Abstract
Few studies have used 24-hour accelerometery to characterise posture and movement patterns in people with chronic obstructive pulmonary disease (COPD). This study aimed to quantify sedentary behaviour (SB), patterns of SB accumulation and physical activity (PA) in people with COPD, and to examine physiological and functional capacity correlates of total SB and patterns of SB accumulation. SB and PA were assessed continuously over seven days using thigh-worn accelerometery in people with COPD. Participants were regarded as “sedentary” if combined sitting/reclining time accounted for ≥70% of waking wear time. Differences in patterns of SB accumulation and PA were compared between “sedentary” and “non-sedentary” participants. Physiological and functional capacity correlates of SB were explored using univariate analysis. Sixty-nine people with COPD (mean (SD) age 74 (9) years, FEV1 55% (19) predicted) had sufficient wear data for analysis. Mean sedentary time was 643 (105) minutes/day (71% (11) of waking wear time), of which 374 (142) minutes/day were accumulated in prolonged bouts of ≥30 min. “Sedentary” participants had a more unfavourable pattern of SB accumulation and spent less time in PA of any intensity. Sedentary time, expressed as a proportion of waking wear time, was inversely correlated with light (r = −0.97, p < .01) and moderate-to-vigorous intensity PA (r = −0.55, p < .01) and exercise capacity (r = −0.33, p < .01), but not with age, body mass index or lung function. People with COPD had high total SB and accumulated the majority of SB in prolonged bouts. High total SB was correlated with low physical activity and exercise tolerance.
Introduction
There is growing recognition of the adverse health effects associated with sedentary behaviour (SB). SB is defined as any waking behaviour undertaken in a sitting or reclining posture and characterised by an energy expenditure of ≤1.5 metabolic equivalents (METs) [Citation1]. Common SBs include watching television, reading and computer use in a sitting/reclining posture, and driving a motor vehicle. Population-based longitudinal studies have demonstrated direct associations between SB and mortality risk [Citation2–4], cardiometabolic disease and cancer [Citation5,Citation6]. Small cohort studies in chronic obstructive pulmonary disease (COPD) have also reported associations of SB with mortality [Citation7,Citation8] and cardiometabolic risk biomarkers such as waist circumference and plasma glucose levels [Citation9,Citation10].
Although SB has previously been characterised in people with COPD using waist- and arm-worn accelerometery, these studies have several limitations. Waist- and arm-worn accelerometers are unable to distinguish between sitting/reclining and upright positions, and subsequently cannot provide robust estimations of posture [Citation11–13]. Most studies have measured SB using 12-hour device wear protocols, in which the monitoring period is initiated on waking in the morning and any activity after the 12 hours is excluded from the analysis [Citation14–16]. Twelve-hour device wear protocols are likely to underestimate total SB, as older adults tend to accumulate most of their SB in the evenings [Citation17] and estimates of SB are more affected by the premature removal of activity monitors compared to physical activity [Citation18–20]. Finally, studies have typically reported time spent in SB in absolute minutes without consideration of waking wear time [Citation12,Citation21]. Variations in waking wear time may confound comparisons of total SB within and between individuals with COPD. To our knowledge, SB has not yet been assessed in people with COPD using thigh-worn inclinometers, which can directly quantify posture and are the recommended measurement tool for SB [Citation22].
The manner in which SB is accumulated also appears to influence health outcomes. Preliminary evidence suggests that accumulating SB in relatively prolonged bouts has a deleterious effect on cardiometabolic risk factors compared to more frequently interrupted bouts of SB [Citation23]. Usual bout duration, frequency of postural transitions and the longest sedentary bout duration are commonly used indicators of SB accumulation [Citation22], but have not yet been described in people with COPD. Usual bout duration is considered to be a particularly useful measure of SB accumulation because it captures the distribution of sedentary bout frequency and bout duration in a single outcome [Citation24], and has demonstrated sensitivity to change following interventions in office workers and older adults [Citation25]. Quantifying patterns of SB accumulation in COPD may help clinicians to determine who could potentially benefit from interventions to reduce SB and/or increase physical activity levels, and improve the effectiveness of such interventions.
Identifying clinical characteristics linked to high total SB and detrimental patterns of SB accumulation may have implications for treatment selection and prognosis of the disease. People with COPD with the highest total SB have previously been characterised by more frequent exacerbations, lower exercise capacity, long-term oxygen use and the presence of physical co-morbidities [Citation11,Citation26]. When taken in consideration with physical activity levels and body composition, high total SB differentiated those with more severe dyspnoea and airway obstruction [Citation27]. However, no correlations were observed between self-reported total SB and exercise capacity measured by six-minute walk distance (6WMD), four-metre gait speed and time taken to perform five sit-to-stands in an Australian sample of COPD [Citation28]. Further exploration of the relationships between SB and clinically relevant outcomes in COPD may help to guide treatment choice for those with poorer clinical profiles.
The primary aim of this study was to quantify total SB, patterns of SB accumulation and physical activity in people with COPD over a 24-hour monitoring period using thigh-worn accelerometery. Secondary aims of this study were as follows:
To examine variations in total SB and patterns of SB accumulation by physical activity levels, exercise-induced oxygen desaturation (EID) and season of the year;
To examine physiological (age, body mass index, lung function) and functional capacity (6MWD, physical activity) correlates of total SB and patterns of SB accumulation.
Methods
Study design and participants
This study was a cross-sectional analysis of baseline assessment data from a randomised controlled trial investigating the effects of a six-week behaviour change intervention on sedentary time in people with COPD (Australian New Zealand Clinical Trials registration number ACTRN12616001534471) [Citation29]. Data collection occurred from December 2016 to April 2019 and comprised a convenience sample of people with COPD on the waiting lists of five pulmonary rehabilitation programmes in metropolitan Sydney, Australia. Participants were included in the study if they (1) had a clinical diagnosis of COPD confirmed by spirometry (post-bronchodilator fixed ratio of forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) < 0.7); (2) were clinically stable, defined as no change in medication in the four weeks preceding recruitment; (3) were able to mobilise independently with or without a walking aid and (4) were expected to wait eight weeks or more on the waiting list for entry to a pulmonary rehabilitation programme. Exclusion criteria were participation in supervised exercise training within the last six months, and conditions likely to impair completion of the assessment procedures (e.g. cognitive impairment, limited spoken and/or written English). Written informed consent was obtained from all participants. This study was approved by the Ethics Review Committee (RPAH Zone) of the Sydney Local Health District (reference number HREC/16/RPAH/400) and was also given institutional approval by the South Eastern Sydney Local Health District and Northern Sydney Local Health District Research Governance Offices.
Setting
Participants lived in metropolitan Sydney, which has a humid subtropical climate with warm summers and mild winters. The temperature usually varies from 8 to 27 °C and is rarely below 5 °C or above 32 °C. The warm season lasts from the end of November to the end of March, with an average temperature above 24 °C. The cool season lasts from the end of May to the end of August, with an average temperature below 18 °C [Citation30].
Assessments
Baseline characteristics included age, sex, height, weight and lung function. Lung function was assessed using a spirometer (EasyOne. Niche Medical, Sydney, Australia) according to standard procedures [31] and compared to predicted normal values [Citation32].
Sedentary behaviour and physical activity
Total SB, patterns of SB accumulation and physical activity were assessed using the activPAL3 activity monitor (PAL Technologies Ltd., Glasgow, UK). The activPAL has demonstrated excellent agreement with direct observation for time spent in SB, standing and stepping and for sit-stand transitions [Citation33,Citation34], as well as sensitivity to small changes in walking speed in people with COPD [Citation35]. The monitor was initialised, waterproofed and secured onto the anterior thigh with Tegaderm adhesive dressing (3 M, Sydney, Australia). Participants were asked to wear the monitor continuously for seven days and to record sleep and periods of non-wear (e.g. activities involving submersion underwater) in a diary. Sleep, non-wear and invalid days (i.e. days with <10-hour waking wear time) were removed from the analysis based on the diary and monitor data. Data were downloaded using activPAL software version 7.2.38 and processed in SAS (version 9.4, SAS Institute, Cary, NC, USA). SB and physical activity data were totalled for each day and then averaged across valid days. Further details of activPAL data collection and processing are described in the online supplement (supplementary Table S1).
Four measures of total SB, seven of SB accumulation and seven of physical activity were assessed (supplementary Table S2). Participants were classified as “sedentary” or “non-sedentary” according to a sedentary time cut-off of ≥70% waking wear time. Such a cut-off has been used to indicate high SB in a Brazilian sample of COPD and was associated with a higher mortality risk even after adjustment for moderate-to-vigorous intensity physical activity (MVPA) [Citation7]. Participants were regarded as physically active if they engaged in ≥30 minute/day of MVPA, a threshold which has been used to guide physical activity prescription in “healthy” adults [Citation36].
Functional exercise capacity
Functional exercise capacity was assessed using the six-minute walk test (6MWT). All participants performed two 6MWTs according to standard procedures [37]. Pulse rate and oxygen saturation were measured using a pulse oximeter (Masimo, Irvine, CA, USA) before, every minute during and for two minutes after the test. The best 6MWD of the two tests was used in the analysis and compared to predicted normal values [Citation38]. Participants were classified as having a low exercise tolerance if their 6MWD was <80% predicted, a cut-off which has been used to indicate poor functionality in the Brazilian COPD population [Citation39,Citation40].
Statistical analysis
Descriptive data were reported as mean and standard deviation for continuous variables, and as counts and percentages for categorical variables. Differences in baseline characteristics between “sedentary” and “non-sedentary” participants were compared using analysis of variance for continuous variables and chi-square tests for categorical variables. Assumptions of homogeneity of variance were tested using Levene’s test for equality of variances. Independent t-tests were performed to explore any differences between “sedentary” and “non-sedentary” participants for total SB, patterns of SB accumulation and physical activity. Univariate analysis was performed with five SB measures as independent variables (sedentary time, sedentary time as a proportion of waking wear time, prolonged sedentary time as a proportion of waking wear time, usual bout duration and frequency of sedentary breaks) and the following dependent variables: age; body mass index (BMI); FEV1, FVC, and FEV1% predicted; absolute 6MWD and 6MWD % predicted; time spent standing, stepping, in purposeful walking, in light activities including and excluding standing, in MVPA, and step count. Correlations between these variables in the linear regression model were identified by analysing and describing r values in terms of strength [Citation41]. No relationship between the variables was indicated by r values <0.3; a weak relationship by r values of 0.3 to <0.5; a moderate relationship by r values of 0.5 to <0.7 and a strong relationship by r values >0.7.
A posteriori, subgroup analyses were performed to examine variations in total SB and patterns of SB accumulation by physical activity levels, EID and season of the year. Participants were classified as “sedentary” or “non-sedentary” using a sedentary time cut-off of 70% waking wear time, and as “inactive” or “active” using a MVPA threshold of 30 min/day, to derive four activity phenotypes. These phenotypes were “couch potatoes” (“sedentary” and “inactive”), “light movers” (“non-sedentary” and “inactive”), “sedentary exercisers” (“sedentary” and “active”) and “busy bees” (“non-sedentary” and “active”). A similar classification of participants into four activity phenotypes has been used in COPD [Citation9,Citation16] and in the general population [Citation42]. Participants were classified as EID or non-EID based on oxygen desaturation to <90% during a 6MWT. The four seasons of the year were defined according to the meteorological seasons in the Southern Hemisphere: winter from June 1 to August 31, spring from September 1 to November 30, summer from December 1 to February 28 and autumn from March 1 to May 31. All statistical analyses were performed using SPSS version 25.0 (IBM). A p value of <.05 was considered significant.
Results
Participant characteristics
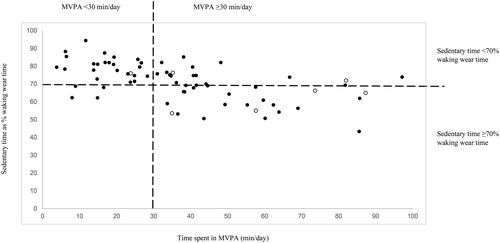
Sixty-nine of the 70 participants had at least four days of valid wear data, and 67 participants did not receive long-term oxygen therapy and were included in the subgroup analysis of EID. In general, the sample consisted of older retired adults with moderate COPD and a high prevalence of cardiometabolic and physical co-morbidities. Baseline characteristics, grouped according to whether or not participants were “sedentary” or “non-sedentary”, are shown in . Of the 69 participants, 57% were classified as “sedentary” and had a significantly lower exercise tolerance compared to “non-sedentary” participants. No other differences were observed between the groups.
Table 1. Baseline characteristics (n = 69).
Distribution of the four activity phenotypes is shown in . Of the 69 participants, 24 (35%) were “couch potatoes”, four (6%) were “light movers”, 15 (22%) were “sedentary exercisers” and 26 (37%) were “busy bees”. Due to violation of the assumption of homogeneity of variance and unequal distribution of participants across the four groups, variations in patterns of SB accumulation and physical activity could not be examined by activity phenotypes. Baseline characteristics and mean SB and physical activity data for the activity phenotypes are shown in supplementary Tables S3 and S4. Participants with EID were older and had more severe airway obstruction, lower exercise capacity and more exposure to pulmonary rehabilitation in the past compared to non-EID participants (supplementary Table S5). No significant differences in baseline characteristics were observed between the seasons, although a higher proportion of participants recruited in spring mobilised with a walking aid (supplementary Table S7).
Figure 1. Scatterplot of sedentary time against moderate-vigorous intensity physical activity (MVPA), stratified by sedentariness (sedentary time ≥70% of waking wear time) and physical activity levels (MVPA ≥30 min/day). Of the 69 participants, 57% were classified as “sedentary” and 43% as “non-sedentary”; 41% were classified as “inactive” and 59% as “active”. Solid circles: 6MWD <80% predicted; blank circles: 6MWD ≥80% predicted.
Patterns of sedentary behaviour accumulation and physical activity
Total SB, patterns of SB accumulation and physical activity are shown in . Overall, participants spent most of their time in SB (71% of waking wear time) and had a low step count and relatively low levels of MVPA. Standing, rather than stepping, comprised the majority of non-sedentary time (76%). The proportions of waking wear time spent in SB, standing, light activities excluding standing and MVPA are illustrated in . Compared to “non-sedentary” participants, those classified as “sedentary” spent more time in prolonged SB and accumulated their SB in relatively fewer, longer bouts, as indicated by a higher usual bout duration. “Sedentary” participants also had a lower step count and spent less time standing and in physical activity of any intensity compared to “non-sedentary” participants. Compared to non-EID participants, those with EID spent 5% (95% confidence interval −0.3 to 10) more of their waking wear time in SB, took fewer steps per day and spent less time in MVPA (supplementary Table S6). No significant variations in SB or physical activity were observed by season of the year (supplementary Table S8).
Figure 2. Proportions of waking wear time spent in sedentary behaviour, standing and physical activity.
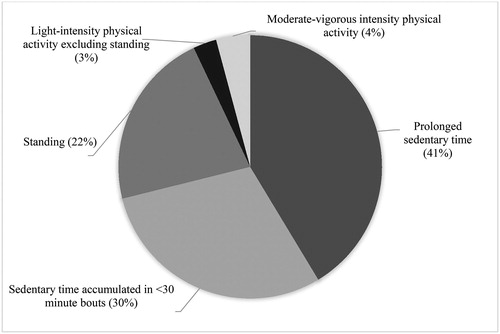
Table 2. Patterns of sedentary behaviour accumulation and physical activity (n = 69).
Physiological and functional capacity correlates of sedentary behaviour
SB was inversely correlated with physical activity, but no consistent correlations were observed for SB with age, BMI, lung function or exercise capacity (). Overall, inverse correlations with physical activity were stronger for total SB and prolonged SB expressed as proportions of waking wear time than for patterns of SB accumulation or when expressed in absolute minutes. Sedentary time as a proportion of waking wear time was strongly correlated with standing time () and time spent in light activities (), moderately correlated with stepping time () and time spent in MVPA (), and weakly correlated with exercise capacity.
Figure 3. Correlation of sedentary time expressed as a proportion of waking wear time with time spent in light activities. Solid circles: 6MWD <80% predicted; blank circles: 6MWD ≥80% predicted.
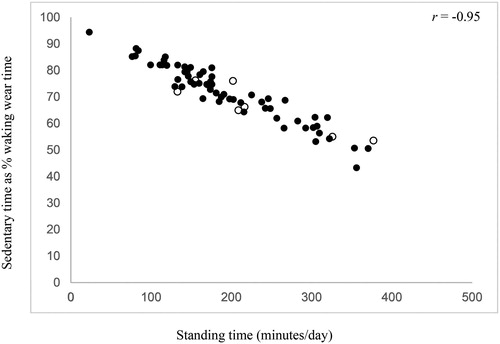
Table 3. Physiological and functional capacity correlates of total sedentary behaviour and patterns of sedentary behaviour accumulation.
Discussion
This study of 69 people with COPD provides a novel and detailed analysis of patterns of SB accumulation, assessed continuously over a minimum of four days using thigh-worn accelerometery. People with COPD in this sample spent a large proportion of the day in SB, but also accumulated the majority of their SB in prolonged bouts of ≥30 min. Values of usual bout duration, alpha and fragmentation index also indicated that SB was accumulated in relatively more prolonged bouts and relatively fewer shorter bouts. Of the 69 participants, 57% were classified as “sedentary” and had a significantly lower exercise tolerance and lower levels of physical activity compared to “non-sedentary” participants. Finally, SB was inversely correlated with physical activity; notably, an almost perfect inverse relationship was observed between sedentary time expressed as a proportion of waking wear time and time spent in light activities (r = −0.97, p < .01).
This is the first study to demonstrate that people with COPD with high total SB (701 min/day) also spent more time in prolonged SB and accumulated their SB in relatively fewer, longer bouts. Other than for frequency of sedentary bouts, significant differences between “sedentary” and “non-sedentary” participants were observed for all indicators of SB accumulation. “Sedentary” participants were also physically inactive with respect to both MVPA (<30 minutes/day of MVPA) and step count (<5000 steps/day). In the general population, estimations of total SB and MVPA using thigh-worn accelerometery were 528 minutes/day (56% of waking wear time) and 72 minutes/day in an Australian cohort [Citation43], and 58% of waking wear time and 27 minutes/day (in those with normal glucose metabolism) in The Maastricht Study [Citation44]. A sedentary time cut-off of ≥70% waking wear time may therefore be useful in guiding treatment selection in COPD. People with COPD identified as “sedentary” using this cut-off should be encouraged to minimise the amount of time spent in SB and to break up SB more often [Citation45], and may benefit from pulmonary rehabilitation and additional interventions, such as physical activity counselling or behaviour change interventions, to reduce SB and increase physical activity levels. Although our study did not show any differences in the prevalence of EID or co-morbidities, BMI or self-reported COPD hospital admissions between “sedentary” and “non-sedentary” participants, people with COPD who are both sedentary and inactive are known to have worse clinical profiles in terms of dyspnoea [Citation16,Citation27], health-related quality of life, body composition and COPD clinical control [Citation27].
Quantification of usual bout duration and alpha is novel in people with COPD. Both measures take into account the concurrent effects of bout frequency and bout duration on total SB; that is, a reduction in sedentary bout frequency does not necessarily lead to a reduction in total SB; it depends on the change in bout duration. For example, it is possible to improve in one dimension (fewer sedentary bouts) but worsen in another (longer sedentary bouts), and depending on which had the greater impact on total SB, an overall net change in sedentary time may not be observed. This illustrates the possible caveats of evaluating change in SB within or between individuals based on the frequency or duration of sedentary bouts, and the advantages of using measures such as usual bout duration and alpha, which are unaffected by this issue. In this sample, participants classified as “sedentary” had a significantly higher usual bout duration and significantly lower alpha values compared to “non-sedentary” participants, but there was no difference in the frequency of sedentary bouts between groups.
Given the sensitivity of usual bout duration to change [Citation25] and its inverse correlations with various physical activity measures, as shown in this study, usual bout duration may be a promising measure of SB in future COPD studies. However, there is limited normative data for usual bout duration at present. Compared to a usual bout duration of 12 minutes in young adults (mean age 22) [Citation46], older adults from the Australian Diabetes, Obesity and Lifestyle Study (mean age 58) [Citation23] and the Objective Physical Activity and Cardiovascular Health Study (mean age 79) [Citation47] had mean usual bout durations of 26 and 18 minutes, respectively. These estimates of usual bout duration are almost half of that observed in our sample of COPD. Examining differences in usual bout duration between COPD and healthy age-matched subjects would help to directly establish whether people with COPD have a more unfavourable pattern of SB accumulation compared to their healthy counterparts.
This study highlights the strong inverse relationship between sedentary time (as a proportion of waking wear time) and time spent in light activities, which suggests that in people with COPD, any increase in light-intensity physical activity is likely to reduce SB. Although an inverse relationship was also observed between SB and time spent in MVPA, the lack of difference in time spent in light activities between EID and non-EID participants suggests that replacing SB with light-intensity physical activity is more physiologically realistic than targeting increases in MVPA alone. The cross-sectional nature of these findings, however, limits their ability to inform future physical activity interventions. A pilot randomised controlled trial found that a behaviour change intervention to replace SB with light activities and break up prolonged SB in people with COPD was feasible and acceptable to most patients immediately following discharge from hospital after an acute exacerbation [Citation48]. Larger trials are needed to determine whether interventions focussed on replacing SB with light-intensity physical activity can act as an interim step to more intense physical activity and increase uptake of pulmonary rehabilitation, particularly in people with COPD and EID. In this sample, only a small proportion of non-sedentary time was spent in ambulatory movement compared to standing. This result highlights the potential for change in people with COPD to increase time spent in stepping rather than standing behaviours and aligns with current public health messages about encouraging movement of any intensity [Citation49–51].
Unlike physical inactivity, which has been linked to more severe airflow obstruction, poor body composition and lower exercise tolerance in people with COPD [Citation52–54], this study observed no correlations for SB with age, BMI or lung function, and inconsistent correlations for SB with exercise capacity. It is possible that these physiological variables, which reduce the capacity to engage in physical activity, are not also determinants of SB in people with COPD. Qualitative studies suggest that sedentary activities are associated with health and mental health benefits in older adults, including companionship, enhanced cognitive functioning, and the provision of meaning and structure in daily life [Citation55]. Sedentary activities are also perceived as meaningful and enjoyable by people with COPD, given the context of a progressive chronic disease that makes it increasingly difficult to enjoy any activity [Citation56]. Other perceived barriers to reducing SB include the weather, low levels of intrinsic motivation and lack of resources to access community-based activities, particularly for people from culturally and linguistically diverse backgrounds [Citation55]. Qualitative and participatory action research methods may provide further valuable insights into the psychological, sociocultural and environmental factors associated with SB, which are difficult to assess in cross-sectional study designs.
Interestingly, seasonal variations did not appear to be associated with total SB, patterns of SB accumulation or physical activity levels in this study. These results are consistent with a study of people with COPD living in Melbourne, Australia, which found no significant variations in SB or physical activity by season of the year [Citation57]. Although studies conducted in Brazil and Belgium have shown that people with COPD are less active in winter compared to summer [Citation58], winters in Australia are relatively mild and there is little variation in temperature over the year. Environmental conditions associated with different seasons, such as precipitation, air quality, daylight time and humidity [Citation59], were not accounted for in the subgroup analysis.
Limitations
The cross-sectional study design does not allow inference of causality between SB and physiological or functional capacity outcomes. In addition, only a small number of participants were classified as “light movers” (6%), which limited the ability to examine variations in SB by activity phenotypes. Since this sample of people with stable COPD had moderate airflow obstruction and a relatively large representation of “busy bees”, the study findings may not be generalisable to people with COPD of greater disease severity, where physiological limitations have a greater influence on activity levels. Other clinical characteristics that may influence SB, such as symptom severity, motivation for exercise and sleep quality (i.e. non-fragmented sleep) [Citation60], were not assessed in this study.
Conclusions
This study found that people with COPD spent the majority of their daily lives in SB, but also accumulated most of their SB in prolonged bouts. Individuals classified as “sedentary” using a cut-off of ≥70% waking wear time had a lower exercise tolerance, lower levels of physical activity and a more detrimental pattern of SB accumulation compared to those who were “non-sedentary”. Identifying “sedentary” people with COPD using this cut-off may help to guide the choice of strategies to improve levels of SB and physical activity in this population. SB and prolonged SB expressed as proportions of waking wear time, as well as usual bout duration, were inversely correlated with physical activity and may be useful measures to evaluate change in SB in people with COPD following interventions. Future studies in COPD should evaluate the feasibility and effectiveness of interventions to replace SB with light-intensity physical activity, and explore the determinants and outcomes of SB using longitudinal study designs.
Declaration of interest
The authors report no conflict of interest.
Supplemental Material
Download PDF (444.2 KB)Acknowledgments
We would like to thank the study participants for their time and the following physiotherapists who assisted in data collection: Renae McNamara, Ling Ling Tsai, Lissa Spencer, Amanda Piggott, Elizabeth Mair, Alexander Alam and Sally Wootton.
Additional information
Funding
References
- Sedentary Behaviour Research Network. Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metabol. 2012;37:540–542. doi:10.1139/h2012-024.
- Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–1310. doi:10.1016/S0140-6736(16)30370-1.
- Stamatakis E, Gale J, Bauman A, et al. Sitting time, physical activity, and risk of mortality in adults. J Am Coll Cardiol. 2019;73(16):2062–2072. doi:10.1016/j.jacc.2019.02.031.
- Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–132. doi:10.7326/M14-1651.
- Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33(9):811–829. doi:10.1007/s10654-018-0380-1.
- Ekelund U, Brown WJ, Steene-Johannessen J, et al. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br J Sports Med. 2019;53(14):886–894. doi:10.1136/bjsports-2017-098963.
- Furlanetto KC, Donária L, Schneider LP, et al. Sedentary behavior is an independent predictor of mortality in with COPD. Respir Care. 2017;62(5):579–587. doi:10.4187/respcare.05306.
- Ukawa S, Tamakoshi A, Yatsuya H, et al. Association between average daily television viewing time and chronic obstructive pulmonary disease-related mortality: findings from the Japan collaborative cohort study. J Epidemiol. 2015;25(6):431–436. doi:10.2188/jea.JE20140185.
- McKeough Z, Cheng S, Alison J, et al. Low leisure-based sitting time and being physically active were associated with reduced odds of death and diabetes in people with chronic obstructive pulmonary disease: a cohort study. J Physiother. 2018;64(2):114–120. doi:10.1016/j.jphys.2018.02.007.
- Park SK, Larson JL. The relationship between physical activity and metabolic syndrome in people with chronic obstructive pulmonary disease. J Cardiovasc Nurs. 2014;29(6):499–507. doi:10.1097/JCN.0000000000000096.
- McNamara RJ, McKeough ZJ, McKenzie DK, et al. Physical comorbidities affect physical activity in chronic obstructive pulmonary disease: a prospective cohort study. Respirology. 2014;19(6):866–872. doi:10.1111/resp.12325.
- Orme MW, Steiner MC, Morgan MD, et al. 24-hour accelerometry in COPD: exploring physical activity, sedentary behavior, sleep and clinical characteristics. COPD. 2019;14:419–430. doi:10.2147/COPD.S183029.
- Wootton SL, Hill K, Alison JA, et al. Effects of ground-based walking training on daily physical activity in people with COPD: A randomised controlled trial. Respir Med. 2017;132:139–145. doi:10.1016/j.rmed.2017.10.008.
- Morita AA, Silva LKO, Bisca GW, et al. Heart rate recovery, physical activity level, and functional status in subjects with COPD. Respir Care. 2018;63(8):1002–1008. doi:10.4187/respcare.05918.
- Munari AB, Gulart AA, Dos Santos K, et al. Modified Medical Research Council Dyspnea Scale in GOLD classification better reflects physical activities of daily living. Respir Care. 2018;63(1):77–85. doi:10.4187/respcare.05636.
- Schneider LP, Furlanetto KC, Rodrigues A, et al. Sedentary behaviour and physical inactivity in patients with chronic obstructive pulmonary disease: two sides of the same coin? COPD 2018;15(5):432–438. doi:10.1080/15412555.2018.1548587.
- Van Cauwenberg J, Van Holle V, De Bourdeaudhuij I, et al. Diurnal patterns and correlates of older adults’ sedentary behavior. PLoS ONE. 2015;10(8):e0133175. doi:10.1371/journal.pone.0133175.
- Tudor-Locke C, Johnson WD, Katzmarzyk PT. U.S. population profile of time-stamped accelerometer outputs: impact of wear time. J Phys Act Health. 2011;8(5):693–698. doi:10.1123/jpah.8.5.693.
- Masse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exercise. 2005;37:S544–S54. doi:10.1249/01.mss.0000185674.09066.8a.
- Choi L, Liu Z, Matthews CE, et al. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exercise. 2011;43(2):357–364. doi:10.1249/MSS.0b013e3181ed61a3.
- Mesquita R, Nakken N, Janssen DJA, et al. Activity levels and exercise motivation in patients with COPD and their resident loved ones. Chest. 2017;151(5):1028–1038. doi:10.1016/j.chest.2016.12.021.
- Byrom B, Stratton G, Mc Carthy M, et al. Objective measurement of sedentary behaviour using accelerometers. Int J Obes. 2016;40(11):1809–1812. doi:10.1038/ijo.2016.136.
- Bellettiere JWE, Chastin SFM, Kerr J, et al. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS ONE. 2017;12(6):e0180119. doi:10.1371/journal.pone.0180119.
- Chastin SFM, Granat MH. Methods for objective measure, quantification, and analysis of sedentary behaviour and inactivity. Gait Posture. 2010;31(1):82–86. doi:10.1016/j.gaitpost.2009.09.002.
- Chastin SFM, Winkler EA, Eakin EG, et al. Sensitivity to change of objectively-derived measures of sedentary behavior. MPEES. 2015;19:138–147. doi:10.1080/1091367X.2015.1050592.
- Hartman JE, Boezen HM, de Greef MH, et al. Physical and psychosocial factors associated with physical activity in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2013;94(12):2396–2402. doi:10.1016/j.apmr.2013.06.029.
- Xavier RF, Pereira AC, Lopes AC, et al. Identification of phenotypes in people with COPD: influence of physical activity, sedentary behaviour, body composition and skeletal muscle strength. Lung. 2019;197(1):37–45. doi:10.1007/s00408-018-0177-8.
- McKeough ZJ, Large S, Spencer LM, et al. An observational study of self-reported sedentary behaviour in people with chronic obstructive pulmonary disease and bronchiectasis. Braz J Phys Ther. 2019;S1413-3555(19)30069-3. [Epub ahead of print].
- Cheng SWM, Alison J, Dennis S, et al. A behaviour change intervention to reduce sedentary time in people with chronic obstructive pulmonary disease: protocol for a randomised controlled trial. J Physiother. 2017;63(3):182. doi:10.1016/j.jphys.2017.04.001.
- Weatherspark.com: Average weather for Sydney, Australia [Internet]. San Francisco (SF): Cedar Lake Ventures, 2019. [cited 2019 Jun 19]. Available from: https://weatherspark.com/y/144544/Average-Weather-in-Sydney-Australia-Year-Round
- Miller MR, Hankinson JL, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi:10.1164/ajrccm.159.1.9712108.
- Ryan CG, Grant PM, Tigbe WW, et al. The validity and reliability of a novel activity monitor as a measure of walking. Br J Sports Med. 2006;40(9):779–784. doi:10.1136/bjsm.2006.027276.
- Grant PM, Ryan CG, Tigbe WW, et al. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med. 2006;40(12):992–997. doi:10.1136/bjsm.2006.030262.
- Ng CLW, Jenkins S, Hill K. Accuracy and responsiveness of the stepwatch activity monitor and ActivPAL in patients with COPD when walking with and without a rollator. Disabil Rehabil. 2012;34:1317–1322. doi:10.3109/09638288.2011.641666.
- Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi:10.1249/MSS.0b013e318213fefb.
- Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi:10.1183/09031936.00150314.
- Jenkins S, Cecins N, Camarri B, et al. Regression equations to predict 6-minute walk distancein middle-aged and elderly adults. Physiother Theory Pract. 2009;25(7):516–522. doi:10.3109/09593980802664711.
- Moreira GL, Donária L, Furlanetto KC, et al. GOLD B-C-D groups or GOLD II-III-IV grades Which one better reflects the functionality of patients with chronic obstructive pulmonary disease? Chron Respir Dis. 2015;12(2):102–110. doi:10.1177/1479972315573528.
- Morakami FK, Morita AA, Bisca GW, et al. Can the six-minute walk distance predict the occurrence of acute exacerbations of COPD in patients in Brazil? J Bras Pneumol. 2017;43(4):280–284. doi:10.1590/s1806-37562016000000197.
- BMJ Publishing Group: Correlation and Regression. 2018. [cited 2019 Jun 19]. Available from: http://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression.
- Bakrania K, Edwardson CL, Bodicoat D, et al. Associations of mutually exclusive categories of physical activity and sedentary time with markers of cardiometabolic health in English adults: a cross-sectional analysis of the Health Survey for England. BMC Public Health. 2015;16(1):25. doi:10.1186/s12889-016-2694-9.
- Healy GN, Winkler EAH, Owen N, et al. Replacing sitting time with standing or stepping: associations with cardio-metabolic risk biomarkers. Eur Heart J. 2015;36(39):2643–2649. doi:10.1093/eurheartj/ehv308.
- van der Berg JD, Stehouwer CDA, Bosma H, et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: The Maastricht Study. Diabetologia 2016;59(4):709–718. doi:10.1007/s00125-015-3861-8.
- Australian Government, Department of Health: Australia’s physical activity and sedentary behaviour guidelines for adults (18-64 years). 2019. [cited 2019 Jun 19]. Available from: https://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines.
- McVeigh JA, Winkler EAH, Howie EK, et al. Objectively measured patterns of sedentary time and physical activity in young adults of the Raine study cohort. Int J Behav Nutr Phys Act. 2016;13(1):41. doi:10.1186/s12966-016-0363-0.
- Bellettiere J, Healy GN, LaMonte MJ, et al. Sedentary behavior and prevalent diabetes in 6,166 older women: the objective physical activity and cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2019;74(3):387–395. doi:10.1093/gerona/gly101.
- Orme MW, Weedon AE, Saukko PM, et al. Findings of the Chronic Obstructive Pulmonary Disease-Sitting and Exacerbations Trial (COPD-SEAT) in reducing sedentary time using wearable and mobile technologies with educational support: randomized controlled feasibility trial. JMIR Mhealth Uhealth. 2018;11:e84. doi:10.2196/mhealth.9398.
- Physical Activity Guidelines Advisory Committee. 2018 Physical activity guidelines advisory committee scientific report. Washington (DC): U.S. Department of Health and Human Services, 2018.
- Chastin SFM, De Craemer M, De Cocker K, et al. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53(6):370–376. doi:10.1136/bjsports-2017-097563.
- Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53(16):1013–1020. doi:10.1136/bjsports-2017-098733.
- Furlanetto KC, Pinto IFS, Sant'Anna T, et al. Profile of patients with chronic obstructive pulmonary disease classified as physically active and inactive according to different thresholds of physical activity in daily life. Braz J Phys Ther. 2016;20(6):517–524. doi:10.1590/bjpt-rbf.2014.0185.
- Mesquita R, Spina G, Pitta F, et al. Physical activity patterns and clusters in 1001 patients with COPD. Chron Respir Dis. 2017;14(3):256–269. doi:10.1177/1479972316687207.
- Pitta F, Troosters T, Probst VS, et al. Potential consequences for stable chronic obstructive pulmonary disease patients who do not get the recommended minimum daily amount of physical activity. J Bras Pneumol. 2006;32(4):301–308.
- Mcewan T, Tam-Seto L, Dogra S. Perceptions of sedentary behavior among socially engaged older adults. Gerontologist. 2016;0:1–10.
- Weedon AE, Saukko PM, Downey JW, et al. Meanings of sitting in the context of chronic disease: a critical reflection on sedentary behaviour, health, choice and enjoyment. Qual Res Sport Exerc Health. 2019;1–14. doi:10.1080/2159676X.2019.1595105.
- Hoaas H, Zanaboni P, Hjalmarsen A, et al. Seasonal variations in objectively assessed physical activity among people with COPD in two Nordic countries and Australia: a cross-sectional study. COPD. 2019;14:1219–1228. doi:10.2147/COPD.S194622.
- Furlanetto KC, Demeyer H, Sant'anna T, et al. Physical activity of patients with COPD from regions with different climatic variations. COPD. 2017;14(3):276–283. doi:10.1080/15412555.2017.1303039.
- Demeyer H, Burtin C, Van Remoortel H, et al. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest. 2014;146(2):318–327. doi:10.1378/chest.13-1968.
- Spina G, Spruit MA, Alison J, et al. Analysis of nocturnal actigraphic sleep measures in patients with COPD and their association with daytime physical activity. Thorax. 2017;72(8):694–701. doi:10.1136/thoraxjnl-2016-208900.
