Abstract
Chronic obstructive pulmonary disease (COPD) is a heterogeneous entity with different clinical phenotypes, such as asthma-COPD overlap (ACO). The aim of this retrospective study was to compare routine blood biomarkers in patients with ACO and the remaning COPD phenotypes. Data were collected from stable COPD patients visited in during 2018, including C-reactive protein (CRP), fibrinogen, neutrophyl/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR).A total of 77 patients with COPD were included, 24 (31%) fulfilled the diagnosis of ACO. Clinically, patients with ACO presented more dyspnoea and wheezing. Regarding laboratory parameters, both groups had low levels of lymphocytes, especially the non-ACO group (24.2% vs. 29.3%; p = 0.031), patients with ACO had significantly higher eosinophil counts (4.7% vs. 1.9%; p < 0.001) but a lower percentage of neutrophils (56.8% vs. 64.7%; p = 0.003), NLR and PLR (2.5 vs. 3.8; p = 0.013 and 115 vs. 160; p = 0.063, respectively). In conclusion, besides the expected eosinophilic inflammation in patients with ACO, both groups had low levels of lymphocytes, especially the non-ACO group. The low levels of lymphocytes, in particular in non-ACO patients, should be confirmed in larger studies.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex, multicomponent and heterogeneous entity. Due to its heterogeneity, different clinical phenotypes have been described, being the asthma-COPD overlap (ACO) phenotype one of the most frequently studied [Citation1]. The term ACO describes patients who present features of both asthma and COPD [Citation2]; however, there are no definitive universal diagnostic criteria [Citation3–5]. Therefore, in the last years there has been increased interest in describing systemic biomarkers that could help to differentiate patients with ACO from patients with asthma or COPD. The aim of this retrospective study was to compare routine blood biomarkers in patients diagnosed with ACO and the remaining patients with COPD.
Methods
Data were retrospectively collected from patients visited for stable COPD in one outpatient clinic during 2018. The inclusion criteria were: 40 years of age or older; current or former smokers of at least 10 pack-years and a post-bronchodilator forced spirometry FEV1(forced expiratory volume in the first second)/FVC (forced vital capacity) ratio <0.7. ACO was diagnosed according to the Spanish diagnostic criteria: confirmed COPD with either a concomitant diagnosis of asthma or with one of the following characteristics: very positive bronchodilator test (BDT) (≥15% and ≥400 ml increase in FEV1) and/or blood eosinophilia (≥300 eosinophils/µL) [Citation5]. The exclusion criteria were the presence of another chronic respiratory disease (e.g., cystic fibrosis, severe bronchiectasis, cancer, or restrictive lung disease) or lack of blood eosinophil count in stable phase.
The study was approved by the Research and Ethics Committee of the Vall d’Hebron Hospital. Since the data were retrospectively collected from the electronic clinical records, informed consent was not required.
Sociodemographic and clinical characteristics were collected. Blood biomarkers (blood cell count, C-reactive protein (CRP), fibrinogen, the neutrophyl/lymphocyte ratio (NLR), and the platelet/lymphocyte ratio (PLR)) were analysed in stable phase. The sample characteristics were shown as mean (standard deviation [SD]), frequencies and percentages. Normal ranges were obtained from our laboratory and from published data for NLR [Citation6] and PLR [Citation7]. Characteristics between ACO and non-ACO were compared with the Student’s t-test if they had a normal distribution or the Mann-Whitney U-test if the variables did not follow a normal distribution. The Chi-squared test (Fisher test for frequencies <5) was used for the comparison of categorical variables. Differences were considered significant with a p-value < 0.05. The statistical package SPSS (V 25) was used for the analyses.
Results
From a population of 118 patients with COPD, a total of 77 (65.3%) patients fulfilled all the inclusion criteria and were included in the study. The mean age was 66 years (SD = 8.7), 56 (72.7%) were male, and 19 (24.6%) were active smokers. Twenty-four (31%) patients fulfilled the diagnostic criteria of ACO; all had blood eosinophilia ≥ 300 eosinophils/µL, and 5 (20.8%) also had a diagnosis of asthma. The Charlson index and different comorbidities did not sigificantly differ between the two groups. Regarding symptoms, the ACO group presented a greater degree of dyspnoea, more frequently having a modified Medical Research Council (mMRC) ≥2 (75% vs. 50.9%, p = 0.047), more wheezing (54.2% vs. 22.6%, p = 0.004) and more frequently received treatment with inhaled corticosteroids (ICS) (79.2% vs. 54.7%, p = 0.04) (). Laboratory findings showed that both groups of patients had an increased number of individuals with leucocytes and neutrophils above the normal range and, in particular, the non-ACO group had 32% and 17% of patients with lymphocytes in percentage and absolute numbers below normal range. Lymphocytes in numbers and percentages were significantly higher in ACO patients and, consequently, they had a lower NLR, and albeit non-significant, a lower PLR compared with patients with COPD ( and ). Other biomarkers, such as fibrinogen and CRP, were not significantly different between ACO and COPD patients ().
Figure 1. (A) Scatterplot of individual data of the neutrophil/lymphocyte ratio (NLR) according to the diagnosis of ACO. Dotted lines correspond to the normal reference values. (B) Scatterplot of individual data the platelet/lymphocyte ratio (PLR) according to the diagnosis of ACO. Dotted lines correspond to the normal reference values.
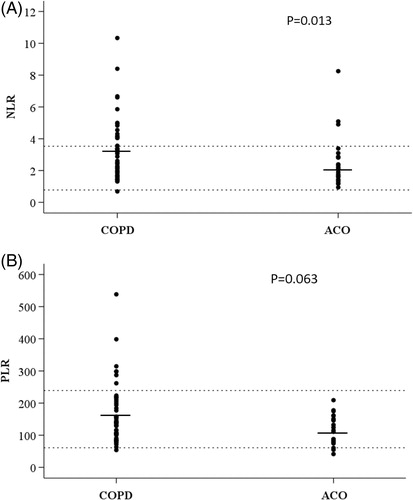
Table 1. Demographic and clinical characteristics of ACO patients and the remaining COPD patients.
Table 2. Laboratory findings of ACO patients and the remaining COPD patients.
Discussion
Almost one third of patients in the present cohort were classified as ACO according to Spanish diagnostic criteria. This finding is consistent with other studies reporting a prevalence of ACO ranging from 13% to 29% of patients with COPD [Citation8–12]. From a clinical point of view, it is well known that patients with ACO more frequently have respiratory symptoms and a higher risk of exacerbations compared to other COPD phenotypes [Citation8, Citation9]. Our results confirm that ACO patients have a greater degree of dyspnoea and more frequently report wheezing as the main symptom. However, regarding exacerbations, no significant differences were found between the two groups, perhaps due to the small sample size of the study.
As expected, ACO patients presented a higher blood eosinophil count than the remaining COPD phenotypes in which the neutrophilic trait prevailed. It is well known that the ACO phenotype is more frequently characterised by a greater degree of bronchial and peripheral eosinophilic inflammation compared to other COPD phenotypes [Citation13]. Moreover, it has been demonstrated that the blood eosinophil count is a reliable marker of Th2 inflammation and is associated with asthma-related characteristics, such as increased bronchodilator responsiveness and more importantly, a favourable response to ICS [Citation13–15]. ACO patients also had significantly increased basophil concentrations, although the absolute difference was small and of uncertain clinical significance.
Beyond eosinophils, we observed low levels of lymphocytes in both grups of patients, but significantly more reduced in non-ACO group. As a consequence, both PLR and especially NLR were reduced in ACO compared to COPD. Elevated NLR can identify COPD patients at increased risk of exacerbations [Citation16] and elevated NLR and PLR are reliable predictors of mortality after discharge due to an exacerbation of COPD [Citation17]. The lower NLR found in our ACO patients may be associated with the better prognosis of this phenotype, since a lower NLR was found to be associated with the better long-term prognosis of patients with ACO demonstrated in prospective studies [Citation18]. Similar to our results, Aksoy et al., also found a lower NLR in COPD patients with an eosinophilic phenotype [Citation19]. Another routine blood biomarker that has been proposed is the PLR, which has demonstrated to be a useful predictor of mortality after discharge in patients with an exacerbation of COPD [Citation17]. Similar to the NLR, patients with ACO in our cohort had a lower, albeit non statistically significant, PLR compared to non-ACO patients; and again, this lower level of PLR is consistent with the lower mortality observed in patients with ACO [Citation18]. These findings might be explained by the fact that patients with an eosinophilic phenotype present a better clinical course than non-eosinophilic COPD patients and therefore, predictors of mortality might be reduced [Citation18]. Finally, we did not find significant differences between groups in CRP or fibrinogen values, in accordance with the results of other studies [Citation20, Citation21].
The main limitation of this study was the cross-sectional design that did not allow analysis of the predictive value of these biomarkers, making larger prospective studies necessary.Since one of the criterion for ACO is a very positive bronchodilator test in COPD, the lack of bronchodilator test results in some of our patients may have induced some misclassification. However, previous results by Perez de Llano et al. [Citation11] showed that no patient was classified with ACO with only the criterion of large reversibility, thereby making misclassification in the present study very unlikely. It is well known that smoking habits and comorbidities can influence inflammatory status; however, no significant differences in these variables were observed between the two groups of patients; thereby suggesting that they did not influence our results. Another limitation was that patients were recruited and treated at a single centre and our cohort may not be representative of all the COPD population.
In conclusion, besides the expected higher eosinophilic inflammation presented by ACO patients, they also showed a lower NLR and PLR compared with the remaining COPD patients. The lower NLR and PLR are consistent with the reduced mortality in patients with ACO described in prospective studies. It would be interesting to investigate if the differences in limphocytes found in our study can be confirmed in larger multicenter sudies.
Conflicts of interest
Giordana Gava from the University of Sassari (Italy) was the recipient of an Erasmus Placement for a short stay at the Pneumology Department of University Hospital Vall d’Hebron (Barcelona, Spain). Alexa Nuñez is the recipient of a Rio Hortega contract in the 2019 Strategic Action Health Call from the Instituto de Salud Carlos III for the years 2020–2022. Miriam Barrecheguren is the recipient of a Rio Hortega contract in the 2017 Strategic Action Health Call from the Instituto de Salud Carlos III for the years 2018–2019. Cristina Esquinas has received speaker fees from CSL Behring. Pietro Pirina has received speaker fees from Chiesi, Menarini, Novartis, Mundifarma, GlaxoSmithKline, all unrelated to this manuscript. Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols, unrelated to this manuscript. Miriam Barrecheguren has received speaker fees from Grifols, Menarini, CSL Behring, GSK and consulting fees from GSK, Novartis and Gebro Pharma. The remaining authors report no other conflicts of interest in this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48(3):86–98. doi:10.1016/j.arbres.2011.10.007.
- Barrecheguren M, Esquinas C, Miravitlles M. The asthma COPD overlap syndrome (ACOS). Opportunities and challenges. Curr Opin Pulm Med. 2015;21(1):74–79. doi:10.1097/MCP.0000000000000118.
- GINA and Global Initiative for Chronic Obstructive Lung Disease (GOLD) [webpage on the Internet]. Asthma, COPD, and asthma-COPD overlap syndrome. GINA and Global Initiative for Chronic Obstructive Lung Disease; 2015 [accessed 2019 Oct 10]. Available from: http://www.goldcopd.org/asthma-copd-overlap.html
- Soler-Cataluña JJ, Novella L, Soler C, et al. Clinical characteristics and risk of exacerbations associated with different diagnostic criteria of asthma-COPD overlap. Arch Bronconeumol. 2019. doi:10.1016/j.arbres.2019.08.023.[Epub ahead of print]
- Plaza V, Alvarez F, Calle M, et al. Consensus on the asthma-COPD overlap syndrome (ACOS) between the Spanish COPD guidelines (GesEPOC) and the Spanish guidelines on the management of asthma (GEMA). Arch Bronconeumol. 2017;53(8):443–449.
- Forget P, Khalifa C, Defour JP, et al. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. doi:10.1186/s13104-016-2335-5.
- Fest J, Ruiter R, Ikram MA, et al. Reference values for white blood-cell-based inflammatory markers in the Rotterdam study: a population-based prospective cohort study. Sci Rep. 2018; 8(1):10566. doi:10.1038/s41598-018-28646-w.
- Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12(1):127. doi:10.1186/1465-9921-12-127.
- Menezes AM, de Oca MM, Perez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype COPD asthma. Chest. 2014;145(2):297–304. doi:10.1378/chest.13-0622.
- Wurst KE, Rheault TR, Edwards L, et al. A comparison of COPD patients with and without ACOS in the ECLIPSE study. Eur Respir J. 2016;47(5):1559–1562. doi:10.1183/13993003.02045-2015.
- Pérez de Llano L, Cosío BG, Miravitlles M, et al. Accuracy of a new algorithm to identify asthma-COPD overlap (ACO) patients in a cohort of patients with chronic obstructive airway disease. Arch Bronconeumol. 2018;54(4):198–204. doi:10.1016/j.arbres.2017.10.007.
- Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060. doi:10.1016/j.rmed.2013.03.007.
- Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758–766. doi:10.1164/rccm.201408-1458OC.
- Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X.
- Nuñez A, Sarasate M, Loeb E, et al. Practical guide to the identification and diagnosis of asthma-COPD overlap (ACO). COPD J Chronic Obstruct Pulm Dis. 2019;16(1):1–7. doi:10.1080/15412555.2019.1575802.
- Acartürk Tunçay E, Karakurt Z, Aksoy E, et al. Eosinophilic and non-eosinophilic COPD patients with chronic respiratory failure: neutrophil-to-lymphocyte ratio as an exacerbation marker. Int J Chronic Obstruct Pulm Dis. 2017;12:3361–3370. doi:10.2147/COPD.S147261.
- Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chronic Obstruct Pulm Dis. 2017;12:2285–2290. doi:10.2147/COPD.S141760.
- Suzuki M, Makita H, Konno S, et al. Asthma-like features and clinical course of chronic obstructive pulmonary disease. An analysis from the Hokkaido COPD cohort study. Am J Respir Crit Care Med. 2016;194(11):1358–1365. doi:10.1164/rccm.201602-0353OC.
- Aksoy E, Karakurt Z, Gungor S, et al. Neutrophil to lymphocyte ratio is a better indicator of COPD exacerbation severity in neutrophilic endotypes than eosinophilic endotypes. Int J Chronic Obstruct Pulm Dis. 2018;13:2721–2730. doi:10.2147/COPD.S170353.
- Fu JJ, McDonald VM, Gibson PG, et al. Systemic inflammation in older adults with asthma-COPD overlap syndrome. Allergy Asthma Immunol Res. 2014;6(4):316–324. doi:10.4168/aair.2014.6.4.316.
- Nuñez A, Marras V, Harlander M, et al. Association between routine blood biomarkers and clinical phenotypes and exacerbations in chronic obstructive pulmonary disease. Int J Chronic Obstruct Pulm Dis. 2020;15:681–690. doi:10.2147/COPD.S240720.
