logistic regression, the existence of coronary artery disease, BMI ≥ 27.5 kg/m2 as we well as snoring were each given 1 point, while witnessed apnea received 2 points (). The questionnaire had a total score of 5 points. This design indicated that severe OSA would be predicted in the COPD patients if they had witnessed apneas alone or any two of snoring, BMI ≥ 27.5 kg/m2 and coronary artery disease. reveals the diagnostic performance of the questionnaire relating to a variety of AHI diagnostic values. Using a cutoff score of ≥ 2/5 for AHI ≥ 30, the questionnaire had a sensitivity of 87.5%, a specificity of 69.8%, a positive predictive value (PPV) of 56.8% and a negative predictive value of 92.5%. When applied to AHI ≥ 5, the specificity and PPV was good (90.1% and 97.1%, respectively).
Table 2. Univariate severe obstructive sleep apnea predictors: (using an AHI cutoff value of 30 h-1).
Table 3. Logistic regression analysis: factors associated with an apnea-hypopnea index ≥30/h as determined by X2 automatic interaction detection analysis.
Table 4. OSA screening questionnaire.
Table 5. Diagnostic performance of OSA sleep questionnaire at a variety of AHI diagnostic criterion values in development group.
Validation group
The diagnostic performance of the questionnaire for a variety of AHI diagnostic values for the validation group is shown in . Using a cutoff score of ≥ 2/5 for AHI ≥ 30, the questionnaire had a sensitivity of 85.2%, specificity of 80.4%, positive predictive value of 69.7% and negative predictive value of 91.1%. When applied to AHI ≥ 5, the specificity and PPV was also good (100% and 100%, respectively). The performance of the questionnaire in the validation group was similar to that in the validation group, with an area under the ROC curve (AUC) of 0.83(95% CI: 0.73-0.93, p < 0.001) ().
Figure 1. Receiver operating characteristic curve showing the performance of the OSA screening questionnaire, using a cutoff value of ≥ 2/5 (blue line) or ≥ 3/5 (pink line) in discriminating patients with severe OSA (AHI ≥ 30/h) in the development group (n = 77) and validation group (n = 78). AUC, area under the ROC curve.
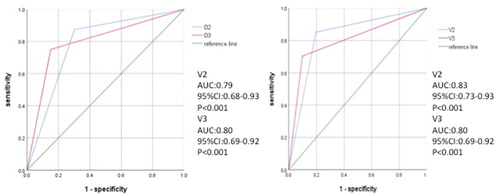
Table 6. Diagnostic performance of OSA sleep questionnaire at a variety of AHI diagnostic criterion values in validation group.
Diagnostic performance for AHI ≥ 30 of the questionnaire at a variety of COPD severity
The questionnaire’s diagnostic performance for AHI ≥ 30 at a variety of COPD severity was listed on . Using a cutoff score of ≥ 2/5 for AHI ≥ 30, the questionnaire had a good NPV for all COPD GOLD stage, especially at stage IV (NPV 95.8%). The specificity and PPV were both 100% in stage IV, while using a cutoff score of ≥ 2/5 or a cutoff score of ≥ 3/5.
Table 7. Diagnostic performance of OSA sleep questionnaire at a variety of COPD severity, AHI ≥ 30.
Discussion
The present study demonstrates that a simplified questionnaire can be used to identify the existence of severe OSA in COPD patients with a sensitivity of approximately 85%−87%. Since the existence of severe OSA in COPD patients has a significant impact on exercise capacity, mortality and exacerbations [Citation3–5], the simplified screening questionnaire could be useful for clinical physicians. To the best of our knowledge, this is the first study to design a simplified screening questionnaire to detect the existence of severe OSA in COPD patients.
Several studies have reported clinical prediction formulae to detect the existence of OSA in the general population or in sleep clinic-based patients, with the most common predictive factors being obesity (BMI, neck and waist circumference), snoring, witnessed apneas, age, gasping, choking and sex [Citation12, Citation15, Citation22]. In the multivariable apnea risk (MAP) index [Citation12], BMI, snoring, gasping and witnessed apneas are used to calculate the probability of OSA with an AUC of 0.79 for AHI ≥ 10. The sensitivity and specificity of the MAP index are 88% and 55%, respectively. However, the formulae are too complicated for clinical physicians to apply in practice. The clinical sleep apnea score (SACS) [Citation15], another useful screening tool for OSA, uses hypertension, neck circumference, snoring and gasping or choking as reported by the patient’s bed-partner. The results of the SACS show a likelihood ratio of 5.17 (95% CI: 2.54–10.51) and post-test probability of 81% when SACS > 15. Another study chose age, snoring, witnessed apnea and neck circumference to design a simplified screening questionnaire to detect moderate to severe OSA with an AUC of 0.84 (13). A recent study demonstrates that cardiovascular diseases (hypertension, coronary artery disease, atrial fibrillation, congestive heart failure and peripheral artery disease) are also significantly associated with OSA [Citation21]. Therefore, age, BMI, snoring and witnessed apneas and cardiovascular diseases were chosen as possible predictive factors for the questionnaire in this study.
Age was not correlated with the severity of OSA in this study; possibly due to the age range of the patients is narrow. This is similar to a recent study [Citation21]. Therefore, age was not included in the questionnaire. With regards to BMI, the cutoff point value was set at 27.5 kg/m2 due to the high risk in Asian population [Citation23] and no large population studies have focused on the cutoff point in Asian COPD patients to date. STOP-BANG questionnaire contains symptoms of snoring, witnessed apnea and tiredness. Both snoring and witnessed apnea revealed significantly association with OSA in current study. In addition, STOP-BANG questionnaire uses hypertension alone as a cardiovascular risk factor for OSA. In contrast, a new COPD-OSA questionnaire uses five cardiovascular diseases (hypertension, coronary artery disease, atrial fibrillation, congestive heart failure and peripheral artery disease) as risk factors for OSA. The present study analyzed all these factors and found that only coronary artery disease was associated with OSA. Therefore, coronary artery disease as a cardiovascular risk factor was chosen as a predictor for OSA.
Pulmonary function is not a good predictor for OSA [Citation21]. The mainly population of present study was moderate to severe COPD and moderate to severe OSA. The interaction between COPD and OSA was quite complicated. Increases in lung volume have been documented to dilate the pharynx and decrease its collapsibility, which suggests that increases in lung volume will ameliorate the severity of OSA [Citation24]. If severe COPD patients have larger lung volume, the severity of OSA may be reduced. However, some severe COPD patients had osteoporosis, which caused the decrease of lung volume. Decreases in lung volume in COPD patients with osteoporosis will deteriorate the severity of OSA [Citation18]. Therefore, the actual lung volume in severe COPD patients is individualized.
STOP-BANG questionnaire (cut-point ≥ 3) was reported to be associated with OSA in moderate to severe COPD patients [Citation21]. The sensitivity and specificity of STOP-BANG questionnaire (cut-point ≥ 3) were 80% and 50% respectively. A new COPD-OSA questionnaire (cut-point ≥ 1) was reported to be associated with OSA with sensitivity (92%) and specificity (33%). However, the definition of OSA was AHI ≥ 5 in both questionnaires. Because the existence of severe OSA in patients with COPD has a significant impact on mortality and exacerbations, it is urgent to screen, to diagnose and to treat those patients. The current questionnaire provides a good sensitivity 85%−87% to screen the existence of severe OSA in COPD patients, who will be benefit from the further CPAP treatment [Citation3].
The major limitations of the present study are its retrospective nature, which may have led to bias in patient selection. Second, those COPD patients referred to the sleep center with complaints or symptoms prompting sleep testing. This is therefore cautious for screening an unselected pool of patient with COPD, such as those being seen in a Chest Clinic or primary care office. Third, the population in this study was relatively small and was enrolled from only one tertiary medical center. Therefore, a large-scale multi-center-based study is warranted to validate the results of this study. Another possible limitation is the ethnic population. All of the patients in this study were Asian, and thus the definition of obesity was different from a Caucasian population. Another potential issue is the male predominance, which means this questionnaire should be applied with caution in a female population.
Conclusions
In conclusion, the existence of severe OSA in patients with COPD has a significant impact on mortality and exacerbations. Therefore, it is crucial to have a simplified screening questionnaire with good sensitivity to detect the existence of severe OSA in COPD patients, as with the current questionnaire. As the proposed questionnaire does not require complex mathematical calculations or complicated physical examinations, we propose that it may be useful for clinical physicians in the future. Further studies are warranted to confirm these findings.
Declaration of interest
The authors have reported to COPD that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Ethics Committee of Chang Gung Memorial Hospital approved the study (IRB 201701665B0C601) and waived the requirement for informed consent due to the retrospective nature of this study.
Supplemental Material
Download PDF (376.5 KB)Additional information
Funding
References
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412. doi:10.1183/09031936.06.00025805.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO.
- Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi:10.1164/rccm.200912-1869OC.
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7.
- Wang TY, Lo YL, Lee KY, et al. Nocturnal CPAP improves walking capacity in COPD patients with obstructive sleep apnoea. Respir Res. 2013;14(1):66. 2013(doi:10.1186/1465-9921-14-66.
- Lopez-Acevedo MN, Torres-Palacios A, Elena Ocasio TM, et al. Overlap syndrome: an indication for sleep studies?: A pilot study. Sleep Breath. 2009; 13(4):409–413. doi:10.1007/s11325-009-0263-5.
- Soler X, Gaio E, Powell FL, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015; 12(8):1219–1225. doi:10.1513/AnnalsATS.201407-336OC.
- Shawon MS, Perret JL, Senaratna CV, et al. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Med Rev. 2017;32:58–68. doi:10.1016/j.smrv.2016.02.007.
- McNicholas WT. COPD-OSA overlap syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest 2017;152(6):1318–1326. doi:10.1016/j.chest.2017.04.160.
- Kapur V, Strohl KP, Redline S, et al. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. doi:10.1007/s11325-002-0049-5.
- Fuhrman C, Fleury B, Nguyen XL, et al. Symptoms of sleep apnea syndrome: high prevalence and underdiagnosis in the French population. Sleep medicine. 2012;13(7):852–858. doi:10.1016/j.sleep.2012.04.005.
- Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18(3):158–166. doi:10.1093/sleep/18.3.158.
- Chai-Coetzer CL, Antic NA, Rowland LS, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax 2011;66(3):213–219. doi:10.1136/thx.2010.152801.
- Tsai WH, Remmers JE, Brant R, et al. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003; 167(10):1427–1432. doi:10.1164/rccm.200112-110OC.
- Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150(5):1279–1285. 1994doi:10.1164/ajrccm.150.5.7952553.
- Flemons WW, Douglas NJ, Kuna ST, et al. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169(6):668–672. doi:10.1164/rccm.200308-1124PP.
- Quanjer PH, the ERS Global Lung Function Initiative, Stanojevic S, Cole TJ, et al. ERS global lung function initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012; 40(6):1324–1343. doi:10.1183/09031936.00080312.
- Wang TY, Lo YL, Chou PC, et al. Associated bone mineral density and obstructive sleep apnea in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:231–237. [Mismatch] doi:10.2147/COPD.S72099.
- EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep1992. 15(2):173–184.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22(5):667–689.
- Soler X, Liao SY, Marin JM, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): The challenge to predict OSA in advanced COPD. PLoS One. 2017; 12(5):e0177289. doi:10.1371/journal.pone.0177289.
- Duran J, Esnaola S, Rubio R, et al. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001; 163(3 Pt 1):685–689. doi:10.1164/ajrccm.163.3.2005065.
- Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363(9403):157–163.
- Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax 2006; 61(5):435–439. doi:10.1136/thx.2005.052084.
