Abstract
Cognitive and motor impairment are well documented in chronic obstructive pulmonary disease (COPD) patients, but their relationship has not been studied. This study evaluated and compared cognitive and motor performance during dual tasks and related dorsolateral prefrontal cortex (PFC) changes in oxygenated hemoglobin (ΔO2Hb), a proxy measure of neural activity, in patients with COPD and age-matched healthy individuals. Participants performed three single tasks: (1) backwards spelling cognitive task; (2) 30 m preferred paced walk; (3) 30 m fast walk, and two dual tasks: (4) preferred paced walk + backwards spelling; (5) fast paced walk + backwards spelling. The ΔO2Hb from left and right dorsolateral PFC were measured using functional near-infrared spectroscopy. Gait velocity was measured using a Zeno walkway. Compared to healthy adults (n = 20), patients with COPD (n = 15) had higher ΔO2Hb during single preferred (-0.344 ± 0.185 vs. 0.325 ± 0.208 µM; p = 0.011) and fast paced walk (-0.249 ± 0.120 vs. 0.486 ± 0.182 µM; p = 0.001) in right PFC. Among healthy adults, ΔO2Hb were higher bilaterally during preferred paced walking dual versus single task (right: 0.096 ± 0.159 vs. −0.344 ± 0.185 µM, p = 0.013; left: 0.114 ± 0.150 vs. −0.257 ± 0.175 µM, p = 0.049) and in right PFC during fast walking dual versus single task (0.102 ± 0.228 vs. −0.249 ± 0.120, p = 0.021). Patients with COPD did not increase O2Hb during dual versus single tasks. Patients with COPD exhibited slower velocity than older adults during all walking tasks. The lack of further increase in O2Hb from single to dual tasks in patients with COPD, may indicate reduced cognitive-motor capacity and contribute to poorer motor performance limiting safe ambulation. Dual tasking rehabilitation may improve neural efficiency to offset these risks.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease that shows a high prevalence and a worldwide impact on morbidity and mortality [Citation1]. In addition to the pulmonary manifestations, it is now well accepted that COPD has systemic effects [Citation2]. Cognitive impairment is well-documented in patients with COPD, with a prevalence ranging from 27 to 61% [Citation3–6]. The underlying pathophysiology has been partially attributed to the effects of hypoxemia on neural tissue, which is particularly vulnerable to injury from prolonged hypoxic stress [Citation3, Citation7]. Although cognitive impairment appears to be worse in hypoxemic patients, findings are inconsistent and abnormalities are still found in stable non-hypoxemic patients [Citation3].
Regarding the brain regions, alterations in the frontal cortex are common in COPD [Citation8] along with the associated cognitive impairments [Citation9]. Activation in the dorsolateral prefrontal cortex (PFC) has been linked to tasks that require attention and working memory, and is involved in motor planning and regulation [Citation10–12]. Cognitive impairment has been linked to an increased risk of falls in patients with COPD [Citation13]. The rate of falls has been reported to be five times higher in individuals with COPD compared to healthy adults [Citation14]. These cognitive functions are required during simple walking and to a greater extent during complex walking that has higher balance demands (e.g., when maneuvering around obstacles) [Citation15]. Structural changes in the PFC, as a result of aging, are found to be greater in patients with COPD compared to older adults [Citation16]. However, relationships between executive function and motor performance have not yet been investigated in people with COPD.
Performing two tasks simultaneously is termed dual tasking. Performing challenging tasks requires greater cognitive resources leading to an increase in blood flow to the area of high neural activity and corresponding increased oxygenated hemoglobin (O2Hb). Since cognitive resources are limited, splitting them amongst tasks during dual tasking can lead to errors in performance of either one or both tasks, termed dual task interference [Citation17]. Although, patients with COPD are known to have both cognitive and motor dysfunction, the dual task effect has not been widely considered [Citation18]; thus, rehabilitation protocols are not focused on assessing or strengthening dual task performance. One approach to evaluate the association between neural activity and motor performance is using a noninvasive neuroimaging technique, functional near-infrared spectroscopy (fNIRS) during dual tasking [Citation19–21].
The fNIRS provides a measure of neural activity by quantifying changes in O2Hb [Citation22]. Although, functional magnetic resonance imaging (fMRI) is the conventional neuroimaging method that indirectly measures neuronal activity using blood-oxygen-level dependent imaging (BOLD), its use is limited during tasks that involve mobility, unlike fNIRS [Citation23]. Examining dual task interference while monitoring dorsolateral PFC ΔO2Hb will provide insight into deficits of cognitive function in patients with COPD during mobility tasks.
Measurement of brain activity from dorsolateral PFC during a dual tasking paradigm in patients with COPD can provide a model of competing demands of daily living activities that require walking and a cognitive task. Moreover, elucidation of factors other than a COPD diagnosis that are linked to the changes in brain activity and cognitive-motor performance especially during dual tasking may be important to inform approaches in pulmonary rehabilitation and other therapies to improve mobility and cognitive function in patients with COPD. The purpose of this study was to compare dual task interference and related dorsolateral PFC oxygenation (to estimate neural activity) in patients with COPD and age-matched healthy individuals. We hypothesized that dual tasks will result in a greater increase in dorsolateral PFC O2Hb and decline in performance compared to single tasks in patients with COPD compared to healthy adults. A second purpose was to determine the association of FEV1 (% predicted), single leg stance duration and Montreal Cognitive Assessment (MoCA) scores to: (i) right PFC ΔO2Hb; and (ii) left PFC ΔO2Hb. We hypothesized that reduced FEV1 (% predicted), shorter single leg stance duration and low MoCA scores would be associated with higher O2Hb and lower cognitive and motor performance.
Materials and methods
Participants
Twenty healthy older adults were recruited from the University of Toronto, University Health Network associated hospitals and community centers in Toronto, Canada. Patients with COPD (n = 15) were recruited from West Park Healthcare Center, Toronto Western Hospital and the Abilities Center in Whitby, Ontario. Participants were age matched within each group. Inclusion criterion for both groups included men and women aged ≥55 years. Individuals in both groups were excluded if they: experienced an acute illness or an acute exacerbation during the last 3 months; were current smokers; had unstable cardiovascular, neurological or musculoskeletal conditions that interfered with independent ambulation or ability to stand on a single leg; consumed oral corticosteroids within the last 3 months; had cognitive impairment or a lack of English fluency that interfered with providing informed consent or following study instructions. In addition, patients with COPD requiring supplemental oxygen or a gait aid during walking were also excluded.
Study protocol
Participants were screened with the American College of Sports Medicine (ACSM) questionnaire to determine their eligibility to safely perform exercise. Using a handheld spirometer (copd-6TM, model 4000, Vitalograph Ltd., Ennis, Ireland) and standardized procedures [24], healthy people had the following measures evaluated and expressed as predicted values: forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio. Post-bronchodilator spirometry data were obtained from charts of patients with COPD.
The following questionnaires were administered: Digit Span test [Citation25], MoCA [Citation26], Medication and Comorbidities Questionnaire [Citation27–29] and International Physical Activity Questionnaire (IPAQ) [Citation30]. Participants performed a single leg stance test, a reliable measure to evaluate balance, an element of walking, [Citation31–34] and then performed three single and two dual tasks, each separated by a 1-minute baseline task. The baseline task asked participants to spell words forward from flashcards to normalize neural activity and minimize the carryover effect onto the subsequent tasks. Single tasks were performed in random order and included: (1) backwards spelling cognitive task, (2) preferred paced walk and (3) fast paced walk. Dual tasks were also randomly ordered and involved (4) preferred paced walk + backwards spelling and (5) fast paced walk + backwards spelling. For the cognitive task, participants spelled five letter words backwards from a list of 100 words for one minute. Care was taken to not include homonymns. During the motor tasks, the participants walked 6 times (∼30 m) across a 5 × 0.88 m pressure sensitive Zeno walkway (ProtoKinetics Movement Analysis Software; http://www.protokinetics.com/) that contains a grid of 13,824 pressure sensors. During preferred paced walk, velocity was measured while participants walked at their usual pace whereas during the fast walk, velocity was measured while they walked as quickly and safely as possible. Scripted instructions were kept neutral to avoid any indication of prioritizing one task over the other during dual tasking.
The two walking speeds were chosen (preferred paced and fast paced) to mimic activities of daily living because these speeds are commonly used in other investigations of older adults and patients with COPD [Citation18, Citation35]. Fast walking might mimic hurried walking required to cross the street whereas preferred paced walking may simulate walking such as in the home or out to the garage.
Questionnaires
During the Digit Span test [Citation25], an investigator recited sequences up to 5 digits that participants were instructed to recall in same (Digit Span Forward) or reverse order (Digit Span Backward). Digit Span Backward is an assessment of working memory and attention that requires an individual to hold and manipulate information before responding [Citation25]. MoCA is a valid and reliable tool to screen for cognitive dysfunction in various populations in which a score below 26 out of 30 suggests mild cognitive impairment [Citation26]. Although, Mini Mental State Examination and MoCA are similar cognitive screening tools, MoCA is more sensitive in detecting mild cognitive impairment [Citation36]. The long form IPAQ [Citation30] used contains 27 questions that pertained to physical activity during various tasks, intensities and settings in the past 7 days: at work, at home (inside and the yard), to get from place to place and during recreational activities. The IPAQ provides an overall physical activity level of participants based on their Metabolic Equivalent Task minutes (MET-min) per week.
Functional near infrared spectroscopy (fNIRS)
The fNIRS device (FNIR100W-1, wireless fNIR Imager System, BIOPAC) was secured over the participants’ forehead [Citation37, Citation38] and the center of each sensor pad was vertically aligned with the iris. Light intensity raw data at two wavelengths (730 nm and 850 nm) were obtained during single and dual tasks from left and right dorsolateral PFC using the Cognitive Optical Brain Imaging (COBI) Studio (fNIR Devices, LLC) at a sampling frequency of 4 Hz. LED current and detector gain settings were adjusted for each participant to prevent low or saturated light signals. A low-pass, finite impulse response, filter with hamming order 57 and cutoff frequency of 0.05 was applied to attenuate physiological artifacts of respiration and pulse. Time markers placed at the onset and end of each task in COBI Studio were used to extract data blocks and calculate relative ΔO2Hb using the modified Beer-Lambert law.
Analysis
The dorsolateral PFC ΔO2Hb data were acquired via fNIRS device and processed in fnirSoft. Statistical analyses were performed using R (R Foundation for Statistical Computing). Normality of the data was tested using Shapiro-Wilk test. Outliers were examined using the Tukey Fences method where values smaller than Q1 - 1.5*IQR and larger than Q3 + 1.5*IQR (Q = quartile; IQR = interquartile range) were removed [Citation39]. Participant characteristics were compared using unpaired t-tests and Mann–Whitney U test for non-normal data. The left and right dorsolateral PFC ΔO2Hb, gait velocity and backwards spelling accuracy were compared between tasks (within subject factors) and groups (between subject factors) using mixed analysis of variance (ANOVA). Within subject factors included pairs of single and dual tasks: (1) preferred paced walk and preferred paced walk + backwards spelling; (2) fast paced walk and fast paced walk + backwards spelling; (3) backwards spelling and preferred paced walk + backwards spelling; (4) backwards spelling and fast paced walk + backwards spelling. Interactions between tasks and groups were also analyzed. Post hoc comparisons were applied using paired or unpaired t-tests. In addition, dual task effects on velocity and backwards spelling accuracy were calculated using:
Dual task effect (%) = [(dual task – single task)/single task] * 100% [Citation17]
With pooled data of both groups, multiple linear regression analyses were performed to determine the association of FEV1 (% predicted), single leg stance duration and MoCA scores to: (i) the right PFC ΔO2Hb; and (ii) the left PFC ΔO2Hb.
Results
Twenty healthy older adults and 15 patients with COPD completed the study. The two groups were similar in age, height, weight and body mass index (). Patients with COPD had a lower FEV1 (% predicted) compared to healthy adults (52.3 ± 20.3 versus 92.6 ± 11.7, respectively, p < 0.001) and 2, 7, 4, and 2 patients with COPD were categorized as mild (FEV1 ≥ 80%), moderate (50% ≤ FEV1 < 80%), severe (30% ≤ FEV1 < 50%) or very severe (FEV1 < 30%), respectively. Patients with COPD had lower MoCA scores (24.2 ± 2.6 vs. 26.1 ± 2.9, p = 0.032) and reported a trend of 64% lower physical activity level on the IPAQ (p = 0.091) compared to healthy older adults. Patients with COPD reported 2.3-fold more comorbidities (p = 0.001) and 3.9-fold more medications (p < 0.001). The single leg stance duration for patients with COPD was 69% lower compared to healthy adults (p = 0.008).
Table 1. Characteristics of healthy older adults and patients with COPD.
For right dorsolateral PFC, a difference of borderline statistical significance was found for ΔO2Hb between single and dual preferred paced walking (p = 0.056) using mixed ANOVA. Notably, a higher ΔO2Hb in right dorsolateral PFC was shown in patients with COPD compared to healthy adults for both single walking tasks (preferred paced walking p = 0.011; fast walking p = 0.001). Within group analyses indicated that in healthy adults, O2Hb increased in right dorsolateral PFC during both dual compared to single tasks (preferred paced walking: 0.096 ± 0.159 vs. −0.344 ± 0.185 µM, p = 0.013; fast walking: 0.102 ± 0.228 vs. −0.249 ± 0.120 µM, p = 0.021) (). In contrast, patients with COPD did not demonstrate increases in right dorsolateral PFC O2Hb when performing dual compared to single tasks (preferred paced walking: 0.519 ± 0.270 vs. 0.325 ± 0.208 µM, p = 0.309; fast walking: 0.522 ± 0.338 vs. 0.486 ± 0.182 µM, p = 0.466). Based on effect sizes from between group differences of right dorsolateral PFC ΔO2Hb (d=.47 for preferred paced walking dual task; d=.36 for fast walking dual task), estimated sample sizes for preferred and fast paced walking dual task were 56 per group and 98 per group, respectively, at a power of 0.80 and alpha of 0.05.
Figure 1. The ΔO2Hb in the right (A) and left (B) dorsolateral PFC during the preferred and fast paced walking single and dual tasks in healthy adults and patients with COPD. The ΔO2Hb during preferred and fast walk were higher in patients with COPD compared to older adults in the right dorsolateral PFC. In older adults, ΔO2Hb was higher during preferred paced walking dual versus single task in the right and left dorsolateral PFC and during fast paced walking dual versus single task in the right dorsolateral PFC (A).
* indicates statistical significance at p < 0.05. BS: backwards spelling; FPW: fast paced walking; PPW: preferred paced walk.
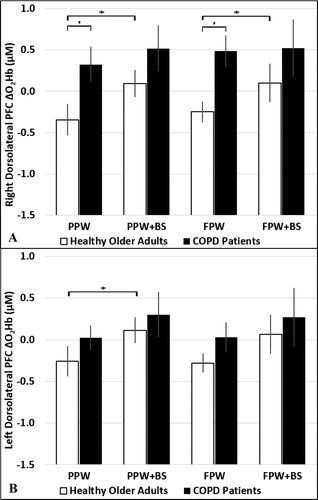
The effect of single and dual preferred paced tasks on left dorsolateral PFC ΔO2Hb was significantly different (p = 0.025) and was of marginal significance for fast walking single and dual tasks (p = 0.053) in healthy adults and patients with COPD, respectively. In healthy adults, post hoc analysis indicated higher left dorsolateral PFC ΔO2Hb during dual versus single preferred (0.114 ± 0.150 vs. −0.257 ± 0.175 µM, p = 0.049), but not fast paced walking (0.064 ± 0.230 vs. −0.279 ± 0.107 µM, p = 0.064) (). Similar to the right dorsolateral PFC ΔO2Hb, patients with COPD did not demonstrate significant increases when performing dual versus single tasks (preferred paced: 0.302 ± 0.269 vs. 0.025 ± 0.141 µM, p = 0.130; fast paced: 0.271 ± 0.346 vs. 0.029 ± 0.178 µM, p = 0.207).
Mixed ANOVA indicated that the effect of preferred (p < 0.001) and fast paced walking (p < 0.001) single and dual tasks was significantly different on velocity for older adults and patients with COPD. Post hoc analysis indicated that patients with COPD had a significantly slower velocity than healthy adults during all single and dual tasks (p < 0.035) (). In addition, within group analyses showed that both older adults and patients with COPD walked more slowly during both dual tasks compared to single tasks (). However, the dual task effects (% decrement) of preferred paced walk dual task (-11.7 ± 12.8% vs. −6.3 ± 15.0%, p = 0.130) and fast paced walk dual task (-19.9 ± 12.4% vs. −17.2 ± 6.6%, p = 0.226) on velocity were not different between older adults and patients with COPD, respectively.
Table 2. Performance of healthy older adults and patients with COPD on cognitive and motor tasks.
The effects of backwards spelling single and preferred paced walking dual task (p < 0.001) as well as backwards spelling single and fast paced walking dual task (p < 0.001) were significantly different on number of words spelled accurately in older adults and patients with COPD. Post hoc analysis indicated that dual compared to single tasks significantly reduced the number of words spelled correctly, but no between group differences were found (). Moreover, the dual task effects on accuracy of backwards spelling did not differ between older adults and patients with COPD during both dual tasks (preferred paced walking: -33.0 ± 28.8% vs. −14.3 ± 89.1%, p = 0.120; fast paced walking: −43.2 ± 18.6% vs. −16.2 ± 116.8%, p = 0.165, respectively). No significant interaction between groups and tasks was found for any of the mixed ANOVA analyses.
Regression analysis indicated that FEV1 explained 25.4% of the variance in ΔO2Hb in the left dorsolateral PFC during preferred paced walking dual task (). The results suggest that for each additional % predicted increase in FEV1, the ΔO2Hb decreased by 0.013 µM. MoCA scores and single leg stance duration did not affect ΔO2Hb in the right dorsolateral PFC.
Table 3. Summary of regression statistics for the predictor variables.
Discussion
Novel findings of this study were that ΔO2Hb in right dorsolateral PFC were significantly higher during preferred and fast paced walking single tasks in patients with COPD compared to healthy older adults. Moreover, for healthy adults, ΔO2Hb increased bilaterally in the dorsolateral PFC during preferred paced walking dual versus single task, but only increased in the right dorsolateral PFC during fast walking dual versus single task. In contrast, for patients with COPD, no significant differences in ΔO2Hb during dual compared to single tasks were found for both the left and the right dorsolateral PFC. Multiple linear regression analyses indicated that lower lung function was associated with greater increases in neural activity during the preferred paced walking dual task in the right dorsolateral PFC. Lastly, patients with COPD exhibited slower velocity than older adults during all walking tasks.
In patients with COPD, the right dorsolateral PFC ΔO2Hb was higher during single task walking compared to older adults but did not increase further during dual task walking. Individuals with COPD have a high prevalence of cognitive impairment, as also indicated by the MoCA scores of the sample in this study. Diminished cognitive ability in patients with COPD may have resulted in these participants requiring a greater level of neural activity to perform single tasks compared to older adults, and thus resulted in a higher ΔO2Hb. Walking fast especially with simultaneous performance of a cognitive task is complex with increased cognitive demands. Thus, over-activation of the PFC in the more cognitively impaired COPD group during fast paced walking dual task may have lead to reaching a ceiling of the available cognitive resources and/or oxygen delivery that may explain leveling off of the ΔO2Hb during dual tasks and the consequent deficits in cognitive and motor performance [Citation40]. Higher cognitive demands to perform single tasks and limited capacity for further increases during dual tasks resulted in a lack of differences from single to dual tasks in patients with COPD and during dual tasks between the two groups.
Neural activity in the left and the right dorsolateral PFC is observed during tasks that involve various cognitive functions (e.g., attention, working memory) [Citation41]. In this study, ΔO2Hb were higher bilaterally during preferred paced walking dual compared to single task and during fast walking dual compared to single task in the right dorsolateral PFC in healthy adults. An increase in O2Hb in the left PFC occurs during tasks involving divided attention (e.g., dual tasking), semantic memory retrieval and language (e.g., generation of words) [Citation41, Citation42], while tasks that involve sustained attention lead to increased O2Hb in the right PFC. Moreover, an overlap in activity in the left and right PFC is observed during tasks that involve manipulation of information in the working memory [Citation43]. Dual tasks, in this study, required greater utilization of working memory to store 5 letter words, while maintaining sustained attention to spell them backwards, unlike the single walking tasks that didn’t require backwards spelling. This led to higher increase in the right dorsolateral PFC O2Hb compared to the left that is involved in cognitive functions such as semantic memory. Holtzer et al. [Citation20] observed greater increase in O2Hb bilaterally during preferred paced walking dual versus single task. The findings of the present study corroborate the previous findings as walking at a preferred pace and performing a cognitively challenging task requires division of attentional resources towards walking, spelling and vocalizing the response, in addition to maintaining sustained attention to minimize the risk of falls from loss of balance and focus on steps. Moreover, backwards spelling requires manipulation of the words to accurately reverse the sequence of letters, hence, eliciting a greater bilateral hemodynamic response during the preferred paced walking dual compared to the single task. Locomotor tasks with increasing cognitive load result in progressive activation of higher brain centers (e.g., dorsolateral PFC) [Citation44], which may be due to higher cognitive demands to maintain balance during this complex fast walking dual task.
Patients with COPD exhibited slower velocity during all single and dual walking tasks compared to healthy older adults; however, backwards spelling accuracy did not differ between the two groups. Reducing gait speed is a potential compensatory strategy to reduce the complexity of the task being performed in order to maintain postural stability and balance [Citation45]. However, reduced gait speed is associated with diminished functional capacity [Citation46] and an increased risk of falls [Citation47]. Decrements in performance during dual tasking can be explained by the resource allocation theory which states that from a limited pool of processing resources, if more resources are allocated to one task, it leaves less resources for the second task [Citation48]. Depending on the task that is prioritized, decrements in the other task’s performance will occur. Moreover, since not all resources are allocated to one task, errors in the performance of both tasks might occur [Citation48]. The results of this study suggest that patients with COPD faced with dual cognitive and motor tasks prioritized the cognitive task, leading to greater decrements in walking compared to healthy older adults and similar performance in the backwards spelling task.
Decrements in gait velocity during dual compared to single tasks may negatively impact quality of life of patients with COPD. Individuals with COPD execute many activities of daily living at a preferred pace and avoid walking fast to conserve energy and cognitive resources. Not being able to cross a long street before the signal changes can induce anxiety and stress. This may force patients with COPD to try walking fast, utilizing their limited resources and potentially increasing their risk of falls. Walking and talking is a significant predictor of falls and a common activity of daily living [Citation49]. Our observation that patients with COPD favored accuracy of backwards spelling over maintaining their walking speed supports the previous findings that patients with COPD are at increased risk of falls. Regularly multitasking, but with decrements in performance can reduce the quality of life. Therefore, determining the neural correlates of cognitive-motor performance during dual tasking can provide insight into the cognitive demands associated with daily tasks and could help to devise appropriate rehabilitation interventions for the elderly and patients with COPD to reduce risk of falls.
The methodology of this study includes some limitations. A convenience sample of older adults and patients with COPD was used creating a potential for sampling bias; thus, the sample may not be representative of population outside of Greater Toronto Area. Moreover, the cognitive task used may not be challenging enough for some participants depending on their education level and experience that may have biased the neural response elicited during single and dual tasks. Lastly, the fNIRS device used in this study provides relative, rather than absolute, concentration of ΔO2Hb; thus, baseline levels of O2Hb and any change in oxygen saturation during tasks, which could not be measured due to distraction while walking, may have confounded the change elicited by the cognitive and motor tasks performed. Future studies should utilize a larger sample size and fNIRS devices with measurement capabilities that span a greater surface area of the head. This will provide a more in-depth understanding of the changes in neural activity during cognitive and motor tasks. Moreover, a more challenging cognitive task may produce a significant dual task effect on its accuracy. Lastly, the potential ceiling effect of neural activity or oxygen delivery observed during dual tasks in patients with COPD can be further explored by administering supplemental oxygen during this experimental protocol.
In conclusion, ΔO2Hb in left and right dorsolateral PFC were higher during single task walking in patients with COPD compared to healthy adults but did not increase further during dual walking tasks in patients with COPD. In contrast, the ΔO2Hb increased during the preferred and fast paced walking dual tasks compared to the respective single tasks in the right dorsolateral PFC in healthy adults. FEV1 predicted the direction of ΔO2Hb in the right PFC during the preferred paced walking dual task. Moreover, patients with COPD walked slower than healthy adults during all walking tasks and tended to have lower accuracy in the performance of the cognitive task. Lower performance in cognitive and motor performance and a higher ΔO2Hb during single tasks in patients with COPD compared to older adults may be indicative of greater proportional cognitive requirement, neural inefficiency or competition for limited cognitive resources to perform the tasks. The reduced cognitive-motor performance during dual tasks suggests that rehabilitation should involve cognitive-motor interventions to improve cognition and enhance physical function to reduce the risk of falls in the elderly and patients with COPD. The cognitive and motor tasks used that mimic activities of daily living (walking and engaging in a cognitively challenging task) may ease the transition of this protocol and findings into clinical rehabilitation settings.
Acknowledgments
The study was approved by University of Toronto’s Research Ethics Board (protocol ID: 33466). The authors would like to acknowledge Dr. Hasan Ayaz for his technical support with fNIRS data, Meeran Manji, coordinator of the Pulmonary Rehabilitation Clinic at Toronto Western Hospital and a registered nurse, for her assistance with participant recruitment and Tyler Saumur for his assistance with the regression analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Global burden of COPD - López‐Campos - 2016 - Respirology - Wiley Online Library [Internet]. [cited 2019 Jul 11]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/resp.12660.
- Malerba M, Radaeli A, Olivini A, et al. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed Res Int. 2014;2014:271918.
- Grant I, Heaton RK, McSweeny AJ, et al. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142(8):1470–1476.
- Grant I, Prigatano GP, Heaton RK, et al. Progressive neuropsychologic impairment and hypoxemia. Relationship in chronic obstructive pulmonary disease. Arch Gen Psychiatry. 1987;44(11):999–1006.
- Villeneuve S, Pepin V, Rahayel S, et al. Mild cognitive impairment in moderate to severe COPD: a preliminary study. Chest. 2012;142(6):1516–1523.
- Torres-Sánchez I, Rodríguez-Alzueta E, Cabrera-Martos I, et al. Cognitive impairment in COPD: a systematic review. J Bras Pneumol. 2015;41(2):182–190.
- Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922.
- Yin M, Wang H, Hu X, et al. Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm Med. 2019;19(1):203.
- Karamanli H, Ilik F, Kayhan F, et al. Assessment of cognitive impairment in long-term oxygen therapy-dependent COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:2087–2094.
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9(4):637–671.
- MacDonald AW, Cohen JD, Stenger VA, et al. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838.
- Ruocco AC, Rodrigo AH, Lam J, et al. A problem-solving task specialized for functional neuroimaging: validation of the Scarborough adaptation of the Tower of London (S-TOL) using near-infrared spectroscopy. Front Hum Neurosci. 2014;8:1–13. [Internet]. [cited 2018 Aug 5]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3975118/.
- Oancea C, Tudorache E, Tudorache V. COPD - An update in pathogenesis and clinical management. In: Cormac M, editor. Neurocognitive impairment as systemic effects of COPD. London: IntechOpen; 2018: 67–86. [Internet]. [cited 2020 Mar 30]. Available from: https://www.intechopen.com/books/copd-an-update-in-pathogenesis-and-clinical-management/neurocognitive-impairment-as-systemic-effects-of-copd.
- Roig M, Eng JJ, MacIntyre DL, et al. Falls in people with chronic obstructive pulmonary disease: an observational cohort study. Respir Med. 2011;105(3):461–469.
- Chen M, Pillemer S, England S, et al. Neural correlates of obstacle negotiation in older adults: An fNIRS study. Gait Posture. 2017;58:130–135.
- Dodd JW, Chung AW, van den Broek MD, et al. Brain structure and function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(3):240–245.
- Plummer P, Eskes G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Lausanne, Switzerland: Front Hum Neurosci; 2015;9. [Internet]. [cited 2018 Jun 16]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4412054/.
- Heraud N, Alexandre F, Gueugnon M, et al. Impact of chronic obstructive pulmonary disease on cognitive and motor performances in dual-task walking. COPD. 2018;15:1–6.
- Metzger FG, Ehlis A-C, Haeussinger FB, et al. Functional brain imaging of walking while talking - An fNIRS study. Neuroscience. 2017;343:85–93.
- Holtzer R, Mahoney JR, Izzetoglu M, et al. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66A(8):879–887.
- Hill A, Bohil C, Lewis J, et al. Prefrontal cortex activity during walking while multitasking: An fNIR Study. Proc Human Factors Ergon Soc Annual Meeting. 2013;57(1):1224–1228.
- Piper SK, Krueger A, Koch SP, et al. A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. Neuroimage. 2014;85:64–80. [Internet]. [cited Sep Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3859838/.
- Silva MA, See AP, Essayed WI, et al. Challenges and techniques for presurgical brain mapping with functional MRI. Neuroimage Clin. 2018;17:794–803.
- Miller MR, Crapo R, Hankinson J, ATS/ERS Task Force, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161.
- Sattler JM, Ryan JJ. Assessment with the WAIS-IV. Jerome M California (CA): Sattler Publisher; 2009.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- HajGhanbari B, Yamabayashi C, Garland RJ, et al. The relationship between pain and comorbid health conditions in people with chronic obstructive pulmonary disease. Cardiopulmonary Phys Ther J. 2014;25:29.
- Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395.
- Friden T, Zätterström R, Lindstrand A, et al. A stabilometric technique for evaluation of lower limb instabilities. Am J Sports Med. 1989;17(1):118–122.
- Ageberg E, Zätterström R, Moritz U. Stabilometry and one-leg hop test have high test-retest reliability. Scandinavian J Med Sci Sports. 2007;8(4):198–202.
- Springer BA, Marin R, Cyhan T, et al. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. 2007;30(1):8–15.
- Mkacher W, Mekki M, Tabka Z, et al. Effect of 6 months of balance training during pulmonary rehabilitation in patients with COPD. J Cardiopulmonary Rehabil Prevent. 2015;35:207–213.
- Beurskens R, Bock O. Does the walking task matter? Influence of different walking conditions on dual-task performances in young and older persons. Hum Mov Sci. 2013;32(6):1456–1466.
- Aggarwal A, Kean E. Comparison of the Folstein Mini Mental State Examination (MMSE) to the Montreal Cognitive Assessment (MoCA) as a cognitive screening tool in an inpatient rehabilitation setting. NM. 2010;1(2):39–726.
- McKendrick R, Mehta R, Ayaz H, et al. Prefrontal hemodynamics of physical activity and environmental complexity during cognitive work. Hum Factors. 2017;59(1):147–162.
- McKendrick R, Parasuraman R, Murtza R, et al. Into the wild: Neuroergonomic differentiation of hand-held and augmented reality wearable displays during outdoor navigation with functional near infrared spectroscopy. Front Hum Neurosci. 2016;10:216.
- Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley Pub. Co.; 1977.
- Hawkins KA, Fox EJ, Daly JJ, et al. Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Hum Mov Sci. 2018;59:46–55.
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47.
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95(3):906–913.
- Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci. 1995;769:85–96.
- Malouin F, Richards CL, Jackson PL, et al. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19(1):47–62.
- Bloem BR, Grimbergen YAM, van Dijk JG, et al. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):196–204.
- Busch T, de A, Duarte YA, Pires Nunes D, et al. Factors associated with lower gait speed among the elderly living in a developing country: a cross-sectional population-based study. BMC Geriatr. 2015;15(1):1–9. [Internet]. [cited 2018 Sep 10]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4391309/.
- Quach L, Galica AM, Jones RN, et al. The nonlinear relationship between gait speed and falls: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston Study. J Am Geriatr Soc. 2011;59(6):1069–1073.
- Pashler H, Johnston JC. Attentional limitations in dual-task performance. In: Pashler H, editor. Attention. Hove, England: Psychology Press/Erlbaum (UK) Taylor & Francis; 1998. p. 155–189.
- Ayers EI, Tow AC, Holtzer R, et al. Walking while talking and falls in aging. Gerontology. 2014;60(2):108–113. 2014
