Abstract
Chronic pain affects up to 88% of people with chronic obstructive pulmonary disease (COPD) and has been associated with comorbidities. However, with pain not evaluated during pulmonary rehabilitation (PR) assessments, it is unclear whether PR impacts pain intensity and coping ability. This study aimed to 1) determine the effect of PR on pain qualities, coping behavior and psychological symptoms in those with COPD and chronic pain; and 2) assess the impact of PR on exercise capacity and quality of life in individuals with COPD and chronic pain compared to those without pain. Patients with COPD and comorbidities enrolling in outpatient PR were assessed for chronic pain. Those with chronic pain completed the Brief Pain Inventory, Coping Strategies Questionnaire-24, Fear Avoidance Behavior Questionnaire and measures of anxiety and depression. Changes in HRQOL and 6-minute walk distance (6MWD) following PR were compared between participants with and without chronic pain. Thirty-four participants with chronic pain and 34 participants without pain were included (mean ± SD, FEV1 47 ± 19% predicted). In those with chronic pain, PR did not affect pain intensity (median[IQR] pre/post PR 3[2–5] vs. 4[2–6] points, p = 0.21), anxiety (7[2–9] vs. 5[3–8] points, p = 0.82) or depression (4[2–8] vs. 3[1–6] points, p = 0.38) and did not change pain coping strategies. Both groups improved in 6MWD (mean difference [95% CI] 17[−39 to 72] m), and those without pain had greater improvement in mastery (p = 0.013). PR was effective in patients with moderate to severe COPD whether or not they reported chronic pain at the time of their initial assessment.
Introduction
Chronic obstructive pulmonary disease (COPD) is a systemic disease associated with dyspnea, fatigue and poor health-related quality of life (HRQOL) [Citation1]. Recent studies have demonstrated that the clinical profile of COPD is further complicated by the presence of pain, with a prevalence ranging from 32 to 88% [Citation2, Citation3]. Although cause and effect has not been established, it has been suggested that pain experienced in people with COPD may be associated with comorbidities [Citation4, Citation5]. Comorbidities have been reported in 50–97% of people with COPD [Citation6–8]. The most common comorbid conditions in COPD are cardiovascular disease, musculoskeletal disease (osteoarthritis and osteoporosis), cancer and metabolic disease [Citation5, Citation9]. As regions of pain in those with moderate-to-severe COPD include the upper and lower back and lower limb joints [Citation10, Citation11], there has been concern regarding the impact of pain on physical activity and HRQOL [Citation10–13].
Pulmonary rehabilitation (PR) is an integral component in the management of COPD, and improves fatigue, dyspnea, exercise tolerance and HRQOL [Citation1, Citation14]. Individuals with COPD and chronic pain undertaking PR report that pain experienced during exercise training was one reason for program attrition [Citation15–17]. It remains unclear whether the presence of chronic pain at the commencement of PR has a negative impact on program outcomes. As the management of many conditions associated with chronic pain in COPD such as osteoarthritis, cardiac disease and diabetes includes exercise training [Citation5, Citation18–22], it is plausible that improvements in strength and endurance might reduce chronic pain in people with COPD who have these comorbidities. However, as pain is not routinely measured as part of PR assessment [Citation17, Citation23], it is unknown whether those patients with COPD, comorbidities and chronic pain achieve comparable benefits to those without pain, or whether exercise training aggravates this symptom. It is similarly unknown whether the effects of PR on HRQOL and functional exercise capacity in COPD are modified by the presence of chronic pain.
The experience of chronic pain has been associated with fear-avoidance, pain catastrophizing, anxiety and depression [Citation24–27]. If pain is perceived to be threatening to wellbeing, avoidance behavior increases [Citation28]. In COPD, fear of injury from movement, pain catastrophizing and higher levels of depression have been described [Citation11, Citation13]. Although it is possible that both pain coping ability and fear avoidance may influence an individual’s response to PR, as pain is not systematically reported as an outcome measure, this response has not been explored.
The primary aim of this study was to determine the effects of PR on qualities of pain and psychological responses to chronic pain in adults with COPD and comorbidities. The secondary aim was to explore differences in exercise capacity and HRQOL before and after PR in adults with COPD who report chronic pain compared to those without pain. An improved understanding of the association between chronic pain and PR will inform whether patients with COPD with chronic pain require specific program modifications.
Materials and methods
Study design
This is a prospective study of adults with moderate to very severe COPD enrolled in an outpatient PR program. All participants gave written informed consent, with the study approved by the Centre’s Institutional Research Ethics Board. The trial was registered on ClinicalTrials.gov Identifier: NCT02979379.
Participants
Participants were eligible for inclusion if they had a diagnosis of COPD (FEV1/FVC < 70) [Citation1], according to post bronchodilator spirometry [Citation1], with spirometry undertaken within 12 months of study enrollment. This is accompanied by symptoms of dyspnea, chronic cough or sputum production and a history of exposure to disease risk factors [Citation1], all noted in the participants’ medical record. All participants were clinically stable, and enrolled in outpatient PR at West Park Healthcare Centre. All participants were recruited consecutively and were screened for the presence of chronic pain at their baseline assessment for PR using standard criteria previously described [Citation13]. Participants were asked about pain currently experienced, the duration (ranging from three months to > 10 years) and the frequency (ranging from constant to less than once a month). The criteria for chronic pain was the presence of any type of pain which occurred constantly or flared up frequently for more than three months. Based on this criteria, participants were classified in the pain or the no pain group. Individuals were excluded if they had a primary respiratory diagnosis other than COPD, known cognitive impairment precluding the accurate completion of surveys, or non-pulmonary comorbidities which prevented their undertaking exercise safely.
Intervention
All participants completed a 10-week outpatient PR program, attending twice-weekly sessions of supervised exercise training, with a total of 30 min of aerobic exercise (walking or cycling) and 15 min of strength training per session. The initial aerobic exercise prescription for walking was set at 80% of the walking speed of the baseline six-minute walk test (6MWT) [Citation29, Citation30]. For cycling, the cycle workload was based on the 6MWT-estimated VO2 maximum [Citation31]. Symptoms were monitored with the aim of achieving a dyspnea rating of 3 and 4 (Borg scale) and a perceived exertion rating of 12–14 (Modified Borg scale) [Citation32]. Oxygen saturation was monitored during exercise training and supplemental oxygen provided for those meeting criteria for significant exertional desaturation.
Resistance training consisted of a mix of functional strengthening for the lower limbs (sit to stand, stair training, squats with free weights) and dumbbells for the upper limbs [Citation29, Citation30, Citation33]. For lower limbs, muscles trained included quadriceps, hamstrings, hip flexors, hip abductors and extensors; for upper limbs, biceps, triceps, deltoid, pectoral muscles, using free weights. The resistance was initially set at 10 repetition maximum for both upper and lower limbs, ranging from one to three sets prior to increasing load, and aiming for a perceived exertion rating of 12-14 [Citation29, Citation30, Citation32]. Exercises were progressed according to participant tolerance and perceived exertion ratings.
Self-management education and training conducted by members of the multidisciplinary team was provided through lectures and discussion groups, with topics consistent with the current recommendations [Citation29, Citation30]. No specific topics covering pain and pain management options were included.
Outcomes
The following outcome measures were completed at baseline and at program completion.
All participants
As a measure of functional exercise capacity, all participants completed the 6MWT according to standardized guidelines; the best result of two tests was recorded [Citation34]. All participants also completed the self-reported version of the Chronic Respiratory Disease Questionnaire (CRDQ). This instrument assesses HRQOL across four domains (dyspnea, fatigue, emotional function and mastery), with a higher score indicating better HRQOL [Citation35].
Demographic factors including age, sex and disease severity were noted. Of those reporting chronic pain at the time of their baseline assessment, current prescription of regular pain treatment (pharmacological therapy), and use of non-pharmacological agents (specific exercises, acupuncture, electrotherapy) were noted. Comorbidities were extracted from the participants’ medical records and converted to comorbidity categories according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) [Citation36].
During the PR program, participants with chronic pain completed a rating of pain on a visual analog scale every second week. Participants classified without pain on initial assessment were asked bi-weekly whether they developed pain over the course of the PR program.
Participants with chronic pain
Participants with chronic pain completed additional questionnaires prior to commencement of PR and at the completion.
The Brief Pain Inventory (BPI) (short form) collates information on pain intensity and its degree of interference with daily activities within the last 24 h using numerical rating scales (0 to 10), with a higher score denoting a greater pain intensity or interference [Citation37]. It is a valid and reliable tool for assessing pain and has been previously applied in COPD [Citation11, Citation13, Citation38]. Pain locations were marked on the body chart of the BPI. The coding of standardized body regions was based on 45 anatomical areas, with a score of 1 or 0 recorded for the presence or absence of pain respectively. The reliability of this method has been previously reported [Citation39] and has been applied in COPD [Citation13].
Coping Strategies Questionnaire (CSQ-24) is a valid measure of pain coping strategies, reported in a mix of patient groups [Citation40–43] that assesses the ability to cope with pain. It consists of 23 items regarding cognitive and behavioral strategies for coping with pain, scored on a seven-point Likert scale, and one item measuring perceived control over pain [Citation40, Citation44]. It has four subscales: catastrophizing, diverting attention, re-interpreting and cognitive coping. From these subscales a dominant strategy is determined, with cognitive coping classed as positive and catastrophizing, diverting attention and re-interpreting classed as negative strategies.
Fear Avoidance Behavior Questionnaire (FABQ) measures fear of pain and avoidance of physical activity. It is composed of 16 items, each scored from 0 to 6, and has two subscales for physical activity and work [Citation45]. Higher scores of the FABQ indicate greater fear and avoidance beliefs [Citation45]. It has shown strong reliability in individuals with low back pain (acute and chronic), shoulder pain and other musculoskeletal disorders [Citation46, Citation47]. For the physical activity subscale, the maximum score is 24 points with scores >15 representing a high level of fear avoidance. For the work subscale, the maximum score is 42 with scores >34 representing a high level of fear avoidance [Citation45].
Hospital Anxiety and Depression (HAD) scale measures anxiety and depression in the last week. Comprised of 14 items measured on a four-point Likert scale with varying anchor statements, the scale is scored from 0 to 21 for both anxiety and depression. A score of 8 to 10 is suggestive of the presence of a mood disorder and a score of 11 or higher indicates probable presence [Citation48, Citation49]. The scale has been validated for individuals with COPD [Citation50].
Centre for Epidemiological Studies for Depression survey (CES-D) (self-administered) measures the presence and severity of depressive symptoms experienced over the preceding week [Citation51, Citation52], with items rating on a four-point scale. Higher scores indicate the presence of depressive symptoms, with a score of 16 or more diagnostic of depression [Citation51, Citation52]. Subscores of the CES-D are depressed affect, somatic symptoms, interpersonal problems and positive affect [Citation51].
Statistical analysis
Data were analyzed using SPSS (Statistical Package for Social Sciences) for Windows version 25.0. Continuous data were reported as mean and standard deviation, or median and interquartile range, depending on data distribution. Comparisons of baseline demographics between groups were analyzed using Independent t-tests or Chi-square test, while comparisons of pre and post rehabilitation for pain coping abilities, fear avoidance and pain qualities were analyzed using paired t-tests or Wilcoxon rank sum test. Due to multiple comparisons, a Bonferroni correction was applied and alpha was set at p < 0.002. Comparison between groups for 6MWD and HRQOL were analyzed using repeated measures analysis of variance, with alpha set at 0.05. For the primary objective, we determined that a sample size of 33 participants with chronic pain would yield 80% power (α = 0.05) to detect a difference of 2 points (minimal important difference of the BPI intensity score) [Citation53, Citation54], with a standard deviation of 3.5 points [Citation13], pre and post PR. For the secondary objective, we determined that a sample size of 68 (34 per group) would yield 80% power (α = 0.05) to detect a difference of 33 m in the 6MWD [Citation34], with a standard deviation of the change in 6MWD of 43 m [Citation55] between those with and without chronic pain.
Results
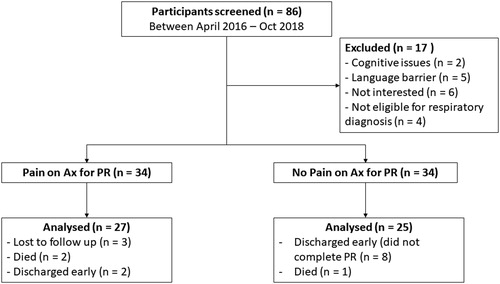
Of the 86 participants screened for this study, 17 were excluded (). The remaining 68 participants (mean age 71 ± 10 years) had moderate to severe COPD (mean FEV1 47.5 ± 19.2% predicted), with 34 participants in the chronic pain and 34 in the no pain group.
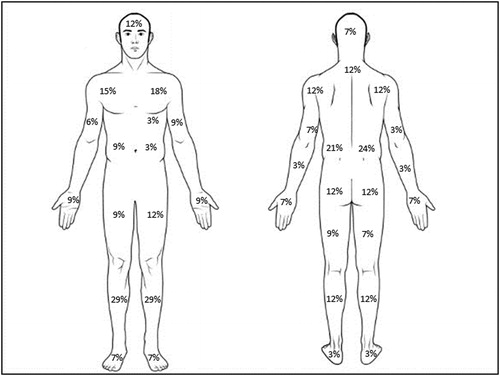
In the pain group, most participants had experienced chronic pain for 1-3 years (29%), followed by 3-5 years (27%), > 10 years (21%), 5-10 years (18%) and 3-6 months (6%). The most common pain frequency was once daily (43%), followed by constant pain (34%) or once per week (23%). Common regions of pain are outlined in , with most participants (88%) reporting two or more areas of pain. A total of 22 participants were taking medication to treat pain, including Tylenol (n = 10), Pregabalin (n = 3), Voltaren (n = 4), Naproxen (n = 1), Percocet (n = 1), Tramadol (n = 1), Advil (n = 1), Methadone (n = 1) and Cortisone (n = 1). Non-pharmacological treatments including hot packs, massage therapy and weekly physiotherapy treatment, used by three participants.
A total of 27 participants completed post-PR assessment in the pain group and 25 in the no-pain group (). Four participants (two in each group) did not complete end PR measures of HRQOL. Only one participant in the no-pain group developed pain during PR. A comparison of baseline characteristics between groups is outlined in . The no pain group had a greater proportion of males (p = 0.03) and of participants using supplemental oxygen (p = 0.04). Those with pain had a higher BMI on average and less severe lung disease compared to those without pain (all p < 0.05). Categories of comorbidities in the participants were circulatory (hypertension, coronary artery disease, atrial fibrillation, peripheral vascular disease), endocrine (Type II diabetes mellitus, dyslipidemia, obesity), digestive (gastroesophageal reflux disease), musculoskeletal and connective tissue diseases (osteoarthritis, osteoporosis, fibromyalgia), nervous system (neuropathy), mental health (anxiety, depression, schizophrenia) and malignant neoplasms. All participants had at least one comorbidity, with no significant difference in the number of comorbidities (p = 0.72) or any of the categories of comorbidities (all p > 0.05) between those with or without pain. There was no difference in exercise capacity or quality of life measures at the commencement of PR.
Table 1. Participant demographics.
Participants with chronic pain – effect of pulmonary rehabilitation
The overall PR program did not worsen pain intensity on average or at its worst and did not have an effect on pain interference with general health, mood, walking ability, sleep, normal work or enjoyment of life ().
Table 2. Effect of PR on pain intensity and interference in those with chronic pain.
At baseline, the dominant strategy for coping was cognitive (68%), followed by diverting attention (21%) and catastrophizing (12%). At the conclusion of PR, cognitive strategies were used in 63%, with 22% using diverting attention, 7% using catastrophizing and 7% using re-interpreting. Four (15%) participants changed from a positive to a negative strategy, 15% changed from a negative to a positive strategy and the remainder were unchanged, with no significant difference in proportions (p = 0.78). There was no difference in the fear avoidance behavior related to physical activity (median [IQR] 12[9–16] vs. 14[10–18] p = 0.79) or work (12[6–28] vs. 8[0–20], p = 0.21). Anxiety scores were unchanged by PR (median[IQR] 7[2–9] vs. 5[3–8], p = 0.82) as were measures of depression based on the HADS-depression subscore (4[2–8] vs. 3[1–6], p = 0.38) and the CES-D total score (12[7–20] vs. 10[5–17], p = 0.86) or any of the CES-D subscores (all p > 0.05).
Comparison between those with chronic pain and no pain
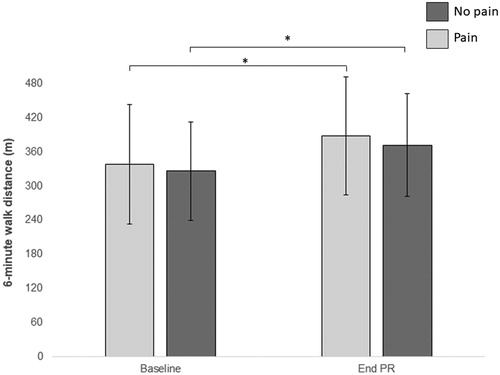
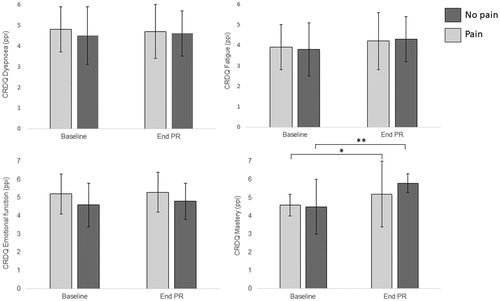
Both groups significantly improved in exercise capacity following PR (), but there was no difference in the magnitude of improvement between groups (mean difference (MD) 17 m [95% CI −39 to 72 m]). For HRQOL domains, while there was improvement in fatigue, the magnitude of change was not statistically significant within groups, and there was no difference between groups (). Both groups improved in mastery following PR, with greater change in the no pain group (MD 0.53ppi [0.12 to 0.94ppi]) ().
Discussion
To our knowledge, this is the first study to report the effects of PR on pain in people with COPD and comorbidities who have chronic pain. PR neither aggravated nor reduced pain intensity or its interference with daily activities. PR had no effect on the coping strategies for those with chronic pain and did not alter fear avoidance behavior or psychological symptoms. Those with and without chronic pain had a similar magnitude of improvement in functional exercise capacity.
It is interesting to note the greater disease severity among those without pain at the commencement of PR. In earlier studies of pain prevalence in people with stable COPD, FEV1 percentage predicted has ranged from a mean of 38% to 48% [Citation11, Citation13, Citation56, Citation57], which is within the range of the current study. While those with more severe COPD have experienced greater interference with daily activities from pain [Citation57], a paradoxical relationship between pain and lung function has been previously noted [Citation3, Citation4]. It is possible that for people with more severe COPD, other symptoms such as dyspnea are noted to be more distressing compared to pain and are afforded a greater focus [Citation3, Citation4]. In addition, reluctance to report pain, worry about being dismissed or excluded from PR and the need to maintain a stoical attitude while exercising has been previously noted in people with COPD [Citation15, Citation17, Citation58]. This hesitance in reporting may be exacerbated by a lack of enquiry regarding pain within typical clinical practice in the management of people with COPD [Citation23].
In a qualitative study, it was suggested by people with COPD that PR is a short-term aggravator but a long-term reliever of pain [Citation17]. Short-term aggravation was reflected by hinderance of full participation in PR or linked to missing sessions or program attrition, while long-term relief was reflected by health care professional observations of improvements in muscle strength aligning with a reduced impact of pain [Citation17]. However, according to the BPI, we found that PR did not aggravate pain intensity or exacerbate the extent of interference in those with chronic pain. This may be influenced by the intensity of pain and its degree of interference at the beginning of the program. The mean average pain intensity on the BPI in our study was 3/10, similar to previous studies of people with pain and stable COPD [Citation4, Citation5, Citation13, Citation17, Citation59] and interference scores ranged from 1/10 to 4/10. This pain intensity could be classed as mild in severity [Citation60] and the efficacy of exercise programs for people with mild pain in previous studies is variable, with some noting improvement and others noting minimal or no effect [Citation61]. The effect of PR in people with COPD reporting moderate or severe pain remains unclear. In addition, the short-form BPI assesses pain over 24 h only and while a valid and reliable measure of chronic pain [Citation62], temporal characteristics of pain, including daily fluctuations in pain intensity have been previously noted [Citation63] and may influence the reports of pain. In addition, when describing the frequency of chronic pain, only 34% of participants reported experiencing constant pain; this is likely to influence what information can be captured by the BPI at the time of measurement. Each of these factors, together with our smaller sample size which affected the power for this analysis due to attrition limit our interpretations of these findings. In the absence of any evaluation beyond immediate completion of PR, it is not possible to determine if PR influences the long-term experience of pain in this study.
The lack of improvement in pain intensity or interference may be linked to the structure of the PR program. While exercise programs targeting chronic pain has been associated with positive improvements [Citation61], evidence suggests that multimodal approaches, which include exercise, cognitive behavioral therapy and self-management education are the most effective in reducing pain symptoms [Citation64]. The content of the PR program in the current study followed standard recommendations, which do not incorporate specific adjustments or treatment approaches to address pain. In addition, the treatment effects of exercise-based therapy for individuals with chronic pain generally increase in proportion to the number of exercise sessions performed [Citation65, Citation66], with interventions greater than three months duration linked with positive improvements [Citation61, Citation64]. A positive impact on pain characteristics may be difficult to accomplish within the 10-week program of the current study. The negligible impact on pain observed may also be linked to the underlying causes of pain. While previous studies have described an association between comorbidities and pain in COPD [Citation5, Citation22], etiology has not been established [Citation3] and we did not identify the cause of participants’ pain. Although some comorbidities identified in the current study are characterized by pain, others are not, and this may account for the lack of difference in the number or categories of comorbidities between those with or without pain.
The lack of impact of PR on pain coping strategies, fear avoidance behavior or psychological symptoms may be attributed to several factors. Firstly, it may be related to the mild intensity and corresponding degree of interference of pain as well as the absence of high levels of fear avoidance behavior in physical activity or work. Whether a similar finding is evident in people classed as experiencing moderate or severe pain is yet to be established. Secondly, those with chronic pain and COPD did not appear to have anxiety or depression prior to commencing PR and there was minimal fear avoidance behavior related to chronic pain. Thirdly, the structure of PR with standard exercise and education applied, with no information provided on pain management may not be equipped to influence pain coping ability. For people with COPD and chronic pain who have maladaptive coping strategies, future studies could explore the possible role of adjunctive therapies on pain management within the education component of PR.
The improvement in functional exercise capacity beyond the minimal important difference (>30 m) [Citation34] regardless of the presence of pain is clinically reassuring. This may be due to the mild impact of pain on walking ability (according to the BPI), which suggests that the level of pain experienced during walking-based exercise prescribed during PR was minimal. Regular pain ratings over the course of PR ranged from 0 to 2, indicating minimal pain during exercise. It may also be due to adaptations to exercises applied by either healthcare professionals at the location of this study, in response to pain reports or by patients themselves to minimize symptoms [Citation17]. These findings are consistent with previous reports of the impact of comorbidities on PR outcomes [Citation67, Citation68]. Achieving a similar magnitude of improvement for exercise capacity, irrespective of the presence or type of comorbidity suggests that any association between those conditions and the mild pain reported in this study appears to be negligible. With low levels of pain intensity reported in this group of participants, it will be interesting to determine if similar effects are observed in those with moderate or severe pain [Citation60].The change in HRQOL domains was variable, with small or negligible changes in dyspnea and emotional function in both groups, while improvements in fatigue and mastery were equal to or greater than the minimal important difference [Citation35]. This may be influenced by attrition from both groups as well as a number of participants (four) not completing the CRDQ at the end of PR. Of those who completed the study, all 20 sessions of supervised rehabilitation were completed, so total dosage is an unlikely cause of the variable effect on HRQOL. Although the findings should be interpreted with caution, the observed greater change in mastery in those without pain may be related to reports in people with COPD and chronic pain of difficulty in concentrating during education sessions and a lack of willingness and ability to engage with other individuals enrolled in PR [Citation15, Citation17]. This may impact on the uptake of mastery elements incorporated within the education sessions within PR or achieved through exercise. While the CRDQ is a disease-specific tool, it does not incorporate a measure of pain as part of the evaluation of HRQOL. An alternative choice in the Short-form 36 which includes a measure of bodily pain may have provided further insight into the effect of PR on pain.
Clinically, our findings suggest that a PR program of this format and exercise prescription can achieve beneficial improvement in exercise capacity for people with COPD, despite the presence of mild pain and irrespective of the comorbidities identified in this study. The structure of the PR program does not influence pain coping strategies and the presence of mild pain appears to have minimal influence on selected HRQOL measures. While health care professionals have responded positively to the idea of a pain management intervention delivered within PR [Citation17], these findings suggest that the role of this strategy and the patients with COPD remains to be determined.
There are limitations to this study. The generalizability of findings is limited to individuals with moderate to severe COPD and mild pain. The collation of comorbidities was extracted from medical records, which may underestimate their presence. Although comorbidities were reported, etiology of chronic pain was not established, therefore we cannot comment on how these findings relate to specific causes of chronic pain. In addition, qualitative studies have reported variable findings in the relationship between the pathophysiological changes and symptoms of COPD and pain experiences, with some individuals directly attributing pain to their COPD, others stating no link and a proportion remain unclear [Citation15, Citation17]. Fluctuations in pain characteristics of intensity and interference and the corresponding impact on pain coping behavior have been observed in individuals with chronic pain [Citation63]. The timing of these fluctuations with respect to completion of outcome measures in this study may have influenced the findings. Measurements of pain during the course of the program were only recorded every second week rather than before and after each session of exercise. Finally, while we recruited the target number for each analysis, the attrition rate during the study influenced the power for both the primary and secondary outcomes and therefore limits the interpretations drawn from these findings. Further clarity is required using larger sample sizes in people with COPD and mild pain.
Conclusions
In conclusion, PR did not aggravate or relieve the sensation of pain or the patients’ response to pain in those with moderate to severe COPD with comorbidities whose pain was mild. Improvements in exercise capacity associated with PR were independent of the presence of pain. Further research to determine if the presence of moderate or severe pain in people with COPD has a similar effect on PR outcomes could be considered.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Acknowledgements
The authors would like to thank all the participants and clinicians within pulmonary rehabilitation who were involved in this study.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2018. Report.
- Lee AL, Harrison SL, Goldstein RS, et al. Pain and its clinical associations in COPD – a systematic review. Chest 2015;147(5):1246–1258. doi:10.1378/chest.14-2690.
- van Dam van Isselt E, Groenewegen-Sipkema K, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898–e005898. doi:10.1136/bmjopen-2014-005898.
- Bentsen SB, Miaskowski C, Cooper B, et al. Distinct pain profiles in patients with chronic obstructive pulmonary disease. COPD. 2018;13:801–811.
- Chen Y-W, Camp P, Coxson H, et al. Comorbidities that cause pain and the contributors to pain in individuals with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2017;98:35–43.
- Crisafulli E, Costi S, Luppi F, et al. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008;63(6):487–492. doi:10.1136/thx.2007.086371.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042–1048. doi:10.1183/09031936.00203809.
- Vanfleteren L, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammations in patients with chronic objective pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC.
- Franssen RME, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev. 2014;23(131):131–141. doi:10.1183/09059180.00007613.
- Borge CR, Wahl AK, Moum T. Pain and quality of life with chronic obstructive pulmonary disease. Heart Lung 2011;40:90–101.
- HajGhanbari B, Holsti L, Road J, et al. Pain in people with chronic obstructive pulmonary disease (COPD). Respir Med. 2012;106(7):998–1005. doi:10.1016/j.rmed.2012.03.004.
- HajGhanbari B, Garland SJ, Road JD, et al. Pain and physical performance in people with COPD. Respir Med. 2013;107(11):1692–1699. doi:10.1016/j.rmed.2013.06.010.
- Lee AL, Goldstein RS, Brooks D. Chronic pain in chronic obstructive pulmonary disease: prevalence, clinical and psychological implications. J COPD F. 2017;4:194–203. doi:10.15326/jcopdf.4.3.2016.0172.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2:CD003793.
- Lee AL, Harrison SL, Goldstein RS, et al. An exploration of pain experiences and their meaning in people with chronic obstructive pulmonary disease. Physiother Theory Pract. 2018;34(10):765–772.
- Keating A, Lee AL, Holland AE. Lack of perceived benefit and inadequate transport influence uptake and completion of pulmonary rehabilitation in people with chronic obstructive pulmonary disease: A qualitative study. J Physiother. 2011;57(3):183–190. doi:10.1016/S1836-9553(11)70040-6.
- Harrison S, Lee A, Button H, et al. The role of pain in pulmonary rehabilitation: a qualitative study. COPD. 2017;12:3289–3299.
- Pedersen B, Saltin B. Exercise as medicine: evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1–72. doi:10.1111/sms.12581.
- Juhl C, Christensen B, Roos E, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis. Rheumatol. 2014;66(3):622–636. doi:10.1002/art.38290.
- Garber C, Blissmer B, Deschenes M, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal and neuromotor fitness in apparently healthy adults. Med Sci Sports Exer. 2011;43(7):1334–1359.
- American Geriatrics Society Panel on Exercise and Osteoarthritis. Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations. A supplement to the AGS clinical practice guidelines on the management of chronic pain in older adults. J Am Geriatr Soc 2001;49:808–823.
- HajGhanbari B, Yamabayashi C, Garland S, et al. The relationship between pain and comorbid health conditions in people with chronic obstructive pulmonary disease. Cardiopulm Phys Therapy J. 2014;25(1):29–35. doi:10.1097/01823246-201403000-00007.
- Lewthwaite H, Williams G, Baldock K, et al. Systematic review of pain in clinical practice guidelines for management of COPD: A case for including chronic pain?. Healthcare (Basel). 2019;7(1):15. doi:10.3390/healthcare7010015.
- Vlaeyen J, Kole-Snijders A, Boeren R, et al. Fear of movement/(re) injury in chronic low back pain and its relation to behavioural performance. Pain 1995;62(3):363–372. doi:10.1016/0304-3959(94)00279-N.
- Linton S, Shaw W. Impact of psychological factors in the experience of pain. Phys Ther. 2011;91(5):700–711. doi:10.2522/ptj.20100330.
- Gatchel RJ, Neblett R, Kishino N, et al. Fear-avoidance beliefs and chronic pain. J Orthop Sports Phys Ther. 2016;46(2):38–43. doi:10.2519/jospt.2016.0601.
- Holmes A, Christelis N, Arnold C. Depression and chronic pain. Med J Aust. 2012;1(4):17–20. doi:10.5694/mjao12.10589.
- Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert. Rev. Neurother. 2009;9(5):745–758. doi:10.1586/ern.09.34.
- Nici N, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390–1413. doi:10.1164/rccm.200508-1211ST.
- Lung Foundation Australia, Pulmonary rehabilitation toolkit, 2006.
- Hill K, Jenkins S, Cecins N, et al. Estimating maximum work rate during incremental cycle ergometry testing from six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2008;89(9):1782–1787. doi:10.1016/j.apmr.2008.01.020.
- Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;4:377–381.
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64.
- Holland AE, Spruit MA, Troosters T, et al. An Official European Respiratory Society/American Thoracic Society Technical Standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi:10.1183/09031936.00150314.
- Williams J, Singh SJ, Sewell L, et al. Development of a self-reported chronic respiratory questionnaire (CRQ-SR). Thorax. 2001;56(12):954–959. doi:10.1136/thorax.56.12.954.
- World Health Organisation. International stastical classification of disesaes and related health problems 10th revision (ICD-10). https://icd.who.int/browse10/2016/en. 2016.
- Keller S, Bann C, Dodd S, et al. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi:10.1097/00002508-200409000-00005.
- Chen Y, HajGhanbari B, Road JD, et al. Reliability and validity of the Brief Pain Inventory in individuals with chronic obstructive pulmonary disease. Eur J Pain. 2018;22(10):1718–1726. doi:10.1002/ejp.1258.
- Margolis R, Tait R, Krause S. A rating system for use with patient pain drawings. Pain 1986;24(1):57–65. doi:10.1016/0304-3959(86)90026-6.
- Harland N, Georgieff K. Development of the coping strategies questionnaire 24, a clinically utilitarian version of the coping strategies questionnaire. Rehabil Psych. 2003;48(4):296–300. doi:10.1037/0090-5550.48.4.296.
- Harland N, Martin D. Exploring the longitudinal stability of the CSQ24 in a back pain population. Rehabil Psych. 2014;59(1):79–84. doi:10.1037/a0034337.
- Rosenstiel A, Keefe F. Pain coping strategy. Pain 1983;17(1):33–44. doi:10.1016/0304-3959(83)90125-2.
- Swartzman L, Gwadry F, Shapiro A, et al. The factor structure of the coping strategies questionnaire. Pain 1994;57(3):311–316. doi:10.1016/0304-3959(94)90006-X.
- Harland N, Ryan C. The value of pain coping constructs in subcategorising back pain patients according to risk of poor outcome. BioMed Res Int. 2013;2013:1–7.
- Waddell G, Newton M, Henderson I, et al. Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993;52(2):157–168. doi:10.1016/0304-3959(93)90127-B.
- George S, Fritz J, McNeil D. Fear-avoidance beliefs as measured by the fear-avoidance beliefs questionnaire: change in fear-avoidance beliefs questionnaire is predictive of change in self-report of disability and pain intensity for patients with acute low back pain. Clin. J. Pain. 2006;22(2):197–203. doi:10.1097/01.ajp.0000148627.92498.54.
- Mintken P, Cleland J, Whitman J, et al. Psychometric properties of the Fear-Avoidance Beliefs Questionnaire and Tampa Scale of Kinesiophobia in patients with shoulder pain. Arch Phys Med Rehabil. 2010;91(7):1128–1136. doi:10.1016/j.apmr.2010.04.009.
- Zigmond A, Snaith R. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x.
- Snaith R. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1:29doi:10.1186/1477-7525-1-29.
- Nowak C, Sievi N, Clarenbach C, et al. Accuracy of the Hospital anxiety and depression scale for identifying depression in chronic obstructive pulmonary disease patients. Pulmon. Med. 2014;2014:973858. doi:10.1155/2014/973858.
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. App Psych Meas. 1977;1(3):385–401. doi:10.1177/014662167700100306.
- Hanania N, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604–611., Müllerova H, Locantore N,. doi:10.1164/rccm.201003-0472OC.
- Mathias SD, Crosby RD, Qian Y, et al. Estimating minimally important differences for the worst pain rating of the brief pain inventory – short form. J. Support. Oncol. 2011;9(2):72–78.
- Mease P, Spaeth M, Clauw D, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res (Hoboken). 2011;63(6):821–826. doi:10.1002/acr.20449.
- Beauchamp MK, O'Hoski S, Goldstein RS, et al. Effect of pulmonary rehabilitation on balance in persons with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(9):1460–1465. doi:10.1016/j.apmr.2010.06.021.
- Bentsen SB, Rustoen T, Miaskowski C. Prevalence and characteristics of pain in patients with chronic obstructive pulmonary disease compared to the Norwegian general population. J. Pain. 2011;12(5):539–545. doi:10.1016/j.jpain.2010.10.014.
- Christensen V, Holm A, Kongerud J, et al. Occurrrence, characteristics, and predictors of pain in patients with chronic obstructive pulmonary disease. Pain. Manag. Nurs. 2016;17(2):107–118. doi:10.1016/j.pmn.2016.01.002.
- Lohne V, Heer HCD, Andersen M, et al. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart. Lung. 2010;39(3):226–234. doi:10.1016/j.hrtlng.2009.08.002.
- Borge CR, Wahl AK, Moum T. Association of breathlessness with multiple symptoms in chronic obstructive pulmonary disease. J Adv Nurs. 2010;66(12):2688–2700. doi:10.1111/j.1365-2648.2010.05447.x.
- Boonstra A, Stewart R, Koke A, et al. Cut-off points for mild, moderate and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol. 2016;7:1466. doi:10.3389/fpsyg.2016.01466.
- Geneen L, Moore R, Clarke C, et al. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279.
- Tan G, Jensen M, Thornby J, et al. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005.
- Fillingim R, Loeser J, Baron R, et al. Assessment of chronic pain: domains, methods and mechanisms. J Pain. 2016;17(9):T10–20. doi:10.1016/j.jpain.2015.08.010.
- Ambrose K, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract Res Clin Rheumatol. 2015;29(1):120–130. doi:10.1016/j.berh.2015.04.022.
- Hagen K, Dagfinrud H, Moe R, et al. Exercise therapy for bone and muscle health: an overview of systematic reviews. BMC Med. 2012;10:167doi:10.1186/1741-7015-10-167.
- Kaleth A, Saha C, Jensen M, et al. Effect of moderate to vigorous physical activity on long-term clinical outcomes and pain severity in fibromyalgia. Arthritis Care Res. 2013;65(8):1211–1218. doi:10.1002/acr.21980.
- Mesquita R, Vanfleteren L, Franssen FME, et al. Objectively identified comorbidities in COPD: impact on pulmonary rehabilitation outcomes. Eur Respir J. 2015;46(2):545–548. doi:10.1183/09031936.00026215.
- Butler SJ, Li LSK, Ellerton L, et al. Prevalence of comorbidities and impact on pulmonary rehabilitation outcomes. Eur Respir J Open Res. 2019;5:00264–02009.