Abstract
In Sub-Saharan Africa, COPD remains prevalent but its association with HIV is not well characterized especially in rural settings. We assessed for COPD prevalence, associated factors and lung function profile among HIV-infected individuals attending ART clinics in rural Nakaseke district of Uganda. We enrolled HIV-positive participants from four HIV treatment centers in rural Uganda. Participants underwent spirometry testing following standard guidelines. We defined COPD as a post-bronchodilator FEV1/FVC ratio less than the fifth percentile of the NHANES III African-American reference. We assessed for factors associated with COPD and lung function profiles using multivariable logistic and linear regression analyses. We analyzed data from 722 HIV-positive participants (mean age 48.0 years, 59.7% women). Over 90% of participants were on ART for a median duration of 4 years (IQR 2–7 years), with a median viral load of 0 copies/mL (IQR 0–0 copies/mL), current and baseline CD4 + T cell count of 478 cells/mm3 (IQR 346–663 cells/mm3) and 335 cells/mm3 (IQR 187–523 cells/mm3) respectively. The prevalence of COPD was 6.22%. COPD was associated with worse respiratory symptoms and health status. History of pulmonary tuberculosis was strongly associated with COPD (adjusted OR = 4.92, 95% CI 1.71 to 14.15, p = 0.003) and reduced lung function. Use of ART, CD4+T cell count and viral load were not associated with COPD or reduced lung function. In conclusion, we report a COPD prevalence of 6.22% in HIV-infected individuals in rural Uganda. Pulmonary tuberculosis remains the strongest predictor of COPD risk and reduced lung function in well-controlled HIV.
Keywords:
Introduction
The association between COPD and HIV has been well described in high-income settings [Citation1–4]. Several studies have demonstrated an association between viral load, CD4+ T cell count, antiretroviral therapy (ART) use and COPD. Access to ART among people living with HIV/AIDS (PLWHA) has resulted in low HIV-associated morbidity and mortality particularly among low- and middle-income countries (LMICs) [Citation5,Citation6]. The reduction in mortality has substantially increased life expectancy with estimates among PLWHA now approaching that of the general population [Citation7]. Consequently, the burden of non-communicable diseases, such as chronic obstructive pulmonary disease (COPD) has increased among PLWHA. The prevalence of COPD among PLWHA varies widely and is estimated at 3–23% globally [Citation1,Citation2].
In sub-Saharan Africa, the prevalence of COPD among PLWHA is not fully known. Until 2015, the prevalence and risk factors for COPD in Uganda had been largely uncharacterized [Citation8]. FRESH AIR Uganda found a 16% prevalence of COPD in a rural population in Uganda though this prevalence was not stratified by HIV status. The reported prevalence is higher compared to other studies in the region and may be explained by other factors [Citation8]. Recently, Siddharthan and colleagues reported a 6.1% prevalence of COPD in rural Ugandan communities of Nakaseke district compared to a 1.5% prevalence in urban communities of Kampala. In this study, it was highlighted that the most important factor associated with COPD was a rural residence, and HIV did not appear to be an important risk factor for COPD. Nevertheless, this population-based study did not explore the effects of HIV viral load and CD4 count on COPD prevalence and lung function outcomes. Also, HIV status was self-reported at 8.1% [Citation9]. Similarly, in a recent study in Southwestern Uganda, North and colleagues did not report any significant association between HIV and respiratory symptoms nor lung function [Citation10]. However, the authors did not explore the effects of HIV-specific characteristics on COPD.
We, therefore, sought to assess the COPD prevalence, lung function profile and associated factors among people living with HIV/AIDs attending ART clinics in rural Nakaseke district of Central Uganda.
Materials and methods
Study design and population
We conducted a cross-sectional study at four HIV/AIDS treatment centers in Nakaseke, Uganda between January 2017 and December 2017. We consecutively enrolled HIV-positive participants from four antiretroviral therapy (ART) clinics. These clinics are centrally located within the district and provide comprehensive HIV services to 2,300 HIV-positive individuals. Most patients live within 20–50 km radius of the clinic. Participants were eligible for inclusion if they: resided within Nakaseke district, were ≥35 years of age, were HIV-positive, were capable of understanding study procedures, able to provide written informed consent, and did not have contraindications for spirometry.
Ethical considerations
We conducted our study in compliance with recognized international standards, including the International Conference on Harmonization (ICH) and the guidelines of the Declaration of Helsinki. We obtained written informed consent from all study participants. Ethical approval was granted by the Makerere University School of Medicine Research and Ethics Committee, Uganda National Council for Science and Technology in Kampala, Uganda, and the Johns Hopkins University School of Medicine in Baltimore, USA.
Study design
All participants were screened for COPD with respiratory symptoms questionnaires and spirometry [Citation9]. HIV-related medical history including baseline and current CD4 + T cell count, viral load, ART drug use, and history of opportunistic infections or pulmonary tuberculosis was recorded from patient medical records. Spirometry was performed by trained research assistants using Easy On-PC spirometer (ndd Medical Technologies, Zurich, Switzerland) before and 15 min after administration of 400 mcg of salbutamol using a spacer. The quality of spirometry test results was assessed by ATS/ERS guidelines [Citation11]. Among participants with COPD, we determined the baseline level of dyspnea using a modified Medical Research Council (mMRC) dyspnea scale [Citation12]. Furthermore, we assessed for aerobic capacity and endurance of COPD participants through administering a six-minute walk test (6MWT) following ATS/ERS guidelines [Citation13]. We determined the average distance walked in six minutes. Finally, we assessed for respiratory health status among COPD patients using St. George Respiratory Questionnaire (SGRQ) [Citation14]. We determined the average scores for symptoms, activity and overall impact of the disease on participants.
Outcome
We defined COPD as a post-bronchodilator (post-BD) FEV1/FVC ratio lower than the fifth percentile of the NHANES III African-American reference population and used post-bronchodilator FEV1 values to define the severity of disease [Citation15].
Exposure variables
Study participants were diagnosed with HIV using an enzyme-linked immunosorbent assay and/or Western blot according to ART clinic guidelines in Uganda. We evaluated whether age, sex, body mass index, use of wood for cooking or heating, daily smoking (defined as self-reported smoking of one or more cigarettes per day), pack-years of smoking, baseline CD4 + T cell count (defined as CD4 count when individuals were first registered at the ART clinic), current CD4 count (defined as CD4 count at recruitment into the study), viral load, use and duration of ART, duration of HIV since diagnosis at the clinic and history of previous tuberculosis or other opportunistic infections including recurrent pneumonia, oral candidiasis, and Kaposi sarcoma were associated with COPD and low lung function profiles (defined as z-scores for FEV1, FVC, and FEV1/FVC ratio).
Biostatistical methods
First, we determined the prevalence of COPD among participants. This was reported as a percentage of the total number of participants with COPD. We then examined the distributions of baseline characteristics and stratified them by COPD status. To determine the association between participant characteristics and COPD, we fitted unadjusted and multivariable logistic regression models with COPD as the outcome variable. We also fitted linear regression models in which we examined unadjusted and adjusted associations of participant characteristics with lung function profiles. We re-scaled independent variables as appropriate to aid interpretability. Finally, we assessed the association between respiratory health status measured by SGRQ and COPD using multivariable logistic regression. Analyses were performed using STATA 14 (Stata Corp, College Station, TX, USA) and R.
Results
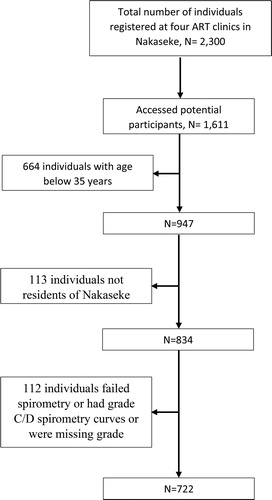
Of 2,300 HIV-positive individuals registered at ART clinics in Nakaseke, we contacted 1,611 potential participants through regular ART clinic visits of whom 834 (51.8%) met eligibility criteria of ≥ 35 years and residence in Nakaseke. Of these, 722 were retained in the final analytic sample (See for the summary).
Population characteristics
The average age of enrolled participants was 48.0 years (95% CI 47.4–48.7) with 59.7% being female. The majority of the participants were nonsmokers (84.2%). Among smokers, the average pack-years was 1.3 (95% CI 0.96–1.56). Over 88.5% of our population reported using wood as a source of cooking () Ninety percent of participants were on ART for a median duration of 4 years (IQR 2–7 years). Among 485 participants for whom viral load information was available, 93.0% were virologically suppressed with a median viral load of 0 copies/mL, current and baseline CD4 + T cell count of 478 cells/mm3 (IQR 346–663 cells/mm3) and 335 cells/mm3 (IQR 187–523 cells/mm3) respectively. The median duration of HIV infection since diagnosis was 6.3 years (IQR 4.0–9.9 years). History of pulmonary tuberculosis was reported at 9.1% (95% CI 7.2–11.5), recurrent bacterial pneumonia at 4.4% (95% CI 3.1–6.2), Kaposi sarcoma at 0.1% (95% CI 0.01–0.1) and oral candidiasis at 4.6% (95% CI 3.3–6.4%) ()
Table 1. Socio-demographic and clinical characteristics of the study participants stratified by COPD.
Table 2. HIV-specific clinical characteristics stratified by COPD status among participants.
Prevalence and factors associated with COPD in the study population
Based on the distribution of Uganda’s population, the age-adjusted prevalence of COPD among the study population was 6.22%. Of these participants, 37.8% (17/45) had mild obstruction, 48.9% (22/45) had moderate obstruction and 13.3% (6/45) had severe obstruction. Participants with COPD were predominantly male (62.2%), above 45 years of age (51.1%). On average, participants with COPD had 3.64 (95% CI 1.58 to 5.70) pack-years of smoking tobacco, a lower BMI (19.3 kg/m2) and higher history of pulmonary tuberculosis (31.1%) compared to individuals without COPD (). We did not find significant differences between median HIV duration (8.12, IQR 6.03 years vs. 6.71, IQR 5.97 years), ART use (88.9% vs. 90.4%, p = 0.74), median ART duration (4.0 years in both groups), median viral loads (0 copies/mL in both groups), median baseline and current CD4+T cell counts (322 vs. 337 cells/mm3, p = 0.720 and 579 vs. 472 cells/mm3, p = 0.18) respectively between participants with COPD, and those without COPD (). In adjusted analysis, history of pulmonary tuberculosis was strongly associated with COPD (adjusted OR = 4.92 (95% CI 1.71 to 14.15, p = 0.003). Each 5 kg/m2 increase in BMI was associated with lower odds of COPD (adjusted OR = 0.27, 95% CI 0.08, 0.80; p = 0.02). Other factors including age, sex, smoking, use of wood as source of cooking, ART use and duration, baseline and current CD4 count, viral load, HIV duration, history of recurrent pneumonia, candidiasis and Kaposi sarcoma were not significantly associated with COPD in this population ()
Table 3. Single variable and multivariable logistic regression results of the associations between participant characteristics and COPD.
Lung function profiles among study participants
Average pre- and post-BD FEV1 among participants with COPD was low when compared to the pre-bronchodilator FEV1 among participants with no COPD (). Participants with COPD had a lower percentage change in FEV1 after the BD challenge compared to participants without COPD (median % change in FEV1 = 6.93, IQR 2.83 to 10.99 vs. 8.05, IQR 3.97 to 14.46). Similarly, the absolute change in FEV1 volume was low in COPD compared to no COPD (median change in FEV1=120mL IQR 49.9 to 199.9 vs. 155 mL IQR 65 to 300). There was a trend though not significant for COPD outcomes (). Each 10-year increase in age was associated with lower FEV1/FVC ratio z-score (adjusted difference= −0.19, 95% CI −0.39 to −0.0076). Having a history of pulmonary tuberculosis was associated with reduced lung function profiles (z-score FEV1 adjusted difference= −0.61, 95% CI −1.04 to −0.19; z-score FEV1/FVC ratio adjusted difference= −0.71, 95% CI −1.21 to −0.211) while each 20-year increase in pack-years of smoking was associated with a lower FEV1/FVC ratio z-score only (adjusted difference −0.93, 95% CI −1.65 to −0.21). Body mass index, sex and the use of wood as a source of fuel/cooking were not associated with significant changes in lung function profiles. Each 5-year increase in HIV duration was associated with a lower FEV1 z-score (adjusted difference −0.067, 95% CI −0.11 to −0.024) and lower FVC z-score (adjusted difference −0.064, 95% CI −0.11 to −0.021) while ART, baseline and current CD4 count, viral load, history of recurrent pneumonia, candidiasis and Kaposi sarcoma were not associated with significant changes in lung function profiles in this population ().
Table 4. Multivariable linear regression analysis results for the association between participant characteristics and pre-bronchodilator lung function z-scores (FEV1, FVC and pre-FEV1/FVC ratio).
Respiratory symptoms and respiratory health status among study participants
Compared to healthy participants (), on most days of the week, COPD patients reported having severe cough (13.3% vs. 0.0%, p < 0.001), troublesome shortness of breath (17.8% vs. 0%, p < 0.001), wheeze (13.3% vs. 0%, p < 0.001) and phlegm (24.4% vs. 0%, p = 0.000). Over 6% of COPD participants reported at least three exacerbations a week and 15.6% hospitalization related to respiratory illness. Overall the level of dyspnea in COPD participants was higher compared to healthy individuals (mMRC scale 4: 4.44% vs. 0.3%; mMRC scale 3: 11.1% vs. 0% and mMRC scale 2: 27.8% vs. 7.4%, p < 0.001), with reduced aerobic capacity and endurance (mean 6MWT, 373 m). The St. George respiratory questionnaire scores for symptoms, activity and overall impact of the disease on participants were higher among individuals with COPD compared to individuals without COPD (SGRQ total score 25.5 vs 4.7; symptoms: 34.6 vs. 7.1; activity: 34.1 vs. 5.4 and impact: 18.6 vs. 3.3, p < 0.001), a marker of worse health. Adjusted analysis showed that COPD (OR = 17.5, 95% CI 12.14–22.83) and history of pulmonary tuberculosis (OR = 7.09, 95% CI 0.63–13.50) were associated with higher SGRQ scores, a marker of worse health.
Table 5. Respiratory symptom and quality of life scores across study participants stratified by COPD.
Discussion
In a rural setting of ART clinics in Uganda, with participants having well-controlled HIV, we found a COPD prevalence of 6.22%. History of pulmonary tuberculosis was strongly associated with COPD and reduced lung function. As expected increasing age was associated with reduced lung function. However, HIV-specific characteristics including use and duration of ART, current and baseline CD4 + T cell counts and viral load were not associated with COPD or reduced lung function both in an adjusted and adjusted analysis. Among those diagnosed, COPD is associated with reduced respiratory health status and severe respiratory symptoms.
We report a prevalence comparable with that of the general Nakaseke population (6.1%) reported by Siddharthan and colleagues in a population-based cross-sectional study conducted in rural Nakaseke communities [Citation9]. Similarly, North and colleagues report similar COPD prevalence among HIV-infected individuals from southwestern rural Uganda (6%), though much higher compared to HIV-negative population (1.0%) [Citation16]. North and colleagues did not find any significant association between COPD and HIV-specific characteristics like ART, CD4 count, and viral load. In their study, pulmonary tuberculosis was strongly associated with COPD as reported in our study. We had several similarities with the previous studies. Over 92% of HIV-positive participants were virologically suppressed with a median CD4 + T cell count of 477 cells/mm3, were predominantly nonsmokers (9%), over 86% reported using wood as source of cooking and in both studies, COPD participants reported severe respiratory symptoms of cough, dyspnea, phlegm and wheeze [Citation16]. Major differences between the two studies, however, have to be noted. North and colleagues completed spirometry in 269 participants of whom only 143 were HIV-positive compared to 722 HIV-positive participants in our study. History of prior pneumonia was higher (9%) compared to our study (6.7%) and the median age was slightly higher (52 years vs. 48yrs) [Citation16]. Similarly, in another population-based survey in southwestern Uganda study, North and colleagues could not find any association between HIV and COPD [Citation17]. In a multi-country, cross‐sectional analysis of adult HIV-positive individuals enrolled in the START trial, Kunisaki and colleagues reported a COPD of 6.8%, similar to the prevalence of our study. In this multi-country analysis, participants from sites in Africa had a worse FEV1/FVC ratio than in other places in the world, despite the low prevalence of smoking [Citation18]. As Siddharthan and colleagues highlight, being in a rural setting may play a role in increasing the risk for COPD in Uganda [Citation9]. This may potentially be associated with biomass early life exposure and poor socioeconomic status in rural areas.
Studies from South Africa have reported similar findings. In a longitudinal cohort study of lung function in 730 HIV-infected black South African adults, Gupte and colleagues reported that obstructive lung disease was not associated with CD4 + T cell count or ART. Factors such as smoking, increased C-reactive protein levels, tuberculosis, and increasing age were reported to be significantly associated with COPD [Citation19]. West Africa studies report higher COPD prevalence as well as significant associations with HIV. We notice clear differences in participant clinical characteristics compared to our population. For instance, in a cross-sectional study by Akanbi and colleagues among HIV-infected adults in Nigeria, which reported a COPD prevalence of 15.4% (95% CI 11.7–19.2) using GOLD criteria, only 67.2% of participants were well-controlled with suppressed viral load. At baseline, the median CD4 + T cell count of their study population was 173 (IQR 29–283) [Citation20]. In a case-control study by Pefura-Yone and colleagues, it is reported that HIV infection was positively associated with COPD. In this study, a clinic-based sample of HIV-infected individuals was compared with a community-based sample of the HIV-negative population and the level of viral suppression and CD4 + T cell count was lower compared to our population [Citation21].
Nevertheless, it is worth mentioning that African COPD studies share some characteristics such as predominant nonsmoker populations and a history of pulmonary tuberculosis being a major driver of COPD prevalence irrespective of HIV status. Also, lower socioeconomic status among African countries has been reported to be associated with COPD [Citation22,Citation23]. It is plausible that the history of pulmonary tuberculosis might be a marker of low socioeconomic status.
Whereas multiple studies have reported the association between pulmonary tuberculosis and COPD [Citation16,Citation21,Citation24,Citation25], the association is complex [Citation26]. Pulmonary tuberculosis and COPD share a number of risk factors including smoking, biomass exposure, diabetes mellitus and vitamin D deficiency [Citation27]. Some studies report that a significant number of individuals diagnosed with pulmonary tuberculosis develop COPD [Citation28]. Mechanistically, it has been suggested that increased expression of lung matrix metalloproteinases, recruitment of CD8+ Lymphocytes and neutrophils as well as increased levels of interleukin 8 (IL-8) and vascular endothelial growth factor in pulmonary tuberculosis might play a significant role in orchestrating structural changes seen in COPD [Citation26]. The resulting volume loss and airway obstruction lead to COPD. Other studies report that individuals diagnosed with COPD are at high risk of developing pulmonary TB [Citation29]. Mechanistically, the impaired innate immunity and corticosteroid therapy in individuals with COPD increases the risk for mycobacterial lung infection [Citation27]. The observed bidirectional relationship between COPD and pulmonary tuberculosis has critical implications for the long term management of COPD and pulmonary tuberculosis and calls for efforts to integrate screening and treatment interventions for both diseases [Citation27].
In contrast to our findings, western studies have reported a higher prevalence of COPD among PLWHA. For example, in a large study of 1,014 HIV-infected veterans in the US [Citation6] the prevalence of COPD by patient self-report was reported at 15%. Additionally, HIV infection was an independent risk factor for COPD after adjusting for age, pack-years of smoking, intravenous drug use, and alcohol abuse although this population differed from the present. Specifically, only 15.6% of participants were on ART, with a higher median viral load among those without COPD. These findings may have been confounded by socio-demographic risk factors. HIV-infected subjects in their study were significantly more likely to be current smokers than our study population (46% vs. 36%; p < 0.001). A history of intravenous drug use was more common in HIV-infected subjects than in HIV-negative subjects (31% vs. 12%, respectively; p < 0.001). These findings are similar to other published studies among HIV-seropositive cohorts in high-income settings. In these cohorts, over 75% of the participants have smoked ≥ 100 cigarettes in their lifetime, with roughly half currently smoking cigarettes [Citation3]. In contrast, our HIV-infected study population was characterized predominantly by nonsmokers (6.2% of our sample identified as daily smokers, and 8.6% as occasional smokers). Others studies have reported similar findings [Citation4,Citation30–32]
In agreement with several studies conducted in sub-Saharan Africa, pulmonary tuberculosis was a strong predictor of poor lung function in our study. However, HIV was not associated with poor lung function profiles, which is in agreement with prior studies in India and Uganda, though contrary to studies in HIC settings. In a longitudinal follow up study, Drummond and colleagues reported declines in lung function profiles among HIV positive individuals. In their study, they show that HIV-positive individuals have a lower absolute FEV1 and FVC at baseline [Citation33]. Authors note that after stratifying participants by CD4 + T cell count, individuals with lower CD4 cell counts experienced a more rapid decline in FEV1 and FVC. Similarly, individuals with viral load >75,000 copies/ml have significantly more rapid annual FEV1 decline compared to HIV-negative individuals irrespective of CD4 count. Of note was the observation that these associations were not existent among individuals with suppressed viral load or CD4 count above 200 [Citation33]. This suggests that if HIV is well-controlled with suppressed viral load and CD4 count above 500, lung function measures are not significantly reduced [Citation33]. Also, Kunisaki and colleagues demonstrated that timing of HIV treatment and hence CD4 and viral load differences had no impact on lung function decline, arguing against the role of viral load and CD4 on lung function/COPD [Citation34].
Our findings suggest that in the setting of well-controlled HIV, the most predictor of COPD risk and poor lung function in rural Ugandan ART clinics is a history of pulmonary tuberculosis. Our study has several strengths. It was conducted in large ART treatment centers serving several rural communities of a district in Uganda. COPD diagnosis was based on spirometry and standards established by ATS/ERS. However, the interpretation of our results must take into account some limitations. We did not use lung diffusion capacity measurements. A major limitation of the present study included missing duration of HIV diagnosis, viral loads, and CD4 counts among a significant (30–40%) of the population. This could potentially bias the results toward health-seeking subgroups of the sample population.
Conclusion
In the setting of well-controlled HIV, the most predictor of COPD risk and poor lung function in rural Ugandan ART clinics is a history of pulmonary tuberculosis.
Declaration of interest
All authors of this manuscript declare that there are no relevant conflicts of interest.
Authors’ contribution
Alex Kayongo: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Original draft writing and editing; Trishul Siddharthan: Conceptualization, Mentorship, Investigation, Design, Funding acquisition and Drafting the manuscript for important intellectual content; Tasmia Naz, Faith Nassali and Adaeze Wosu: Data curation and Statistical analysis; Robert Kalyesubula: Investigation, Methodology and Project administration; Bruce Kirenga: Investigation, Methodology and Project administration; Robert Wise: draft review and editing and William Checkley: Conceptualization, Mentorship, Investigation, Design, Funding acquisition, Statistical analysis and Drafting the manuscript for important intellectual content
Acknowledgments
The authors wish to express gratitude to the following people for data collection: Mr. Dennis Mawanda, Ms. Daka Grace and Ms. Nansamba Margaret; and for data management: Mr. Mathew Grigsby and Mr. Brooks Morgan.
Funding
This study was sponsored and funded by the National Institutes of Health, Fogarty International Center, USA, grant number R25TW009340.
References
- Drummond M, Kunisaki K, Huang L, et al. Obstructive lung diseases in HIV: a clinical review and identification of key future research needs. Semin Respir Crit Care Med. 2016;37(2):277–288. doi:10.1055/s-0036-1578801.
- Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395. doi:10.1164/rccm.201006-0836OC.
- Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011;8(3):320–325. doi:10.1513/pats.201006-045WR.
- Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182(6):790–796. doi:10.1164/rccm.200912-1858OC.
- Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–793. doi:10.1001/jama.296.7.782.
- World Health Organization (WHO). Fact sheet: HIV treatment and care: what’s new in HIV treatment. Geneva: WHO; 2015.
- Asiki G, Reniers G, Newton R, et al. Adult life expectancy trends in the era of antiretroviral treatment in rural Uganda (1991–2012)). AIDS. 2016;30(3):487–493. doi:10.1097/QAD.0000000000000930.
- van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3(1):e44–e51. doi:10.1016/S2214-109X(14)70337-7.
- Siddharthan T, Grigsby M, Morgan B, et al. Prevalence of chronic respiratory disease in urban and rural Uganda. Bull World Health Organ. 2019;97(5):318–327. doi:10.2471/BLT.18.216523.
- North CM, Kakuhikire B, Vořechovská D, et al. Prevalence and correlates of chronic obstructive pulmonary disease and chronic respiratory symptoms in rural southwestern Uganda: a cross-sectional, population-based study. J Glob Health. 2019;9(1):010434.
- Miller MR, Hankinson J, Brusasco V, Burgos F. ATS/ERS task force: standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Munari AB, Gulart AA, Dos Santos K, et al. Modified Medical Research Council dyspnea scale in GOLD classification better reflects physical activities of daily living. Respir Care. 2018;63(1):77–85. doi:10.4187/respcare.05636.
- Society AT. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117.
- Jones P, Quirk F, Baveystock C. The St George’s respiratory questionnaire. Respir Med. 1991;85:25–31. doi:10.1016/S0954-6111(06)80166-6.
- Mirza S, Clay RD, Koslow MA, et al. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502.
- North CM, Allen JG, Okello S, et al. HIV infection, pulmonary tuberculosis, and COPD in rural Uganda: a cross-sectional study. Lung. 2018;196(1):49–57. doi:10.1007/s00408-017-0080-8.
- North CM, Kakuhikire B, Vořechovská D, et al. Prevalence and correlates of chronic obstructive pulmonary disease and chronic respiratory symptoms in rural southwestern Uganda: a cross-sectional, population-based study. J Glob Health. 2019;9(1):10434.
- Kunisaki KM, Niewoehner DE, Collins G, et al. Pulmonary function in an international sample of HIV‐positive, treatment‐naïve adults with CD4 counts> 500 cells/μL: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16:119–128. doi:10.1111/hiv.12240.
- Gupte AN, Wong ML, Msandiwa R, et al. Factors associated with pulmonary impairment in HIV-infected South African adults. PLoS One. 2017;12(9):e0184530. doi:10.1371/journal.pone.0184530.
- Akanbi MO, Taiwo BO, Achenbach CJ, et al. HIV associated chronic obstructive pulmonary disease in Nigeria. J AIDS Clin Res. 2015;6(5):453. doi:10.4172/2155-6113.1000453.
- Pefura-Yone E, Fodjeu G, kengne AP, et al. Prevalence and determinants of chronic obstructive pulmonary disease in HIV infected patients in an African country with low level of tobacco smoking. Respir Med. 2015;109(2):247–254. doi:10.1016/j.rmed.2014.12.003.
- Gershon AS, Dolmage TE, Stephenson A, et al. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9(3):216–226. doi:10.3109/15412555.2011.648030.
- Grigsby M, Siddharthan T, Chowdhury MA, et al. socioeconomic status and COPD among low- and middle-income countries . Int J Chron Obstruct Pulmon Dis. 2016;11:2497–2507. doi:10.2147/COPD.S111145.
- Byrne AL, Marais BJ, Mitnick CD, et al. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–146. doi:10.1016/j.ijid.2014.12.016.
- Ehrlich R, Adams S, Baatjies R, et al. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis. 2011;15(7):886–891. doi:10.5588/ijtld.10.0526.
- Jain NK. Chronic obstructive pulmonary disease and tuberculosis. Lung India. 2017;34(5):468–469. doi:10.4103/lungindia.lungindia_183_17.
- O'Toole RF, Shukla SD, Walters EH. TB meets COPD: An emerging global co-morbidity in human lung disease. Tuberculosis (Edinb). 2015;95(6):659–663. doi:10.1016/j.tube.2015.08.005.
- Zakaria MW, Moussa HA. Chronic obstructive pulmonary disease in treated pulmonary tuberculous patients. Egypt J Bronchol. 2015;9(1):10.
- Lee C-H, Lee M-C, Shu C-C, et al. Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect. Dis. 2013;13(1):194. doi:10.1186/1471-2334-13-194.
- Varkila MR, Vos AG, Barth RE, et al. The association between HIV infection and pulmonary function in a rural African population. PLoS One. 2019;14(1):e0210573. doi:10.1371/journal.pone.0210573.
- Fitzpatrick ME, Gingo MR, Kessinger C, et al. HIV infection is associated with diffusing capacity impairment in women. J Acquir Immune Defic Syndr. 2013;64(3):284–288.
- Ronit A, Lundgren J, Afzal S, et al. Airflow limitation in people living with HIV and matched uninfected controls. Thorax. 2018;73(5):431–438. doi:10.1136/thoraxjnl-2017-211079.
- Drummond MB, Merlo CA, Astemborski J, et al. The effect of HIV infection on longitudinal lung function decline among injection drug users: a prospective cohort. AIDS. 2013;27(8):1303–1311. doi:10.1097/QAD.0b013e32835e395d.
- Kunisaki KM, Niewoehner DE, Collins G, et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med. 2016;4(12):980–989. doi:10.1016/S2213-2600(16)30319-8.