Abstract
Frequent exacerbators of Chronic Obstructive Pulmonary Disease (COPD) is a distinct clinical phenotype characterised by systemic inflammation. Study objectives were to determine clinical outcomes of pulmonary rehabilitation in frequent exacerbators and the impact this has on the key surrogate markers of this phenotype. Eighty-five mild-very severe COPD patients (FEV1 pred, 52 ± 18%) were categorised as frequent (≥2 exacerbations per year, n = 50) or infrequent exacerbators (≤1 exacerbation per year, n = 35). The primary outcomes were completion rates of pulmonary rehabilitation (clinical) and plasma fibrinogen (biological). Secondary outcomes were: incremental shuttle (ISWT) & endurance shuttle walk tests (ESWT), chronic respiratory disease questionnaire (CRQ), hospital anxiety and depression scale (HADS), plasma C-reactive protein (CRP), blood leukocyte counts, blood neutrophil activation (CD11b, CD62L, CD66b) and subsets (mature, immature, suppressive, progenitor). Fibrinogen and CRP concentrations were determined via ELISA’s with neutrophil activation markers assessed using flow cytometry. Frequent exacerbators were less likely to complete pulmonary rehabilitation (44% vs 69%; p = 0.025). Both groups experienced improvements in ISWT (p < 0.001), ESWT (p < 0.001), all domains of the CRQ (p < 0.001) and Depression (p = 0.017). Pulmonary rehabilitation reduced resting concentrations of fibrinogen (frequent exacerbators = 12%, infrequent exacerbators = 4%, p = 0.033) and % of progenitor blood neutrophils (p = 0.015) in both groups, with reductions in total blood leukocyte (p = 0.018) and neutrophil counts (p = 0.018) also observed in frequent exacerbators. No significant reductions in CRP concentration (p = 0.937), neutrophil activation (CD11b, p = 0.553; CD62L, p = 0.070; CD66b, p = 0.317), or other neutrophil subsets (mature, p = 0.313; immature, p = 0.756; suppressive, p = 0.259) were observed. Frequent exacerbators of COPD were less likely to complete pulmonary rehabilitation, but those who complete experience similar benefits to infrequent exacerbators. Pulmonary rehabilitation may serve to have immune-modulatory properties for frequent exacerbators.
Introduction
Pulmonary rehabilitation is defined as “a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behaviour change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviours” [Citation1]. The benefits of pulmonary rehabilitation are profound in the Chronic Obstructive Pulmonary Disease (COPD) population, especially when assessing outcomes such as exercise capacity and quality of life [Citation2]. However, not all patients who enrol onto, and complete pulmonary rehabilitation see the same benefits [Citation3]. This has led to proposals that clinical factors such as comorbidities or phenotypes might play an influential role in responses to pulmonary rehabilitation [Citation4–6].
One of the most established clinical phenotypes of COPD is the frequent exacerbator phenotype [Citation7,Citation8]. Frequent exacerbators have a worse quality of life, increased risk of hospitalisation and a greater chance of recurrent exacerbations believed to be attributed to an altered immune profile [Citation9]. Systemic inflammatory parameters such as; fibrinogen, C-reactive protein (CRP), total leukocyte, and blood neutrophil counts have been seen to be elevated in frequent exacerbators [Citation7–9]. A variety of pharmacological therapies have been shown to be ineffective or have conflicting results in relation to arresting long-term persistent systemic inflammation in COPD [Citation10].
Limited research has assessed responses to pulmonary rehabilitation in COPD patients according to exacerbation history. Previous findings have reported an association between exacerbations and non-completion of pulmonary rehabilitation [Citation11]. Others [Citation12,Citation13] have suggested that in patients who manage to complete pulmonary rehabilitation, improvements in exercise capacity can be seen to be larger in exacerbators compared to non-exacerbators or that subgroups of ‘responders’ are made up of a higher percentage of frequent exacerbators [Citation13]. Hence, there remains an uncertainty regarding the impact of frequent exacerbators on clinical outcomes of pulmonary rehabilitation.
Completion of pulmonary rehabilitation is considered to lower the risk of experiencing an exacerbation or hospitalisation [Citation14]. However, the mechanisms underpinning a reduced incidence of hospital admissions in those who complete pulmonary rehabilitation are unclear. It has been proposed that an increased resilience following completion of pulmonary rehabilitation may play a role in reducing exacerbation incidence [Citation15]. Evidence demonstrating independent effects of exercise interventions (e.g. without the educational component of pulmonary rehabilitation) on exacerbation risk [Citation16], has led to proposals of other unexplored (biological) mechanisms [Citation17]. One potential area is that of exercise as an immunomodulatory intervention, based on extensive evidence of the anti-inflammatory effects of exercise in healthy individuals [Citation18]. Chronic exercise training programmes, similar in length to pulmonary rehabilitation have been seen to reduce systemic inflammation (e.g. CRP) in other clinical (e.g. type 2 diabetes) populations [Citation19]. Furthermore, walking exercise programmes have been seen to have anti-inflammatory effects in an older but otherwise healthy population with attenuation of CD66b expression on blood neutrophils and decreased shedding of CD62L [Citation20]. Previous research has suggested that pulmonary rehabilitation does not affect systemic inflammation as characterised by a lack of change in plasma CRP, plasma cytokine concentrations or total leukocyte count even in the presence of improved exercise capacity [Citation21,Citation22]. Contrary to previous findings, Silva et al. [Citation23] recently found that an exercise programme of a similar duration to pulmonary rehabilitation induced significant reductions in circulating cytokines. However, no studies have yet assessed whether pulmonary rehabilitation can play a role in reducing other markers of systemic inflammation that are also widely recognised for their reliability as a surrogate marker of exacerbation risk (e.g. fibrinogen) [Citation24].
This study aimed to assess the differences in clinical outcomes and inflammatory responses pre- and post-pulmonary rehabilitation in frequent and infrequent exacerbators of COPD. It was hypothesised that frequent exacerbators would: be less likely to complete pulmonary rehabilitation, achieve comparable clinical benefit to infrequent exacerbators, and experience reductions in markers of systemic inflammation (fibrinogen and CRP).
Materials and methods
Study design and approvals
This study was conducted as part of a prospective cohort study approved by the Health Research Authority research ethics committee (16/LO/0865) and registered on clinicaltrials.gov (NCT02740686). All patients provided written consent.
Study population
85 patients with a clinical diagnosis of COPD (age 69.5 ± 7.1 years; FEV1 pred 52 ± 18%; frequent exacerbators = 50; infrequent exacerbators = 35) and referred to community pulmonary rehabilitation were recruited over a 2-year period. COPD patients presenting with any other active inflammatory (e.g. rheumatoid arthritis or cancer) or respiratory conditions (e.g. asthma, bronchiectasis or pulmonary fibrosis) as confirmed by medical records were excluded from this study.
Pulmonary rehabilitation
COPD patients were screened and recruited upon initial assessment for conventional pulmonary rehabilitation between June 2016 and June 2018. Two sites were identified in close proximity of each other within the City of Lincoln (UK). The pulmonary rehabilitation programme was standardised at both sites. This was not an early pulmonary rehabilitation programme and recruited COPD patients were clear of an exacerbation (i.e. not being treated with antibiotics or oral steroids within 4 weeks) upon study enrolment. Eligible patients participated in a twice weekly supervised community pulmonary rehabilitation programme for 8-weeks. Each pulmonary rehabilitation session consisted of 1 h of exercise and 1 h of education. The exercise programme was tailored in a progressive manner and utilised minimal resources encompassing cardiorespiratory fitness and muscular strengthening exercises. COPD patients were categorised as frequent (≥2 exacerbations requiring treatment with antibiotics and/or steroids in the previous 12 months) or infrequent exacerbators (≤1 exacerbation) [Citation7], based on self-report with verification via medical records [Citation25].
Clinical outcomes such as: attendance, incremental shuttle walk (ISWT) & endurance shuttle walk tests (ESWT), chronic respiratory disease questionnaire (CRQ), and hospital anxiety and depression scale (HADS) were collected at initial and final assessment for pulmonary rehabilitation.
Blood sampling
Participants were advised to remain seated performing minimal movement for 5 min prior to each blood sample. Blood samples (∼ 10 ml) were collected at the 1st (Pre-Rehab) and 16th classes (Post-Rehab) into K3EDTA (6 ml) & Sodium Citrate (4.5 ml) treated vacutainers (Greiner Bio-One, Kremsmünster, Austria) from the brachiocephalic vein. All blood samples were maintained at room temperature to reduce the risk of potential temperature influences on cellular processing.
Analysis of blood biomarkers
Measures of blood biomarkers were only reported in patients who reported to not have had a recent exacerbation. Fibrinogen and CRP concentrations were quantified via ELISA as per manufacturers’ recommendations (AssayPro LLC, Missouri, USA). Total and differential leukocyte counts were measured in whole blood using an automated haematology analyser within 4 h of collection (ABX Pentra 60 C+, HORIBA Medical, Montpellier, France). Markers of activation (CD11b, CD62L, CD66b – see Table S1 for further details) and maturity (mature, CD16bhigh/CD62Lhigh; immature, CD16blow/CD62Lhigh; suppressive, CD16bhigh/CD62Llow; progenitor, CD16blow/CD62Llow) were assessed in blood neutrophils (CD16bhigh/CD45high) using flow cytometry (FACSVerse, Becton Dickinson, New Jersey, USA) within 4 h of collection (see supplementary material).
Study outcomes
Given the aims of the study, we had a primary outcome for both the clinical and biological outcomes. The primary clinical outcome was completion rates of pulmonary rehabilitation as defined by attending 12 or more sessions [Citation26,Citation27]. The primary biological outcome was change in fibrinogen concentration post-pulmonary rehabilitation in those who completed final assessment of pulmonary rehabilitation [Citation28] and were clear of exacerbation. Secondary clinical outcomes included: ISWT & ESWT, CRQ & HADS. Secondary biological outcomes included: CRP concentrations, total and differential leukocyte count, neutrophil activation markers (CD11b, CD62L, CD66b), and neutrophil phenotypes (mature, immature, suppressive, progenitor).
Statistical analysis
Statistical analyses were performed using SPSS (v22.00; SPSS Inc., Chicago, IL, USA). All data were assessed for normal distribution prior to analysis using Shapiro-Wilk and if necessary, were normalised using log transformation. Data on the proportion of patients within each group (frequent vs infrequent exacerbators) who did not complete pulmonary rehabilitation was reported using chi-squared analysis. Clinical outcomes (ISWT, ESWT, HADS, CRQ), fibrinogen and CRP concentrations, total and differential leukocyte counts, expression of neutrophil activation markers, and neutrophil maturity were analysed pre- and post-rehabilitation in both groups (frequent vs infrequent exacerbators; Pre-Rehab vs Post-Rehab) using a two-way mixed ANOVA. Any significant main effects observed in the ANOVA were further analysed with post-hoc t-tests with Bonferroni correction. Daily beclomethasone equivalent between groups was analysed using a two-tailed independent t-test. Statistical significance was accepted at p < 0.05.
Results
Clinical outcomes
Patient characteristics
The patient characteristics of frequent and infrequent exacerbators are detailed in . All frequent exacerbators were categorised as GOLD grade D (100%) with the majority of infrequent exacerbators classified as GOLD grade B (93%). The majority of frequent and infrequent exacerbators were categorised as either mMRC grade 2, grade 3, or grade 4.
Table 1. Patient characteristics.
Completion rates
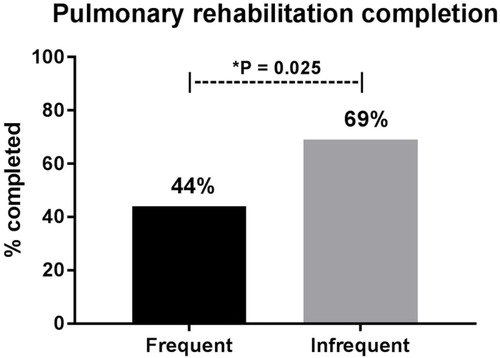
Of the 85 COPD patients who consented to take part in the study (frequent exacerbators, n = 50; infrequent exacerbators, n = 35), 54% of COPD patients completed 12 sessions of pulmonary rehabilitation (total, n = 46; frequent exacerbators, n = 22; infrequent exacerbators, n = 24). Frequent exacerbators were significantly less likely to complete pulmonary rehabilitation when compared to infrequent exacerbators (44% vs 69%; p = 0.025) ().
Exercise capacity and quality of life measures
Statistically significant improvements in exercise capacity were seen in both frequent and infrequent exacerbators (ISWT, p < 0.001; ESWT, p < 0.001). No independent effects of group were observed (ISWT, p = 0.860; ESWT, p = 0.276), nor were there any indications of a time × group interaction (ISWT, p = 0.390; ESWT, p = 0.207) ().
Table 2. Clinical outcomes following pulmonary rehabilitation.
Statistically significantly improvements in the CRQ were observed across all domains (p < 0.001) post-rehabilitation in both frequent and infrequent exacerbators. There were no significant effects of group (Dyspnoea, p = 0.899; Emotion, p = 0.861; Fatigue, p = 0.291; Mastery, p = 0.345) or time × group interaction (Dyspnoea, p = 0.453; Emotion, p = 0.452; Fatigue, p = 0.206; Mastery, p = 0.837) on CRQ subdomains ().
Depression (p = 0.017) scores were significantly decreased following pulmonary rehabilitation in both frequent and infrequent exacerbators. However, anxiety (p = 0.197) was not significantly reduced because of pulmonary rehabilitation. There were no significant differences between frequent and infrequent exacerbators (Anxiety, p = 0.503; Depression, p = 0.348), nor were any time × group interactions observed (Anxiety, p = 0.142; Depression, p = 0.538) ().
Biological outcomes
Plasma inflammatory markers
30 patients (frequent, n = 16; infrequent, n = 14) provided blood samples. Plasma samples were not obtainable for 2 patients (frequent, n = 1; infrequent, n = 1) for the analysis of fibrinogen and CRP. Analysis of neutrophil activation was only analysed in a small subset of COPD patients due to issues surrounding a lack of CD16 expression (frequent, n = 8; infrequent, n = 7).
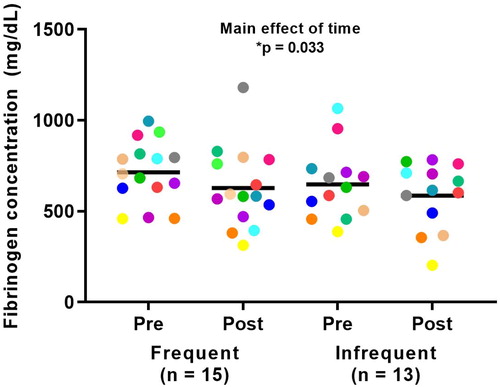
There was a significant main effect of time showing a reduction in plasma fibrinogen following pulmonary rehabilitation (p = 0.033). No significant differences were observed between frequent and infrequent exacerbators for fibrinogen (p = 0.414) nor were any time × group interactions observed (p = 0.702) ().
Figure 2. Fibrinogen concentrations in response to pulmonary rehabilitation in frequent and infrequent exacerbators. *Significant overall main effect of time between pre- and post-rehabilitation (p < 0.05).
Pulmonary rehabilitation did not significantly reduce the concentration of plasma CRP (p = 0.937). No significant differences for CRP concentration were observed between frequent and infrequent exacerbators (p = 0.701), nor were any time × group interactions observed (p = 0.815).
Blood leukocyte counts
There was a significant main effect of time on total leukocyte counts (p = 0.032). There was also a significant time × group interaction with total leukocyte counts (p = 0.041). Post-hoc analyses showed that frequent exacerbators experienced a significant reduction in total leukocyte count by the end of pulmonary rehabilitation (p = 0.018), whereas infrequent exacerbators did not (p = 0.910). There was no significant main effect of group for total leukocyte count (p = 0.222) ().
Table 3. Blood cell counts pre- and post-pulmonary rehabilitation.
There was a tendency towards a main effect of time on neutrophil counts, but this did not reach statistical significance (p = 0.057). However, there was a significant time × group interaction with neutrophil counts (p = 0.036). Post-hoc analyses showed that frequent exacerbators experienced a significant reduction in neutrophil count by the end of pulmonary rehabilitation (p = 0.018), whereas infrequent exacerbators did not (p = 0.849). There was no significant main effect of group for neutrophil count (p = 0.485) ().
No effects of time (lymphocytes, p = 0.115; eosinophils, p = 0.723; neutrophil/lymphocyte, p = 0.524), group (lymphocytes, p = 0.454; eosinophils, p = 0.479; neutrophil/lymphocyte, p = 0.916), or time × group interaction (lymphocytes, p = 0.570; eosinophils, p = 0.846; neutrophil/lymphocyte, p = 0.123) () were observed for other leukocyte counts.
Blood neutrophil activation and subsets
Pulmonary rehabilitation did not appear to significantly alter the cell surface expression of CD11b (p = 0.553) or CD66b on blood neutrophils (p = 0.317). However, there was a suggestion towards an increase in the expression of CD62L by the end of pulmonary rehabilitation, but this was not found to be statistically significant (p = 0.070). There was no significant main effect of group on blood neutrophil expression of CD11b (p = 0.161), CD62L (p = 0.503), or CD66b (p = 0.906). Additionally, there were no significant time × group interactions (CD11b, p = 0.936; CD62L, p = 0.491; CD66b, p = 0.168) (Table S2).
A significant main effect of time was observed with reductions in the progenitor neutrophil subset by the end of pulmonary rehabilitation (p = 0.015). There were no main effects of time observed with any of the other neutrophil subsets (mature, p = 0.313; immature, p = 0.756; suppressive, p = 0.259). There were no main effects of group (mature, p = 0.517; immature, p = 0.172; suppressive, p = 0.261; progenitor, p = 0.999) or time × group interactions observed with any of the neutrophil subsets (mature, p = 0.805; immature, p = 0.780; suppressive, p = 0.809; progenitor, p = 0.193) (Table S3).
Discussion
The main findings from this study suggest that frequent exacerbators are less likely to complete pulmonary rehabilitation compared to infrequent exacerbators but those who complete pulmonary rehabilitation experience similar absolute changes in clinical outcomes compared to infrequent exacerbators. Findings from this study suggest that pulmonary rehabilitation can reduce the concentration of plasma fibrinogen and % of progenitor neutrophils in all COPD patients and can reduce blood total leukocyte and neutrophil counts in frequent exacerbators.
There is a depth of research assessing how clinical and/or socio-demographic factors may predict completion of pulmonary rehabilitation in a mixed cohort but a paucity of studies prospectively investigating the frequent exacerbator phenotype in pulmonary rehabilitation. The lower completion rates in frequent exacerbators observed in the present study further adds to the notion that exacerbation risk may increase the likelihood of non-completion [Citation11,Citation29–32]. There was also a demonstrable high prevalence of frequent exacerbators in this study, agreeing with recent evidence [Citation33], showing the increased presence of frequent exacerbators in pulmonary rehabilitation studies compared to other study settings in COPD [Citation34]. The average completion rate for the current study was 54%, which is lower than the national average (62%) reported in the recent UK National COPD Pulmonary Rehabilitation Audit [Citation27]. However, the National COPD Pulmonary Rehabilitation Audit defined completion as attending final assessment, whereby the present study used the criterion of attendance at 12 or more sessions [Citation26,Citation27]. If the current study adopted the National COPD Pulmonary Rehabilitation Audit definition of completion, overall completion rates would be comparable (64% in the current study vs. 62% national average), but the completion rates of frequent exacerbators remains lower than the national average. A future consensus on the definition of completion of pulmonary rehabilitation would help to interpret findings of patient compliance across different studies.
This study has shown that frequent exacerbators benefit from pulmonary rehabilitation in a similar manner to infrequent exacerbators. Improvements in traditional outcomes such as ISWT, ESWT, CRQ and HADS-Depression were observed in both groups in line with previous findings in the COPD population [Citation35]. These findings also agree with those of Bohn et al., [Citation12] and Zanini et al., [Citation13] showing that frequent exacerbators can experience significant benefits because of pulmonary rehabilitation, although the current study was unable to support the notion that frequent exacerbators respond better to pulmonary rehabilitation. Taken together, the available evidence suggests COPD patients achieve benefits in most traditional clinical outcomes from completion of pulmonary rehabilitation irrespective of exacerbation history.
Fibrinogen, CRP, total leukocyte and neutrophil counts have been established as surrogate biomarkers for exacerbations of COPD [Citation7,Citation36]. This is the first study to demonstrate that pulmonary rehabilitation can be effective in modulating resting concentrations of plasma fibrinogen in COPD. These findings draw comparisons from lung cancer patients where pulmonary rehabilitation has been seen to reduce fibrinogen levels [Citation37]. Fibrinogen has been demonstrated to have pro-inflammatory properties [Citation38] and reductions in this marker can be interpreted as favouring an anti-inflammatory response by the end of the pulmonary rehabilitation programme. Whilst the observed changes are relatively small, it is important to consider the context given that this was a short 8-week intervention and that the early-observed benefits may stem to larger effects that are more meaningful if exercise is maintained. It is plausible to suggest that this reduction in fibrinogen concentration may provide mechanistic evidence towards a reduction in exacerbation risk following pulmonary rehabilitation in COPD [Citation14], but this requires further study. CRP, however, in line with previous findings [Citation21,Citation22], was not significantly reduced following pulmonary rehabilitation.
Pulmonary rehabilitation reduced total leukocyte and neutrophil counts in frequent exacerbators, which is in contrast with work of others that did not demonstrate any changes in these measures [Citation22,Citation39]. The findings suggest that in clinical phenotypes where there is scope for anti-inflammatory benefits (e.g. COPD frequent exacerbators) total blood leukocyte or neutrophil counts are also amenable to change by pulmonary rehabilitation. However, this requires further research on a larger scale and inclusion of a concurrent control arm.
It has been acknowledged that assessing markers of neutrophil activation provides additional insight into inflammatory responses than that of neutrophil counts alone [Citation40]. This is the first study in COPD to assess these neutrophil activation markers in the context of pulmonary rehabilitation. This study did not find any significant changes in neutrophil activation markers (CD11b, CD62L and CD66b) following pulmonary rehabilitation. However, the observed change in CD62L expression by the end of pulmonary rehabilitation suggests that this may be a promising marker to explore further in the context of exercise and COPD. Such findings are in agreement with those of Takahasi et al. [Citation20] who found that exercise training in a healthy aged population increased the resting expression of CD62L. Neutrophils shed CD62L upon activation, suggesting that pulmonary rehabilitation may have the ability to reduce neutrophil activation [Citation20,Citation41–44].
For the first time in an exercise and COPD setting, phenotyping of blood neutrophils [Citation45], was performed in this study. This study identified that pulmonary rehabilitation can reduce the amount of progenitor neutrophils in the circulation. The progenitor subset is a newly identified small subset of neutrophils, considered to have increased degranulation [Citation46]. Progenitor neutrophils have also been suggested to have a poorer migration due to reduced CD62L expression [Citation45]. Therefore, a reduction in this subset could be deemed as a beneficial effect of exercise with a shift towards neutrophil subsets with enhanced migration and reduced degranulation [Citation45]. However, no significant differences were observed in any of the other neutrophil subsets at the end of pulmonary rehabilitation, so whether this decrease results in more mature, immature or suppressive neutrophils is unclear. These findings were based on observations made in a limited sample size with further research warranted.
There are some important factors to consider when interpreting the results of this study. Although this study suggests frequent exacerbators are less likely to complete pulmonary rehabilitation, this study did not provide evidence on the reasons for drop-out which may directly or indirectly be related to incidence of exacerbations (e.g. transportation issues) [Citation20,Citation31]. This study aimed to investigate outcomes on a clinical phenotype basis and the contribution of other factors linked to poor completion were not considered in this study (e.g. frailty, social deprivation) [Citation30,Citation31,Citation46,Citation47]. However, the current study observed comparable characteristics across groups in terms of proportion of smokers (which can impact on inflammation [Citation48]), depression and other characteristics (e.g. number of comorbidities). In relation to inflammation, no strict restrictions were put in place to standardise the exercise training across participants which could impact the effect of exercise training on inflammation. Also, it is important to highlight that blood samples were only provided in a subset of the full study population and this needs to be considered in the interpretation of the study findings. In addition, the sample size utilised for the assessment of neutrophil activation markers was smaller than planned due to an unforeseen issue with a lack of neutrophil CD16 expression in a proportion of the selected sample reducing the ability to confidently isolate neutrophils. No control group was used in this study hence the observed effects over time cannot be wholly attributed to the effect of the components of pulmonary rehabilitation only, but given the evidence base in healthy individuals, it is plausible to assume that the exercise component of pulmonary rehabilitation has played a role in this observed effect. COPD patients with co-existing lung conditions were excluded to avoid confounding effects of these on exacerbation risk which must be considered when interpreting the results of the study. However, a strength of the study was that the findings reflect ‘real-world’ effects of pulmonary rehabilitation.
The findings presented require further investigation by the respiratory research community and consideration of how this may impact on clinical management of COPD frequent exacerbators. Future studies should assess whether it is the incidence of exacerbations during pulmonary rehabilitation or the clinical factors that may ensue during recovery from these recurrent episodes (e.g. fatigue) that are responsible for poorer completion rates of pulmonary rehabilitation in frequent exacerbators. Going forwards, the early findings of potential immune-modulatory and anti-inflammatory properties need to be further investigated as underlying mechanisms of pulmonary rehabilitation and other exercise-based interventions [Citation14,Citation16] on exacerbations of COPD. Evidence of biological mechanisms (i.e. biomarkers relating to exacerbations) in COPD may provide a major selling point for these interventions which are currently underfunded and underutilised [Citation30–32,Citation49].
In conclusion, frequent exacerbators were less likely to complete pulmonary rehabilitation compared to infrequent exacerbators. Frequent exacerbators can expect to see the same benefits in terms of exercise capacity and quality of life measures. Pulmonary rehabilitation appears to have positive effects in reducing plasma fibrinogen concentrations and % of progenitor neutrophils in COPD and may provide more anti-inflammatory benefits to frequent exacerbators characterised by reduced total blood leukocyte and neutrophil counts. Further research is required to assess the full impact that exercise/pulmonary rehabilitation has on clinical outcomes and biological mechanisms of the frequent exacerbator phenotype.
Declaration of interest
The authors declare to have no conflicts of interest relating to the production of this manuscript.
Acknowledgements
We would like to thank the Countywide Respiratory Service of Lincolnshire Community Health Services NHS Trust for supporting the study and allowing access to the pulmonary rehabilitation service.
References
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST.
- Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390–1413. doi:10.1164/rccm.200508-1211ST.
- Spruit MA, Augustin IML, Vanfleteren LE, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J. 2015;46(6):1625–1635. doi:10.1183/13993003.00350-2015.
- Ambrosino N, Clini EM. Response to pulmonary rehabilitation: toward personalised programmes? Eur Respir J. 2015;46(6):1538–1540. doi:10.1183/13993003.01125-2015.
- Kaul V, Farokhi MR, Megally M. Pulmonary rehabilitation, an update: clinical phenotypes and effect of comorbidities and echocardiographic abnormalities. Am J Respir Crit Care Med. 2018;197(4):517–519. doi:10.1164/rccm.201703-0528RR.
- Hassan M, Mourad S, Abdel Wahab NH, et al. Effect of comorbidities on response to pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2016;65(1):63–69. doi:10.1016/j.ejcdt.2015.11.006.
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883.
- Donaldson GC, Mullerova H, Locantore N, et al. Factors associated with change in exacerbation frequency in COPD. Respir Res. 2013;14(1):79. doi:10.1186/1465-9921-14-79.
- Wedzicha JA, Mackay AJ, Singh R. COPD exacerbations: impact and prevention. Breathe. 2013;9(6):434–440. doi:10.1183/20734735.002913.
- King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med. 2015;4(26). doi:10.1186/s40169-015-0068-z.
- Fischer MJ, Scharloo M, Abbink JJ, et al. Drop-out and attendance in pulmonary rehabilitation: the role of clinical and psychosocial variables. Respir Med. 2009;103(10):1564–1571. doi:10.1016/j.rmed.2008.11.020.
- Bohn I, Huber dos Santos Á, Costa CC, et al. Pulmonary rehabilitation in COPD exacerbator phenotype [abstract]. Eur Respir J. 2017;50(Suppl 61):PA760. doi:10.1183/1393003.congress-2017.PA760.
- Zanini A, Chetta A, Gumiero F, et al. Six-minute walking distance improvement after pulmonary rehabilitation is associated with baseline lung function in complex COPD patients: a retrospective study. Biomed Res Int. 2013; 2013:1–6. doi:10.1155/2013/483162.
- Moore E, Palmer T, Newson R, et al. Pulmonary rehabilitation as a mechanism to reduce hospitalizations for acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2016;150(4):837–859. doi:10.1016/j.chest.2016.05.038.
- Evans RA, Steiner MC. Pulmonary rehabilitation: the lead singer of COPD therapy but not a “one-man band”. Chest. 2017;152(6):1103–1105.
- Jenkins AR, Gowler H, Curtis F, et al. Efficacy of supervised maintenance exercise following pulmonary rehabilitation on health care use: a systematic review and meta-analysis. COPD. 2018;13:257–273. doi:10.2147/COPD.S150650.
- Jenkins AR, Holden NS, Jones AW. Pulmonary Rehabilitation, Exercise, and Exacerbations of COPD: Known Clinical Efficacy and the Unknown Mechanisms. Chest. 2018;153(5):1281–1282. doi:10.1016/j.chest.2018.01.054.
- Woods JA, Wilund KR, Martin SA, et al. Exercise, inflammation and aging. Aging Dis. 2012;3(1):130–140.
- Giannopoulou I, Fernhall B, Carhart R, et al. Effetcs of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54(7):866–875. doi:10.1016/j.metabol.2005.01.033.
- Takahashi M, Miyashita M, Kawanishi N, et al. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur J Appl Physiol. 2013;113(5):1117–1126. doi:10.1007/s00421-012-2531-5.
- Canavan J, Garrod R, Marshall J, et al. Measurement of the acute inflammatory response to walking exercise in COPD: effects of pulmonary rehabilitation. Int J COPD. 2007;2:347–353.
- Sciriha A, Lungaro-Mifsud S, Bonello A, et al. Systemic inflammation in COPD is not influenced by pulmonary rehabilitation. Eur J Physiother. 2017;19(4):194–200. doi:10.1080/21679169.2017.1332682.
- Silva BSA, Lira FS, Rossi FE, et al. Inflammatory and metabolic responses to different resistance training on chronic obstructive pulmonary disease: a randomized control trial. Front Physiol. 2018;9(262). doi:10.3389/fphys.2018.00262.
- Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. doi:10.1136/thoraxjnl-2012-201871.
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi:10.7326/0003-4819-106-2-196.
- Boutou AK, Tanner RJ, Lord VM, et al. An evaluation of factors associated with completion and benefit from pulmonary rehabilitation in COPD. BMJ Open Resp Res. 2014;1(1):e000051. doi:10.1136/bmjresp-2014-000051.
- Department of Health and Social Care. 2012. COPD commissioning toolkit. Available from: https://www.gov.uk/government/publications/commissioning-toolkit-for-respiratory-services.
- Steiner M, McMillan V, Lowe D, et al. Pulmonary rehabilitation: beyond breathing better. National Chronic Obstructive Pulmonary Disease (COPD) audit programme: outcomes from the clinical audit of pulmonary rehabilitation services in England 2015. National supplementary report. London: RCP; 2017.
- Cecins N, Geelhoed E, Jenkins SC. Reduction in hospitalisation following pulmonary rehabilitation in patients with COPD. Aust Health Review. 2008;32(3):415–422. doi:10.1071/AH080415.
- Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8(2):89–99. doi:10.1177/1479972310393756.
- Hayton C, Clark A, Olive S, et al. Barriers to pulmonary rehabilitation: characteristics that predict patient attendance and adherence. Respir Med. 2013;107(3):401–407. doi:10.1016/j.rmed.2012.11.016.
- Jones SE, Green SA, Clark AL, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax. 2014;69(2):181–182. doi:10.1136/thoraxjnl-2013-204227.
- Vanfleteren L, Boonen LMC, Spruit MA, et al. The superexacerbator phenotype in patients with COPD: a descriptive analysis. ERJ Open Res. 2019;5(2):00235-2018. doi:10.1183/23120541.00235-2018.
- Le Rouzic O, Roche N, Cortot AB, et al. Defining the “Frequent Exacerbator” phenotype in COPD. Chest. 2018;153(5):1106–1115. doi:10.1016/j.chest.2017.10.009.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;23:CD003793.
- Chen YW, Leung JM, Sin DD. A systematic review of diagnostic biomarkers of COPD exacerbation. PloS One. 2016;11(7):e0158843. doi:10.1371/journal.pone.0158843.
- Morano M, Mesquita R, Da Silva GPF, et al. Comparison of the effects of pulmonary rehabilitation with chest physical therapy on the levels of fibrinogen and albumin in patients with lung cancer awaiting lung resection: a randomized clinical trial. BMC Pulm Med. 2014;14(1):121. doi:10.1186/1471-2466-14-121.
- Wang S, Liu J, Wu DI, et al. Pro-inflammatory effect of fibrinogen on vascular smooth muscle cells by regulating the expression of PPARα, PPARγ and MMP-9. Biomed Rep. 2015;3(4):513–518. doi:10.3892/br.2015.459.
- Gammal AIE, O’Farrell R, O’Mahony L, et al. Systemic inflammatory markers and disease severity in chronic obstructive pulmonary disease - the effect of acute exercise and pulmonary rehabilitation. OJRD. 2015;05(01):19–31. doi:10.4236/ojrd.2015.51003.
- Oudijk EJ, Nijhuis EH, Zwank MD, et al. Systemic inflammation in COPD visualised by gene profiling in peripheral blood neutrophils. Thorax. 2005;60(7):538–544. doi:10.1136/thx.2004.034009.
- Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi:10.1038/nri2156.
- Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–3521. doi:10.1182/blood.V89.10.3503.
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi:10.1038/nri1785.
- Wittmann S, Rothe G, Schmitz G, et al. Cytokine upregulation of surface antigens correlates to the priming of the neutrophil oxidative burst response. Cytometry. 2004;57A(1):53–62. doi:10.1002/cyto.a.10108.
- Cortjens B, Ingelse SA, Calis JC, et al. Neutrophil subset responses in infants with severe viral respiratory infection. J Clin Immunol. 2017;176:100–106. doi:10.1016/j.clim.2016.12.012.
- Steiner MC, Lowe D, Beckford K, et al. Socioeconomic deprivation and the outcome of pulmonary rehabilitation in England and Wales. Thorax. 2017;72(6):530–537. doi:10.1136/thoraxjnl-2016-209376.
- Maddocks M, Kon SSC, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. doi:10.1136/thoraxjnl-2016-208460.
- Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91(2):142–149. doi:10.1177/0022034511421200.
- Jones AW, Taylor A, Gowler H, et al. Systematic review of interventions to improve patient uptake and completion of pulmonary rehabilitation in COPD. ERJ Open Res. 2017;3(1):00089-2016. doi:10.1183/23120541.00089-2016.