Abstract
Alpha-1 antitrypsin deficiency (AATD) is a rare and underdiagnosed disease that is associated with the development of liver disease in adults and children and pulmonary emphysema in adults. Several studies have shown that there is limited knowledge about the disease and its diagnosis among health care providers, and there is an important inequity in the access to specialized care and appropriate treatment across Europe. The European Commision and the European Respiratory Society (ERS) recommend that the care of patients with AATD must be organized in reference centers at national or regional levels. These reference centers must provide optimal clinical care in terms of adequate diagnostic techniques, such as phenotyping and genotyping, and ensure access to treatment according to guidelines. Reference centers should also provide continuous medical education for health care professionals, genetic counseling, collaboration with patient associations and promote collaborative research and clinical trials with new and existing treatments for the disease. These centers must have a registry of their activity and collaborate with large, international, multicenter registries, such as the European Alpha-1 antitrypsin Deficiency Research Collaboration (EARCO) international registry, which is endorsed by the ERS, and aims to recruit up to 3,000 patients over a period of three years and prospectively follow them to better understand the natural history of the disease and the impact of different treatments on outcomes in a real life setting. International collaboration and standardized collection of high-quality prospective data will provide new insights into the clinical manifestations and prognosis of AATD.
Introduction
According to the European Union (EU) the definition of a rare disease is a life-threatening or chronically debilitating condition of low prevalence, affecting 5 or fewer people in every 10,000 [Citation1]. The United States (US) Rare Diseases Act of 2002 established the National Institutes of Health (NIH) Office of Rare Diseases Research. For this purpose, a rare disease was defined as one that affects fewer than 200,000 individuals in the US (less than 1 in approximately 1500) [Citation2]. There are over 7000 such diseases and conditions in the US [Citation3]. About one half of these rare diseases affect children, and about 80% are the result of a genetic defect (others being degenerative or proliferative disorders). The total number of rare diseases is estimated to be in the order of 5000 to 8000 within the EU, and it is thought that 6 to 8% of the population will be affected by a rare disease at some stage in their life. Overall, 25 million North Americans and 30 million Europeans are estimated to be suffering from a rare disease [Citation1]. Examples of rare respiratory disease are cystic fibrosis with a prevalence in Europe of 0.74/10,000, or idiopathic pulmonary fibrosis with a prevalence in men of 2/10,000 [Citation4].
Alpha-1 antitrypsin deficiency (AATD) fulfills the criteria to be considered a rare disease with the particularity that its main clinical expression, pulmonary emphysema, affects adults [Citation5]. It is considered the most frequent potentially severe rare disease affecting adults, and its prevalence is around 2/10,000 in Europe [Citation6]. AATD may predispose to liver disease in children and adults and pulmonary emphysema in adults. In this review we will focus on the pulmonary disease associated with AATD as the most frequent clinical manifestation of the deficiency.
It is of note that there is great variability in the clinical expression of pulmonary disease associated with AATD. Most of this variability is due to exposure to other risk factors, mainly cigarette smoking [Citation7]; but even among smokers with similar smoking exposure, the severity of the lung disease may be very variable [Citation8] suggesting that other factors, either genetic or environmental, may play a role [Citation9–11]. On the other hand, although the majority of patients with lung disease will develop chronic obstructive pulmonary disease (COPD) in the form of panlobular basal emphysema, there are cases with predominantly centrilobular emphysema in the upper lobes [Citation12] and other phenotypes [Citation13], such as chronic bronchitis, asthma-COPD overlap (ACO) [Citation14, Citation15] or associated bronchiectasis [Citation16, Citation17]. In fact, current guidelines suggest testing for AATD in not only individuals with COPD, but also in adults with non-completely reversible asthma or with bronchiectasis of unknown etiology [Citation18].
The low prevalence of AATD and the extreme variability of its respiratory clinical manifestations implies that a single center will never attend enough patients to acquire experience in the management of this condition. Therefore, the European Council [Citation19] and the European Respiratory Society (ERS) [Citation18] recommend organizing the care for AATD patients in reference centers that can accumulate experience in management and lead the clinical and basic research in AATD. In this review, we will address the challenges in diagnosis and treatment of AATD and the characteristics and future of the reference centers and registries as key organizations for care and research in AATD.
Challenges in the diagnosis of AATD
The first challenge in the management of AATD patients is early diagnosis. AATD is an underdiagnosed disease and patients usually have several contacts with health care providers before diagnosis is achieved [Citation20]. Recent epidemiological studies have established that 0.12% of cases of COPD in Europe may be associated with AATD [Citation21]. These numbers may be even higher in other countries, such as Argentina where the prevalence of AATD among COPD patients is 0.83% [Citation22]. In any case, different studies have demonstrated that testing for AATD is clearly insufficient. Soriano et al [Citation23] analyzed trends in AATD testing in United Kingdom (UK) primary care between 1990 and 2014. They found the incidence of AATD diagnosis to be generally increasing over time, but also identified a low frequency of AATD testing, where only 2.2% of patients diagnosed with COPD before the age of 60 years were tested. In Spain, Barrecheguren et al [Citation24] identified an increase in AATD testing in primary care over two periods (2007–2008 and 2010–2011). However, their study showed that the number of alpha-1 antitrypsin (AAT) determinations performed was low compared with the prevalence of COPD, with a rate of AAT determinations ranging from 4.33 per 10,000 inhabitants in 2007 to 6.85 per 10,000 in 2008 and with no increase after 2008 [Citation24].
The reasons for this low frequency of testing are diverse, but predominantly there is a lack of awareness of the deficiency. Several surveys conducted in Europe have shown a low level of knowledge about AATD among respiratory and internal medicine specialists, as well as among primary care physicians [Citation25, Citation26]. The most frequent reason for not testing for AATD was the misbelief of the high economic cost of testing, and only 30% of respiratory specialists and less than 5% of primary care physicians reported testing all patients with COPD [Citation26] as recommended by the World Health Organization (WHO) [Citation27] and international guidelines [Citation28].
The diagnosis of AATD is based on serum quantification of the protein by nephelometry and the determination of the phenotype or genotype in cases with low serum levels [Citation29, Citation30]. Since AAT is a phase reactant protein, its serum levels may increase during inflammatory processes, including COPD [Citation31–33], and the simultaneous determination of C-reactive protein is recommended [Citation34, Citation35]. Despite nephelometry being a widely accessible and cheap diagnostic technique, some new tests have been developed with the objective of making the diagnosis even more accessible, in particular for primary care physicians. The AlphaKit® QuickScreen is a novel qualitative in vitro screening test with the characteristics of a point-of-care system, developed for protein Z detection in capillary whole blood [Citation36]. The AlphaKit® QuickScreen is based on lateral flow assay technology to deliver results within 15-30 min [Citation37]. More recently, a multiplex technology has been developed, which is able to simultaneously identify 14 mutations in the SERPINA1 gene of AAT, using dried blood spot samples as a way to simplify and shorten the diagnostic workup of patients with suspected AAT [Citation38]. Additionally, the possibility of carrying out this genetic study using a sample taken by buccal swab has recently been described [Citation39]. Interestingly, the possibility of complete diagnostic assessment of the deficiency from a buccal swab may facilitate the development of large-scale detection programs including family screening. This technology has been implemented by the Spanish Registry of Patients with AATD and in October 2019 more than 6,000 samples had been analyzed, and 41.6% samples were identified as carrying a deficient allele (S or Z) and 3.6% carried a rare allele [Citation39]. Finally, reference laboratories can offer the option of whole exon sequencing, especially if no deficient variant has been detected by the previous methods or when null variants are expected [Citation40–42].
These new diagnostic techniques may complement the simple nephelometric quantification of AAT serum levels and facilitate the diagnosis of AATD at every level of health care. It will be necessary for reference centers to disseminate information about the diagnostic strategies and coordinate the implementation of the different diagnostic techniques according to the characteristics of their area of influence.
Challenges in augmentation therapy
Treatment of emphysema associated with AATD consists of two components; the first part is the symptomatic treatment of the respiratory disease, based on the same principles as the treatment of COPD, both pharmacological [Citation43–45] and non-pharmacological [Citation46]. The second component is the treatment of the deficiency, which consists in weekly intravenous infusion of AAT purified from plasma donors, the so-called augmentation therapy, which is the only disease-modifying therapeutic approach for patients with AATD-associated lung disease [Citation47, Citation48]. The majority of patients receive this treatment in day hospital facilities, but some countries also have programs for home administration, and there is even the possibility of self-administration of augmentation therapy by the patients themselves [Citation49]. There are different products available for clinical use with small differences in AAT concentrations, specific activity and purity, although no clinical differences in outcomes have been demonstrated among these products [Citation50, Citation51].
Although augmentation therapy has demonstrated to reduce the rate of loss of lung density compared to placebo [Citation47, Citation48], there is still controversy about its clinical efficacy [Citation52, Citation53]. Basically, this controversy is due to the evaluation of the efficacy of augmentation with the same parameters used to evaluate other (symptomatic) treatments for COPD; namely the degree of dyspnea, the frequency of exacerbations and quality of life [Citation54]. Since augmentation therapy is not a symptomatic treatment, we should not expect to see an effect on the traditional outcomes used in clinical trials on COPD. Rather, its clinical efficacy should be evaluated in terms of slowing down the progression of emphysema and reducing mortality [Citation55, Citation56].
Augmentation therapy has not changed for more than 30 years [Citation57], and it is clear that it is not the end of the road for the treatment of AATD-related emphysema. It is becoming more and more evident that the approach of a single dosis for every patient may not be adequate, and some patients with a high protease burden in the lungs may need higher doses of AAT [Citation58]. In fact, recent studies have demonstrated significantly higher antiprotease and anti-inflammatory effects of double doses of AAT [Citation59]. Other controversies in the administration of augmentation therapy include the possible use of this treatment during pregnancy [Citation60] or for the treatment of individuals under 18 years of age [Citation61]. However, in addition to intravenous augmentation therapy, other ways of administration, such as inhaled AAT have been tested [Citation62]. Furthermore, other therapies including oral inhibitors of neutrophil elastase [Citation63] and new gene therapies for AATD [Citation64] are also under investigation. All these new treatment approaches require the collaboration of reference centers that have access to a large number of patients who can potentially participate in clinical trials.
Importance of reference centers
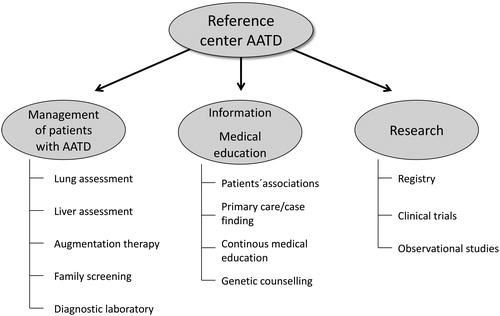
The care of patients with rare diseases, such as AATD, is best organized in reference centers that can provide the highest standard of care and advice to the individuals affected and their families whilst also contributing to the accumulation of knowledge. The different activities that a reference center in AATD should develop are outlined in . In addition to the diagnosis and follow-up of patients and relatives, these activities also include aspects of continuous medical education and research. An optimized format of service provision by a reference center in AATD was proposed in the ERS statement for the management of respiratory disease in AATD and is shown in [Citation18].
Figure 1. European Respiratory Society proposal for the provision of services by a AATD reference center.
Footnote: CXR: Chest X-ray; CT: Computed tomography; MDT: multidisciplinary team. Reproduced with permission of the © ERS 2020: European Respiratory Journal 2017 50: 1700610; DOI: 10.1183/13993003.00610-2017
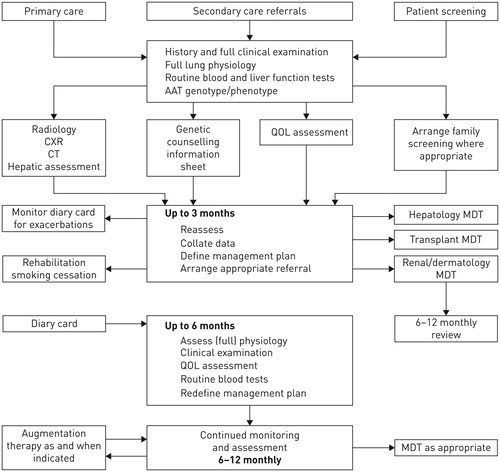
Table 1 Tasks of a AATD reference center.
The development of reference centers for rare diseases has also been recommended by the European Commission [Citation1, Citation65], and these centers should also play a role in ensuring a timely, better and more equitable access to high quality care, including augmentation therapy. In Europe, health care policy is largely a devolved matter and decisions related to the provision of health care services, disease management and prescription medicines lie with national, regional or local policy makers, health technology assessment agencies and payers. This results in different standards of care for AATD and contributes to geographic inequalities in access to optimal health care services, clinical expertise and effective therapies. Notably, access to high-cost, rare disease therapies, such as augmentation therapy for AATD, can significantly vary across jurisdictions [Citation65]. Augmentation therapy is fully reimbursed in some countries such as Italy, Germany, Portugal, France, Spain and others, but is not reimbursed in the majority of Eastern European countries and in some Western European countries such as the Sweden, Ireland or the UK [Citation18]. Moreover, even in countries where augmentation is reimbursed, there are inequalities in the access to treatment in different regions and areas, sometimes depending on aspects such as the personal subjective opinion of the attending physician toward the benefits of the therapy [Citation49].
In the absence of harmonized legislation that regulates AATD health care provision and access to augmentation therapy and other specific treatments across Europe, only multi-stakeholder collaboration and continuous improvement of the available evidence-based efficacy and cost-effectiveness of AATD therapies is likely to achieve greater equity in the implementation of best practice. In this respect the relationship between the reference centers and patient associations may be essential to ensure timely access to augmentation therapy and treatment according to guidelines.
Organization of the different activities that should be developed by an AATD reference center is shown in .
National and international registries of AATD
Reference centers must establish a registry of their activity and prospectively collect information about the natural history of the disease. These data can be shared at national and international levels and be the foundation of the AATD registries. The development of registries is crucial as being the only way for successful accumulation of knowledge on the clinical characteristics, evolution, natural history and response to treatment in the real life of patients with rare diseases, such as AATD [Citation66].
Some of the Nordic European countries were the first to set up a national registry of AATD. In the early 80 s, Denmark and Sweden started to collect prospective data about their patients diagnosed with AATD that provided very relevant information about the natural history of the disease [Citation67, Citation68]. Later, other European countries followed their example and established their own registries. Soon it became evident that due to the low prevalence of the disease, a large international registry would provide more relevant information that individual country data. In addition, the WHO recommended to establish a prospective international registry of AATD [Citation27]. Following this recommendation, the Alpha One International Registry (AIR) was founded in 1997 [Citation69]. Over a period of more than 20 years it has collected information on more than 4,000 patients and has been a platform for the development of clinical trials [Citation48, Citation55, Citation62] and the validation of new outcome measures of treatment such as lung densitometry measured by computed tomography (CT) [Citation55, Citation70]. However, AIR was not designed to prospectively collect reliable follow-up data of the patients, and there is still a need for a large database of standardized follow-up data to understand better the natural history of the disease and the impact of treatments on outcomes.
In the US, the National Heart, Lung and Blood Institute (NHLBI) initiated a registry of patients with severe AATD in 1988 with the objective of comparing the evolution of patients with and without augmentation therapy followed for 3.5 to 7 years [Citation71]. This registry provided very important data about the effectiveness of augmentation therapy in slowing down the decline in forced expiratory volume in 1 s (FEV1) in patients with an FEV1 between 35% to 70% predicted and in reducing mortality in patients with an FEV1 < 50% [Citation71]. After the NHLBI registry was closed, the Alpha One Foundation (AOF) started a new AOF Research Network Registry, which basically consists of a registry of affected individuals willing to be approached to participate in clinical studies, but no prospective collection of clinical data has been reported [Citation72].
European alpha-1 antitrypsin research collaboration (EARCO)
The unmet need of a prospective, international, long-term registry of individuals with AATD prompted the ERS to endorse the development of the European Alpha-1 antitrypsin Research Collaboration (EARCO) [Citation73]. EARCO is one of the ERS Clinical Research Collaborations (CRC), which are programs dedicated to gathering together researchers from all over Europe to work on different fields of respiratory diseases [Citation74]. EARCO brings together multiple stakeholders including researchers, health care providers, patients and industry with the aim of advancing understanding through clinical and scientific research and improving the quality of life of patients with AATD [Citation73]. This initiative currently involves 44 participants from 24 countries and has developed one registry, and four research projects are being conducted. The EARCO Registry is the main project of the EARCO research collaboration and is a non-interventional, multi-center, prospective, international, observational cohort study enrolling patients with severe AATD, genotypes PI*ZZ, PI*SZ or rare deficient or null genotypes [Citation75]. Patients will be managed according to their local procedures and without any clinical intervention from the study team. All patients will provide written informed consent to participate, and participating investigators will collect data prospectively. Data collection includes demographics, proteinase inhibitor genotype and other laboratory analyses, lung function, respiratory symptoms, comorbidities, liver function tests and imaging, exacerbations of respiratory disease, physical activity, chest CT scans, patient-reported outcomes such as the COPD Assessment Test (CAT) and the Five-dimension, European Quality of life Questionnaire (EQ-5D), and treatment [Citation75].
The objectives of EARCO registry are 1) To develop an prospective, international AATD registry. 2) To harmonize the data collection process between existing national registries and to ascertain the high quality of the data by monitoring entered data closely. 3) To generate longitudinal long-term, high-quality clinical data. 4) To better understand the natural history and prognosis of AATD. 5) To investigate the effect of AAT augmentation and other therapies on the progression of emphysema and to examine their potential impact on clinical and functional outcomes, such as FEV1, quality of life and mortality in a “real-life” population. 6) To learn more about the course of the disease in patients with severe AATD with genotypes other than PI*ZZ [Citation75].
There is a long tradition of national AATD registries in Europe. Several countries have active registries that have provided very important information about different aspects of the disease [Citation66, Citation67, Citation76–79]. However, the pooling of data into one large dataset will accelerate the research in different aspects of severe AATD. EARCO is an independent, international registry in which any individual investigator from any country can join and include data. There will be national coordinators, designated by their respective national scientific societies, who will have access to the full dataset from each participating country. Since national registries use different datasets, it was agreed not to include historical data from other registries in EARCO, but to start with the inclusion of current data obtained at the time of enrollment in the registry, from both new and previously diagnosed patients. Some countries with existing or newly developed registries, such as Spain and Portugal, have moved their national registry to EARCO, and the patients included in EARCO from each of these countries will constitute their national dataset in the future [Citation80, Citation81]. Other countries with a different structure may decide to adapt their dataset to EARCO and periodically export their data to EARCO. For efficient transfer of data, the national datasets must include the same type of information (data fields) and with the same structure. Another important consideration is that EARCO provides quality control of the data registered at the individual investigator level. For countries centralizing the download of data to EARCO, the quality control must be performed at a national level.
The EARCO registry was launched in February 2020 and by March 2020 included 38 patients from 3 centers, when recruitment was abruptly stopped due to the COVID-19 pandemic. After the restrictions of patients to attend clinical visits ended, recruitment was restarted in June 2020 with the addition of 5 new recruiting centers from 3 more countries. At the end of June 2020, 70 centers from 24 countries were in the process of obtaining ethical approval and signing contracts with the coordinating institution in order to start recruitment to the registry during the next months.
Conclusions
AATD is an underdiagnosed disease with a very diverse clinical expression and prognosis. Reference centers are indispensable to provide high quality clinical care for patients and promote medical education and research. The accumulation of sufficiently high quality clinical data requires the setting up of a prospective, international registry that will allow investigators to better understand the natural history of the disease and the impact of therapies in a real life setting [Citation82].
Acknowledgment
Alexa Núñez is the recipient of a Rio Hortega contract in the 2019 Strategic Action Health Call from the Instituto de Salud Carlos III for the years 2020-2022. Miriam Barrecheguren is the recipient of a Rio Hortega contract in the 2017 Strategic Action Health Call from the Instituto de Salud Carlos III for the years 2018-2019. Marc Miravitlles is the Director of the Catalan Center for Alpha-1 antitrypsin Deficiency (Barcelona, Spain); Juan Luis Rodriguez-Hermosa and Myriam Calle are the Directors of the Madrid Center for Alpha-1 antitrypsin Deficiency (Madrid, Spain); Francisco Casas-Maldonado is the Director of the Andalusian Center for Alpha-1 antitrypsin Deficiency (Granada, Spain); José Luis López-Campos is the Director of the Sevilla Center for Alpha-1 antitrypsin Deficiency (Sevilla, Spain); Maria Torres is the Director of the Galizian Center for Alpha-1 antitrypsin Deficiency (Vigo, Spain); Alexa Nuñez, Cristina Esquinas, Esther Rodríguez and Miriam Barrecheguren are members of the Catalan Center for Alpha-1 antitrypsin Deficiency (Barcelona, Spain). The Spanish centers for Alpha-1 antitrypsin Deficiency are funded by unrestricted research grants from Grifols.
Disclosure statement
Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols. José Luis López-Campos has received honoraria during the last 3 years for lecturing, scientific advice, participation in clinical studies or writing for publications for (alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi, and Teva. María Torres-Durán has received speaker fees from Chiesi, CSL Behring, Grifols and Resmed and consulting fees from CSL Behring and Grifols. Francisco Casas-Maldonado has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Gebro Pharma, GlaxoSmithKline, Laboratorios Esteve, Laboratorios Ferrer, Menarini, Novartis, Rovi, TEVA, VERTEX, Zambon, CSL Behring and Grifols and consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline, CSL Behring and Grifols. Myriam Calle has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Gebro Pharma, GlaxoSmithKline, Menarini, Novartis, CSL Behring and Grifols and consulting fees from Chiesi and GlaxoSmithKline. Juan Luis Rodriguez-Hermosa has received speaker fees from Zambon, Bial, Gebro Pharma, GlaxoSmithKline, Boehringer Ingelheim, CSL Behring and Grifols. Miriam Barrecheguren has received speaker fees from Grifols, Menarini, CSL Behring, GSK and consulting fees from GSK, Novartis, Boehringer Ingelheim and Gebro Pharma. The remaining authors report no conflicts of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- European Commission Expert Group on Rare Diseases. 2014. https://ec.europa.eu/health/sites/health/files/rare_diseases/docs/ev_20140211_mi_en.pd; 11–12 February 2014. [Accessed 2020 Jun 19].
- Public Law 107-280-NOV. 6, 2002. US Rare Diseases Act of 2002; 2002.
- National Institutes of Health Office of Rare Diseases Research. 2020. http://rarediseases.info.nih.gov/Resources/Rare_Diseases_Information.aspx. [Accessed 2020 Jun 19].
- Luisetti M, Balfour-Lynn IM, Johnson S, et al. Perspectives for improving the evaluation and access of therapies for rare lung diseases in Europe. Respir Med. 2012;106(6):759–768. DOI:10.1016/j.rmed.2012.02.016
- Perciaccante A, Charlier P, Negri C, et al. Lessons from the past: Some histories of Alpha-1 antitrypsin deficiency before its discovery. COPD. 2018;15(1):1–3. DOI:10.1080/15412555.2017.1421151
- Blanco I, Bueno P, Diego I, et al. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis. 2017;12:561–569. DOI:10.2147/COPD.S125389
- Dowson LJ, Guest PJ, Stockley RA. Longitudinal changes in physiological, radiological, and health status measurements in alpha(1)-antitrypsin deficiency and factors associated with decline. Am J Respir Crit Care Med. 2001;164(10):1805–1809. DOI:10.1164/ajrccm.164.10.2106036
- Demeo DL, Sandhaus RA, Barker AF, et al. Determinants of airflow obstruction in severe alpha-1-antitrypsin deficiency. Thorax. 2007;62(9):806–813. DOI:10.1136/thx.2006.075846
- Matamala N, Lara B, Gómez-Mariano G, et al. miR-320c Regulates SERPINA1 expression and is induced in patients with pulmonary disease. Arch Bronconeumol. 2020;S0300-2896(20):30084. DOI:10.1016/j.arbres.2020.03.006
- Esquinas C, Janciauskiene S, Gonzalo R, et al. Gene and miRNA expression profiles in PBMCs from patients with severe and mild emphysema and PiZZ alpha1-antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2017;12:3381–3390. DOI:10.2147/COPD.S145445
- Piitulainen F, Tornling G, Eriksson S. Effect of age and occupational exposure to airway irritants on lung function in non-smoking individuals with alpha-1 antitrypsin deficiency (PiZZ). Thorax. 1997;52(3):244–248. DOI:10.1136/thx.52.3.244
- Holme J, Stockley RA. Radiologic and clinical features of COPD patients with discordant pulmonary physiology: Lessons from alpha-1 antitrypsin deficiency. Chest. 2007;132(3):909–915. DOI:10.1378/chest.07-0341
- Piras B, Ferrarotti I, Lara B, et al. Clinical phenotypes of Italian and Spanish patients with α1-antitrypsin deficiency. Eur Respir J. 2013;42(1):54–64. DOI:10.1183/09031936.00104712
- Soler-Cataluña JJ, Novella L, Soler C, et al. Clinical characteristics and risk of exacerbations associated with different diagnostic criteria of asthma-COPD overlap. Arch Bronconeumol. 2020;56(5):282–290. DOI:10.1016/j.arbres.2019.08.023
- Nuñez A, Sarasate M, Loeb E, et al. Practical guide to the identification and diagnosis of asthma-COPD overlap (ACO). COPD. 2019;16(1):1–7. DOI:10.1080/15412555.2019.1575802
- Gramegna A, Aliberti S, Confalonieri M, et al. Alpha-1 antitrypsin deficiency as a common treatable mechanism in chronic respiratory disorders and for conditions different from pulmonary emphysema? A commentary on the new European Respiratory Society statement. Multidiscip Respir Med. 2018;13:39. DOI:10.1186/s40248-018-0153-4
- Cazzola M, Stolz D, Rogliani P, et al. α1-Antitrypsin deficiency and chronic respiratory disorders. Eur Respir Rev. 2020;29(155):190073. DOI:10.1183/16000617.0073-2019
- Miravitlles M, Dirksen A, Ferrarotti I, et al. European respiratory society statement: Diagnosis and treatment of pulmonary disease in alpha-1 antitrypsin deficiency. Eur Respir J. 2017;50(5):1700610. DOI:10.1183/13993003.00610-2017
- http://ec.europa.eu/health/archive/ph_threats/non_com/docs/contribution_policy.pdf. [Accessed 2020 Jun 19].
- Campos MA, Wanner A, Zhang G, et al. Trends in the diagnosis of symptomatic patients with alpha1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128(3):1179–1186. DOI:10.1378/chest.128.3.1179
- Blanco I, Diego I, Bueno P, et al. Prevalence of alpha-1 antitrypsin genotypes in patients with COPD in Europe: A systematic review. Eur Respir Rev. 2020;29(157):200014. https://doi.org/10.1183/16000617.0014-2020
- Menga G, Fernandez Acquier M, Echazarreta AL, et al. Prevalence of Alpha-1 Antitrypsin Deficiency in COPD patients in Argentina. The DAAT.AR Study. Arch Bronconeumol. 2019;S0300-2896(19):30572–30578. DOI:10.1016/j.arbres.2019.11.010
- Soriano JB, Lucas SJ, Jones R, et al. Trends of testing for and diagnosis of α1-antitrypsin deficiency in the UK: more testing is needed. Eur Respir J. 2018;52(1):1800360. DOI:10.1183/13993003.00360-2018
- Barrecheguren M, Monteagudo M, Simonet P, et al. Diagnosis of alpha-1 antitrypsin deficiency: a population-based study. Int J Chron Obstruct Pulmon Dis. 2016;11:999–1004. DOI:10.2147/COPD.S108505
- Greulich T, Ottaviani S, Bals R, et al. Alpha1-antitrypsin deficiency - diagnostic testing and disease awareness in Germany and Italy. Respir Med. 2013;107(9):1400–1408. DOI:10.1016/j.rmed.2013.04.023
- Esquinas C, Barrecheguren M, Sucena M, et al. Practice and knowledge about diagnosis and treatment of alpha-1 antitrypsin deficiency in Spain and Portugal. BMC Pulm Med. 2016;16(1):64. DOI:10.1186/s12890-016-0222-4
- [No authors listed]. Alpha 1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Organ. 1997;75(5):397–415.
- Attaway A, Majumdar U, Sandhaus RA, et al. An analysis of the degree of concordance among international guidelines regarding alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2019; 14:2089–2101. DOI:10.2147/COPD.S208591
- Miravitlles M, Herr C, Ferrarotti I, et al. Laboratory testing of individuals with severe alpha1-antitrypsin deficiency in three European centres. Eur Respir J. 2010;35(5):960–968. DOI:10.1183/09031936.00069709
- Franciosi AN, Carroll TP, McElvaney NG. Pitfalls and caveats in α1-antitrypsin deficiency testing: a guide for clinicians. Lancet Respir Med. 2019;7(12):1059–106731. DOI:10.1016/S2213-2600(19)30141-9
- Janciauskiene S, DeLuca DS, Barrecheguren M, et al. Serum Levels of Alpha1-antitrypsin and their relationship with COPD in the general Spanish population. Arch Bronconeumol. 2020;56(2):76–83. DOI:10.1016/j.arbres.2019.03.001
- Ellis P, Turner A. What Do Alpha-1 Antitrypsin levels tell us about chronic inflammation in COPD? Arch Bronconeumol. 2020;56(2):72–73. DOI:10.1016/j.arbres.2019.06.010
- Topic A, Milovanovic V, Lazic Z, et al. Oxidized Alpha-1-Antitrypsin as a potential biomarker associated with onset and severity of chronic obstructive pulmonary disease in adult population. COPD. 2018;15(5):472–478. DOI:10.1080/15412555.2018.1541448
- Sanders CL, Ponte A, Kueppers F. The effects of inflammation on alpha 1 antitrypsin levels in a national screening cohort. COPD. 2018;15(1):10–16. DOI:10.1080/15412555.2017.1401600
- Ottaviani S, Gorrini M, Scabini R, et al. C reactive protein and alpha1-antitrypsin: relationship between levels and gene variants. Transl Res. 2011;157(6):332–338. DOI:10.1016/j.trsl.2010.12.014
- Greulich T, Rodríguez-Frias F, Belmonte I, et al. Real world evaluation of a novel lateral flow assay (AlphaKit® QuickScreen) for the detection of alpha-1-antitrypsin deficiency. Respir Res. 2018;19(1):151. DOI:10.1186/s12931-018-0826-8
- López-Campos JL, Carrasco Hernandez L, Marquez-Martín E, et al. Diagnostic performance of a lateral flow assay for the detection of alpha-1-antitrypsin deficiency. Arch Bronconeumol. 2020;56(2):124–126. DOI:10.1016/j.arbres.2019.09.009
- Ottaviani S, Barzon V, Buxens A, et al. Molecular diagnosis of alpha1-antitrypsin deficiency: A new method based on Luminex technology. J Clin Lab Anal. 2020:e23279. DOI:10.1002/jcla.23279
- López-Campos JL, Casas-Maldonado F, Torres-Duran M, et al. Results of a diagnostic procedure based on multiplex technology on dried blood spots and buccal swabs for subjects with suspected alpha1 antitrypsin deficiency. Arch Bronconeumol. 2020;S0300-2896(20)30131–9. https://doi.org/10.1016/j.arbres.2020.04.014
- Martínez Bugallo F, Figueira Gonçalves JM, Martín Martínez MD. Molecular detection of the frequent Allele F52del in alpha 1 antitrypsin deficiency. Arch Bronconeumol. 2018;54(4):236. DOI:10.1016/j.arbres.2017.10.006
- Tubío-Pérez RA, Blanco-Pérez M, Ramos-Hernández C, et al. Alpha-1 antitrypsin deficiency associated with the PI*Q0ourém Allele in a 2-year-old girl and family study. An Unusual Case. Arch Bronconeumol. 2018;54(4):228–230. DOI:10.1016/j.arbres.2017.09.011
- Hernández Pérez JM, Pérez Pérez JA. Changes in the melting point of hybridization probes used for genotyping in alpha-1 antitrypsin deficiency do not always imply errors. Arch Bronconeumol. 2019;55(6):339–340. DOI:10.1016/j.arbres.2018.09.009
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. DOI:10.1183/13993003.00164-2019
- Erro Iribarren M, Alonso Pérez T, Soriano JB, et al. Adjusting the level of intervention in patients with chronic obstructive pulmonary disease according to the risk stratification proposed by the Spanish COPD Guidelines (GesEPOC) Version 2017. Arch Bronconeumol. 2020;56(3):183–185. DOI:10.1016/j.arbres.2019.09.016
- Alcázar Navarrete B, Ancochea Bermúdez J, García-Río F, et al. Patients with chronic obstructive pulmonary disease exacerbations: Recommendations for diagnosis, treatment and care. Arch Bronconeumol. 2019;55(9):478–487. DOI:10.1016/j.arbres.2019.02.020
- Pleguezuelos E, Gimeno-Santos E, Hernández C, et al. Recommendations on non-pharmacological treatment in chronic obstructive pulmonary disease from the Spanish COPD Guidelines (GesEPOC 2017). Arch Bronconeumol. 2018;54(11):568–575. DOI:10.1016/j.arbres.2018.06.001
- Edgar RG, Patel M, Bayliss S, et al. Treatment of lung disease in alpha-1 antitrypsin deficiency: a systematic review. Int J Chron Obstruct Pulmon Dis. 2017;12:1295–1308. DOI:10.2147/COPD.S130440
- Chapman KR, Burdon JG, Piitulainen E, et al. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–368. DOI:10.1016/S0140-6736(15)60860-1
- Horvath I, Canotilho M, Chlumsky J, et al. Diagnosis and management of alpha1-antitrypsin deficiency in Europe: an expert survey. ERJ Open Res. 2019;5(1):00171-2018. DOI:10.1183/23120541.00171-2018
- Boerema DJ, An B, Gandhi RP, et al. Biochemical comparison of four commercially available human α1-proteinase inhibitors for treatment of α1-antitrypsin deficiency. Biologicals. 2017;50:63–72. DOI:10.1016/j.biologicals.2017.08.010
- Esquinas C, Miravitlles M. Are there differences between the available treatments for emphysema associated with alpha-1 antitrypsin deficiency? Arch Bronconeumol. 2018;54(9):451–452. DOI:10.1016/j.arbres.2018.01.006
- Barrecheguren M, Miravitlles M. Augmentation therapy for emphysema due to alpha-1 antitrypsin deficiency: Pro. Arch Bronconeumol. 2018;54(7):363–364. DOI:10.1016/j.arbres.2018.02.002
- Gáldiz Iturri JB. Augmentation therapy nowadays: Con. Arch Bronconeumol. 2018;54(7):361–362. DOI:10.1016/j.arbres.2017.12.017
- Gotzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. Cochrane Database Syst Rev. 2010;(7):CD007851. Epub 2010/07/09.
- Dirksen A, Piitulainen E, Parr DG, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;33(6):1345–1353. DOI:10.1183/09031936.00159408
- Chorostowska-Wynimko J, Barrecheguren M, Ferrarotti I, et al. New patient-centric approaches to the management of Alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2020;15:345–355. DOI:10.2147/COPD.S234646
- Wewers MD, Casolaro MA, Sellers SE, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316(17):1055–1062. DOI:10.1056/NEJM198704233161704
- Stockley RA, Miravitlles M, Vogelmeier C, Alpha One International Registry (A.I.R.). Augmentation therapy for alpha-1 antitrypsin deficiency: Towards a personalised approach. Orphanet J Rare Dis. 2013;8:149. DOI:10.1186/1750-1172-8-149
- Campos MA, Geraghty P, Holt G, et al. The biological effects of double-dose alpha-1 antitrypsin augmentation therapy. A pilot clinical trial. Am J Respir Crit Care Med. 2019;200(3):318–326. DOI:10.1164/rccm.201901-0010OC
- Gaeckle NT, Stephenson L, Reilkoff RA. Alpha-1 antitrypsin deficiency and pregnancy. COPD. 2020;17(3):326–332. DOI:10.1080/15412555.2020.1754778
- Esteves Brandão M, Conde B, Seixas S, et al. Pulmonary Emphysema in a child with Alpha-1 antitrypsin deficiency: evaluation of 2 years of intravenous augmentation therapy. Arch Bronconeumol. 2019;55(9):502–504. DOI:10.1016/j.arbres.2019.01.009
- Stolk J, Tov N, Chapman KR, et al. Efficacy and safety of inhaled α1-antitrypsin in patients with severe α1-antitrypsin deficiency and frequent exacerbations of COPD. Eur Respir J. 2019;54(5):1900673. DOI:10.1183/13993003.00673-2019
- Pye A, Turner AM. Experimental and investigational drugs for the treatment of alpha-1 antitrypsin deficiency. Expert Opin Investig Drugs. 2019;28(10):891–902. DOI:10.1080/13543784.2019.1672656
- Stiles KM, Sondhi D, Kaminsky SM, et al. Intrapleural Gene Therapy for Alpha-1 antitrypsin deficiency-related lung disease. Chronic Obstr Pulm Dis. 2018;5(4):244–257. DOI:10.15326/jcopdf.5.4.2017.0160
- The Council of the European Union; Council recommendation of 8 June 2009 on an action in the field of rare diseases, 2009/C 151/02, available at http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C. :2009:151:0007:0010:EN:PDF; [Accessed 2020 Jun 19].
- Chorostowska-Wynimko J, Wencker M, Horváth I. The importance of effective registries in pulmonary diseases and how to optimize their output. Chron Respir Dis. 2019;16:1479973119881777. DOI:10.1177/1479973119881777
- Seersholm N, Kok-Jensen A, Dirksen A. Survival of patients with severe alpha 1-antitrypsin deficiency with special reference to non-index cases. Thorax. 1994; 49(7):695–698. DOI:10.1136/thx.49.7.695
- Piitulainen E, Tanash HA. The clinical profile of subjects included in the Swedish National Register on individuals with severe alpha-1 antitrypsin deficiency. COPD. 2015;12(sup1):36–41. DOI:10.3109/15412555.2015.1021909
- Stockley RA, Luisetti M, Miravitlles M, Alpha One International Registry (AIR) group, et al. Ongoing research in Europe: Alpha One International Registry (AIR) objectives and development. Eur Respir J. 2007;29(3):582–586. DOI:10.1183/09031936.00053606
- Stolk J, Stockley RA, Piitulainen E, et al. Relationship between change in lung density and long-term progression of lung function. Am J Respir Crit Care Med. 2015;192(1):114–116. DOI:10.1164/rccm.201502-0370LE
- Survival and FEV1 decline in individuals with severe deficiency of alpha-1 antitrypsin. The Alpha-1 Antitrypsin Deficiency Registry Study Group. Am J Respir Crit Care Med. 1998;158:49–59.
- Stoller JK, Brantly M, Fleming LE, et al. Formation and current results of a patient-organized registry for alpha(1)-antitrypsin deficiency. Chest. 2000;118(3):843–848. DOI:10.1378/chest.118.3.843
- Miravitlles M, Chorostowska-Wynimko J, Ferrarotti I, et al. The European Alpha-1 Research Collaboration (EARCO): a new ERS Clinical Research Collaboration to promote research in alpha-1 antitrypsin deficiency. Eur Respir J. 2019;53(2):1900138. DOI:10.1183/13993003.00138-2019
- Brightling C, Genton C, Bill W, et al. ERS Clinical Research Collaborations: underpinning research excellence. Eur Respir J. 2018;52(3):1801534. DOI:10.1183/13993003.01534-2018
- Greulich T, Altraja A, Barrecheguren M, et al. Protocol for the EARCO Registry: a pan-European observational study in patients with α1-antitrypsin deficiency. ERJ Open Res. 2020;6(1):00181-2019. DOI:10.1183/23120541.00181-2019
- Stockley RA. Antitrypsin deficiency assessment and programme for treatment (ADAPT): The United Kingdom registry. COPD. 2015;12(sup1):63–68. DOI:10.3109/15412555.2015.1021911
- Lara B, Blanco I, Martínez MT, et al. Spanish registry of patients with alpha-1 antitrypsin deficiency: Database evaluation and population analysis. Arch Bronconeumol. 2017;53(1):13–18. DOI:10.1016/j.arbres.2016.05.003
- Chorostowska-Wynimko J, Struniawski R, Sliwinski P, et al. The national alpha-1 antitrypsin deficiency registry in Poland. COPD. 2015;12(Suppl 1):22–26. DOI:10.3109/15412555.2015.1021915
- Koczulla R, Bittkowski N, Andress J, et al. [The German registry of individuals with alpha-1-antitrypsin deficiency-a source for research on patient care]. Pneumologie. 2008;62(11):655–658. DOI:10.1055/s-2008-1038263
- Barrecheguren M, Torres-Duran M, Casas-Maldonado F, et al. Spanish Implementation of the New International Alpha-1 antitrypsin deficiency international registry: The European Alpha-1 Research Collaboration (EARCO). Arch Bronconeumol. 2020; Mar 17. pii: S0300-2896(20)30061-2. DOI:10.1016/j.arbres.2020.02.003[Epub ahead of print].
- Sucena M, Gomes J, Guimarães C, et al. Implementation of European Alpha-1 Research Collaboration (EARCO) in Portugal: the future starts now. Pulmonology. 2020;26(4):181–183. pii: S2531-0437(20)30003-9. [Epub ahead of print]. DOI:10.1016/j.pulmoe.2020.01.003
- Bilton D, Caine N, Cunningham S, et al. Use of a rare disease patient registry in long-term post-authorisation drug studies: a model for collaboration with industry. Lancet Respir Med. 2018;6(7):495–496. DOI:10.1016/S2213-2600(18)30192-9