Abstract
This study described the participation in daily and social activities and the perceived barriers and facilitators to participation of individuals with chronic obstructive pulmonary disease (COPD). Individuals, recruited from outpatient clinics, responded to a survey on their participation in, and barriers and facilitators towards, 26 daily and social activities, divided into 3 categories: (1) physical activity and movement (PAM); (2) self-care; and (3) social engagement. For each activity, chi-square analyses were used to examine participation differences by individuals’: quartiles of airflow obstruction [percent predicted forced expiratory volume in 1 second (FEV1%predicted)] and breathlessness burden and exacerbation risk. Of the 200 participants (47% women; mean ± standard deviation age = 68 ± 9 years), most wanted to increase their participation in PAM activities (range 21–75%) and significant differences were found in 5/10 PAM activities for individuals’ breathlessness burden and exacerbation risk (e.g., more individuals than expected in group A (modified Medical Research Council breathlessness score <2 and 0–1 exacerbations in past 12 months) participated in regular exercise as much as they wanted (χ(9) 2=20.43, Cramer’s V=.23)). Regardless of the degree of airflow obstruction or breathlessness burden and exacerbation risk, the most common barrier to participation was breathlessness (p<.001, η2 p=.86) and the most common facilitator was engaging as part of their routine (p<.001, η2 p=.75). Individuals with COPD want to increase their participation in daily and social activities but are limited by breathlessness. Strategies to alleviate breathlessness should be identified/prioritized and incorporated into individuals’ daily routines to meet their self-reported participation objectives in daily and social activities.
Introduction
The adverse physiological and psychological impact of living with chronic obstructive pulmonary disease (COPD) is well documented [Citation1]. Individuals with COPD report not fully engaging in all aspects of their lives [Citation2–4]. Individuals who do not participate as much as they want in their daily lives report a lower quality of life than those who meet their personal participation objectives [Citation5,Citation6]. Thus, it is imperative to understand participation in daily and social activities among adults with COPD to improve both clinical and patient-reported health outcomes.
According to the International Classification of Functioning, Disability and Health (ICF) [Citation3], activity and participation are defined as “the execution of a task or action by an individual” and “involvement in a life situation”, respectively. Activity and participation are intertwined concepts and most often described and categorised together. The ICF domains most commonly impaired in specific diseases can be classified together to create core sets [Citation3].
The ICF obstructive pulmonary disease core set covers 71 categories, 24 of which fall under the activities and participation category (e.g., walking, moving around) [Citation2]. This ICF core set has been validated in people with COPD and healthcare providers [Citation2,Citation7,Citation8]. Across a variety of studies, research has highlighted that COPD can impair participation in all activities included in the obstructive disease ICF core set [Citation9–13]. However, the primary focus of the ICF obstructive pulmonary disease core set is individuals’ ability to move around their homes and communities. Categories focussing on social and community integration (i.e., interpersonal interactions, informal social relationships, family relationships) and daily activities (i.e., preparing and eating meals) are missing, according to individuals with COPD [Citation7]. Therefore, there lacks a full description of the participation in daily and social activities of adults with COPD to understand the activities they want to engage in more.
The primary aim of this exploratory study was to describe the participation in daily and social activities of individuals living with COPD. To this end, the Activities, Healthcare, and Research Priorities Survey (AHRPS) was co-created with individuals living with COPD and healthcare professionals. Through this survey, we sought to develop a more comprehensive understanding of the participation in daily and social activities of individuals living with COPD. The secondary aim of this study was to describe individuals’ present barriers and facilitators of participation in daily and social activities.
To gather a more complete description of individuals’ participation in daily and social activities, we further explored participation rates by grouping individuals based on: the degree of their airflow obstruction [percent predicted forced expiratory volume in 1-second (FEV1%predicted)]; breathlessness burden and exacerbation history risk; age; sex; and previous pulmonary rehabilitation (PR) participation status. These variables were explored because they are known to affect clinical and patient-reported outcomes in COPD [Citation14–17].
Methods
Participants and recruitment
Individuals with COPD were recruited from five secondary and tertiary outpatient care clinics in the Greater Montreal Area from December 2016 to October 2017. Eligible participants were (a) diagnosed with COPD by a respirologist; (b) above the age of 35; and (c) able to read and write in English or French. Ethics approval was obtained for the study from the corresponding hospital research ethics board (ethical approval #:15-203-MUHC) and the study was registered (clinicaltrials.gov protocol ID#: NCT02970422).
Potential participants were approached by their respirologist, a respiratory therapist, or a research assistant and asked if they were interested in participating in a study on their participation in daily activities. Interested individuals were first screened for eligibility and, if eligible, provided informed consent. Once enrolled, participants were sent a link to the AHRPS via email or they completed the AHRPS by telephone with a research assistant. As this was an exploratory study, sample size estimations were not performed. However, we aimed to recruit a sample of 200 participants so that we would have a relatively equal distribution of individuals based on their degree of airflow obstruction (e.g., FEV1%predicted), thus permitting exploration of responses across the disease severity spectrum; that is, from mild to very severe COPD.
Measures
Participation
Individuals’ participation in daily and social activities was measured by the AHRPS, which was modelled (structurally) after the Person-Perceived Participation in Daily Activities Questionnaire [Citation18]. To create the AHRPS, consultations with a panel of eight researchers and respirologists with clinical and/or research expertise in COPD were undertaken to generate a list of potential daily and social activities. Focus groups were then conducted with 22 individuals with COPD (27% women) to understand the daily and social activities they participated in regularly. A final list of 26 daily and social activities was created with these individuals. The daily and social activities were grouped into three categories: (i) physical activity and movement-related activities (n = 10); (ii) self-care activities (n = 8); and (iii) social engagement activities (n = 8). Individuals who responded to the survey reported their participation and satisfaction with their current level of participation for each activity by responding to the prompt “do you participate in this activity?” They responded with either: yes, as much as I want; yes, but less than I want; no, but I would like to do it; or no, and I don’t want to do it (see Supplementary material Appendix A for list of 26 activities). Individuals were said to participate as much as they wanted if they selected yes, as much as I want. Wanting to increase their participation was represented by those who selected either, yes, but less than I want or no, but I want to do it. Lastly, disengagement was said to occur when individuals selected no, and I don’t want to do it. The details of the six-step approach used to develop the AHRPS can be found in the supplemental material (Supplementary material Appendix A). The AHRPS was created as a new COPD survey, rather than a standardised measurement tool (e.g., a tool to assess changes in activity outcomes over time), to help us better understand and describe the activities individuals participate in on a regular basis and those in which they would like to increase their participation.
Barriers and facilitators
Individuals who wanted to increase their participation in any of the 26 daily and social activities were asked to select as many of the 17 listed barriers that applied to their participation in that activity (“why don’t you do the activity as much as you want and/or what makes it difficult to do this activity?” e.g., breathlessness). Individuals who participated as much as they wanted were asked to select from the list of 11 facilitators, which aided their participation in the activity (“why do you do the activity as much as you want?” e.g., I have medication to reduce breathlessness). Both the barriers and facilitators list underwent the same consultation process as the participation measure and the items were presented in a random order.
Demographics
Individuals reported their ethnicity, marital status, employment status, total household income, highest level of education achieved, year of their COPD diagnosis, tobacco smoking habits, and if they had completed a PR programme. Participants also completed the COPD assessment test (CAT) [Citation19] and the modified Medical Research Council scale (mMRC) [Citation20] to assess disease (symptom) and breathlessness burden, respectively. Comorbidities were assessed with the Charlson co-morbidity index [Citation21].
Medical charts
Data collected from medical charts included: sex; age; height; body mass; spirometric data (FEV1, FEV1-to-forced vital capacity (FVC) ratio), and exacerbation history from the past 12-months. Some medical charts were incomplete due to various hospital and clinics own charting practices and/or the loss of patient information. All available medical chart information was obtained. However, not having medical chart information did not prevent individuals from participating in the exploratory study. FEV1 was referenced to the age, sex, and height predicted normal values of Tan et al. from the Canadian population [Citation22]. Exacerbation history was defined as a change in medication due to a COPD exacerbation (i.e., the prescription of prednisone or a medical action plan) as indicated in the medical chart or hospital admission due to a COPD exacerbation. The data were used to divide respondents into groups based on their: degree of airflow obstruction (FEV1%predicted); breathlessness burden (mMRC) and exacerbation risk (exacerbation history from past 12 months); age (above/below the total sample median); sex; and PR participation status (i.e., ever vs never participated in PR).
Data analysis
To assess individuals’ airflow obstruction (disease severity), quartiles of FEV1%predicted were used. Quartiles of FEV1%predicted were used because we could not confirm that individuals’ spirometry was done post-bronchodilator. Thus, we were unable to use the Global Initiative for Obstructive Lung Disease (GOLD) COPD staging system [Citation23]. Nevertheless, all individuals self-reported a physician diagnosis of COPD and were recruited through their healthcare providers who confirmed the diagnosis. Individuals were divided into quartiles based on their FEV1% predicted, with quartiles 1, 2, 3 and 4 representing those with mild (Q1), moderate (Q2), severe (Q3) and very severe (Q4) airflow obstruction, respectively. Only individuals with a FEV1/FVC ratio <0.70 were including in the quartiles of airflow obstruction. Individuals’ breathlessness burden and exacerbation risk groups were based on their mMRC breathlessness scores and 12-month exacerbation history [Citation20]: group A (low breathlessness + low exacerbation risk), mMRC ≤1 with ≤1 exacerbation that did not lead to hospital admission; group B (high breathlessness + low exacerbation risk), mMRC ≥2 with ≤1 exacerbation not leading to hospital admission; group C (low breathlessness + high exacerbation risk), mMRC ≤1 with ≥2 exacerbations or ≥1 exacerbation that led to hospital admission; and group D (high breathlessness + high exacerbation risk), mMRC score ≥2 and ≥2 exacerbations or >1 exacerbation that led to hospital admission.
Descriptive statistics were used to report individuals’ daily and social participation and provide a picture of how respondents participated in each activity. The distribution of the scores for each activity was assessed based on quartiles of airflow obstruction, breathlessness burden and exacerbation risk, sex, age, and PR participation status. Using IBM SPSS Statistical software (v.23), chi-square analysis, with standardised residuals post hoc analyses, were performed on the participation items individually to determine if the observed value differed from the statistically expected value for quartiles of airflow obstruction, breathlessness burden and exacerbation risk, sex, age, and PR participation status. To examine the barriers and facilitators to participation, descriptive statistics were used to identify the most common barrier/facilitator. A repeated measures analysis of variance (ANOVA) was used to determine if there was a significant difference between the barrier/facilitator that was selected the most often compared to all other barriers/facilitators.
Results
Two-hundred individuals completed the AHRPS (47% women; M age=68, SD = 9; ). Of the 200 participants that completed the AHRPS (see for flow chart), a medical chart diagnosis of COPD using FEV1/FVC ratio of <0.70 could be confirmed for 148 or 74% (). These 148 individuals were divided into the four quartiles of airflow obstruction using their FEV1%predicted. The 52 other individuals were either missing medical chart information or did not have a FEV1/FVC ratio <0.70 (). Participation between those who had a FEV1/FVC ratio <0.70 (n = 148) and those that did not (presumptive COPD; n = 52), was only significantly different in one activity, Activity 16: Taking your COPD medication as prescribed by your doctor/health care provider (χ2 (3)=12.86, Cramer’s V=.25). Fewer individuals in the presumptive COPD group than expected who took their medication as prescribed as much as they wanted (Observed (O)=43, Expected (E)=48.1, standard residuals (SR)=–3.1). Given this activity was the only one that differed, the overall results are presented with the total sample.
Figure 1. Participant flow chart. Abbreviations: Activities, Healthcare and Research Priorities Survey (AHRPS); Chronic ObstructivePulmonary Disease (COPD); Quartiles 1-4 of airflow obstruction (Q1-4); modified Medical Research Council breathlessness questionnaire (mMRC); breathlessness burden and exacerbation risk groups A-D (Group A-D).
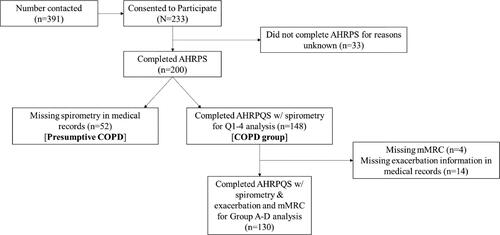
Table 1. Participants demographic information by quartiles of disease severity.
Table 2. Participant spirometry data by comparison groups.
The quartiles of airflow obstruction and the breathlessness burden and exacerbation history groups examined the portion of the sample with confirmed COPD, using FEV1/FVC ratio of <0.70 (n = 148), and with complete mMRC and 12-month exacerbation history data (n = 130). Forty-seven of the 148 individuals self-reported a diagnosis of asthma. There was no difference in the number of individuals with asthma between airflow obstruction quartiles (χ2 (3)=2.09, Cramer’s V=.12; ). When looking at participation differences between those who self-reported a diagnosis of asthma (n = 47) and those that did not (n = 101), participation in the 26 daily and social activities only significantly differed on one activity, Activity 2: Moving from one place to another using motorised transportation (χ2 (3)=8.77, Cramer’s V =.25). More individuals than expected with co-existent asthma did not participate in moving from one place to another using motorised transportation but wanted to (O = 5, E = 1.9, SR = 2.8).
Lastly, 18 individuals from the 148 were missing either mMRC or exacerbation history data (); therefore, 130 individuals were included in the breathlessness burden and exacerbation risk groups, which were based off of the GOLD ABCD grading system [Citation23]. Sixty-nine percent of these individuals fell into group B (38%) and D (31%) representing those with high breathlessness burden + low exacerbation risk and high breathlessness burden + high exacerbation risk, respectfully.
Participation in activities
On average, 45% of our total sample of 200 (range = 6 − 96%) reported participation in activities as much as they wanted across the 26 activities, while 21% (range = 0 − 55%) reported wanting to participate more in the activities (). When looking at individuals’ participation based on the quartiles of airflow obstruction, we visually observed patterns of participation according to airflow obstruction. For instance, the white-colored bar of each activity in is less prominent in participants with more severe airflow obstruction (Q2–4) than those with milder airflow obstruction (Q1), indicating lower participation in the activities and less satisfaction in that participation for those with more severe airflow obstruction. A similar trend was observed when looking across breathlessness burden and exacerbation risk groups, where those with high breathlessness burden (groups B and D) appeared to be less likely to report participating in activities as much as they wanted (i.e., white-colored bars) than those with lower breathlessness burden (groups A and C) (). These visual patterns were statistically investigated per activity as described below.
Figure 2. Overall participation frequencies (%) for each of the 26 daily and social activities (N = 200).
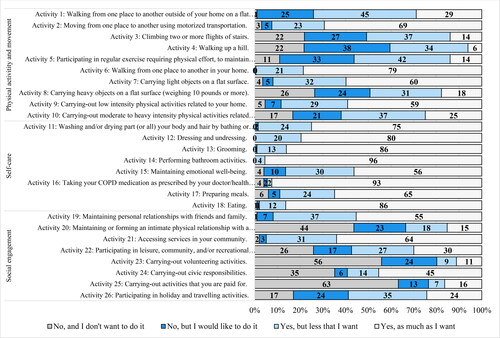
Figure 3. Participation frequencies (%) for each the 26 daily and social activities separated by quartiles of airflow obstruction (FEV1%predicted). Note: The analyses presented in text and in were performed within each activity, rather than across activities. The results are displayed by quartiles of airflow obstruction (FEV1%predicted) to simplify the presentation of results. Comparisons should be made by looking at one activity across the quartiles. Bolded red numbers indicate a standardised residual (SD) of ±2. A = Activity. Quartile 1 (mild airflow obstruction), n = 37; Quartile 2 (moderate airflow obstruction), n = 37; Quartile 3 (severe airflow obstruction), n = 37; Quartile 4 (very severe airflow obstruction), n = 37.
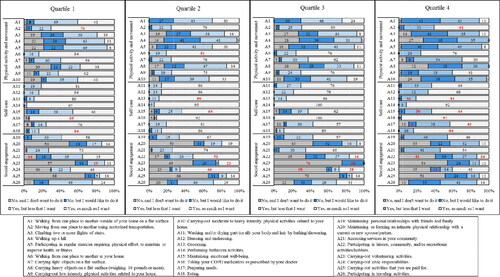
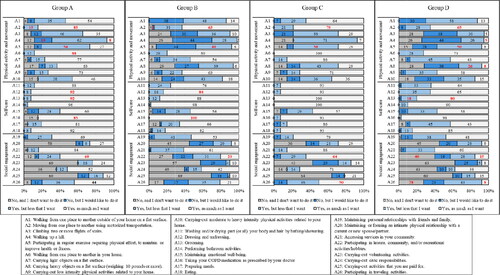
Figure 4. Participation frequencies (%) for the 26 daily and social activities separated by breathlessness burden and exacerbation risk (groups A–D). Note: The analyses presented in text and in were performed within each activity, rather than across activities. The results are displayed by levels of breathlessness burden and exacerbation risk to simplify the presentation of results. Comparisons should be made by looking at one activity across the 4 groups. Bolded red numbers indicate a standardised residual (SD) of ±2. A = Activity. Group A (low breathlessness + low exacerbation risk), n = 27; Group B (high breathlessness + low exacerbation risk), n = 50; Group C (low breathlessness + high exacerbation risk), n = 14; Group D (high breathlessness + high exacerbation risk), n = 40.
Physical activity and movement-related activities
Only 6% of all respondents reported walking up a hill (Activity 4) as much as they wanted; whereas, 72% reported wanting to increase their participation in this activity (). Participation only differed significantly for this activity when looking at the breathlessness burden and exacerbation history risk. There were more individuals than expected in groups A and C who participated but wanted to increase their participation (O = 16, E = 10.6, SR = 2.7; O = 10, E = 5.4, SR = 2.7, respectively; ); whereas, there were fewer individuals in groups B and D who participated but wanted to increase their participation than expected (O = 14, E = 19.4, SR=–2.0; O = 10, E = 15.1, SR= −2.0, respectively). Similarly, 75% of all participants wanted to increase their participation in regular exercise (Activity 5), but only 14% participated as much as they wanted. There were more individuals than expected in the low breathlessness + low exacerbation risk group (group A) that participated as much as they wanted (O = 7, E = 3.6; SR = 2.2; ); as, well as more people than expected in Q2 with moderate airflow obstruction (O = 11, E = 5.3, SR =3.1). More than 60% of individuals wanted to increase their participation in climbing up two or more flights of stairs (Activity 3) and walking from one place to another outside their home on a flat surface (Activity 1; ). More individuals than expected with low breathlessness + low exacerbation risk (group A) participated in both Activity 1 and 3 as much as they wanted (SRs ≥ 3.0; ; ). Yet, fewer individuals than expected in the high exacerbation risk groups (groups B and D) participated as much as they wanted in walking from one place to another outside their home on a flat surface (O = 7, E = 13.5, SR= −2.6; O = 5, E = 10.8, SR=–2.5, respectively; ; ). As well, there were more individuals in Q4 with very severe airflow obstruction than expected who did not participate in the activity but wanted to (O = 16, E = 10.0, SR = 2.6).
Table 3. Chi-squared (χ2) for each quartiles of airflow obstruction and breathlessness burden.
Individuals also wanted to increase their participation in carrying heavy objects on a flat surface (Activity 8; 56%) and carrying-out moderate to heavy intensity physical activities related to their home (Activity 10; 58%). For Activity 10, we found that there were more individuals in Q4 with very severe airflow obstruction than expected that did not participate but wanted to (O = 14, E = 8.3, SR = 2.6; ; ). For Activity 8, there were also more individuals in Q4 with very severe airflow obstruction than expected that did not participate but wanted to (O = 14, E = 9.1, SR = 2.2); while, more individuals in Q2 with moderate airflow obstruction than expected participated, but wanted to increase their participation (O = 17, E = 11.8, SR = 2.1). Furthermore, there were more individuals in Q1 with mild airflow obstruction than expected who participated as much as they wanted (O = 14, E = 5.8, SR = 4.3). Similarly, we found that more individuals than expected in the low breathlessness + low exacerbation risk group (group A) and fewer in the high breathlessness + low exacerbation risk (group B) participated in the activity as much as they wanted (O = 12, E = 4.5, SR = 4.4; O = 3 E = 8.4, SR=–2.6, respectively).
Over 59% of individuals indicated participating in walking from one place to another in their home (Activity 6), carrying light objects on a flat surface (Activity 7), and carrying-out low intensity physical activity related to their homes (Activity 9) as much as they wanted (). There were more individuals in Q4 with very serve airflow obstruction who participated in carrying light objects but wanted to increase their participation than expected (O = 20, E = 12.5, SR = 3.0). For low intensity physical activity (Activity 9), there were more individuals in Q4 than expected that did not participate but wanted to (O = 7, E = 2.8, SR = 3.1).
Self-care activities
Most respondents were satisfied with their participation in self-care activities (range 56–96%; ). Differences were found for dressing and undressing, preparing meals, and taking your COPD medication. For dressing and undressing (Activity 12), there were fewer individuals in the high breathlessness + high exacerbation risk group (group D) than expected who participated as much as they wanted (O = 26, E = 32.3, SR=–3.1; ; ). A similar difference was found for preparing meals (Activity 17): fewer individuals in group D than expected participated as much as they wanted (O = 17, E = 25.5, SR=–3.4); more individuals in Q2 with moderate airflow obstruction and fewer in Q4 with very severe airflow obstruction than expected who participated as much as they wanted in preparing meals (O = 30, E = 23.8, SR = 2.5; O = 16, E = 23.8, SR=–3.1, respectively).
For taking COPD medication as prescribed by your healthcare provider (Activity 16), there were fewer individuals in the low breathlessness + low exacerbation group (group A) and more individuals in the high breathlessness + low exacerbation group (group B) than expected participated as much as they wanted (O = 22, E = 24.8, SR=-2.9; O = 50, E = 47.7, SR = 2.0, respectively; ).
Social engagement activities
Most individuals participated as much as they wanted in maintaining personal relationships with friends and family (Activity 19; 55%) and accessing services in their communities (Activity 21; 64%; ). Individuals (63%) did not want to participate in carrying-out activities they were paid for (Activity 25) and 56% did not want to participate in carrying-out volunteering activities (Activity 23). For volunteering activities and carrying-out civic responsibilities (Activity 24), there were more individuals than expected in Q3 with serve airflow obstruction that neither participated nor wanted to increase their participation (O = 28, E = 21.8, SR = 2.4; O = 21, E = 13.2, SR = 3.1).
Participants indicated they wanted to increase their participation in holiday and travelling activities (Activity 26; 59%) and leisure, community, and/or recreational hobbies/activities (Activity 22; 54%). For holiday and leisure activities, there were more individuals with low breathlessness (groups A and C) who participated as much as they wanted than expected (O = 11, E = 6.1, SR = 2.5; O = 7, E = 3.4, SR = 2.4, respectively). However, there were fewer individuals than expected in the high breathlessness + high exacerbation risk group (group D) who participated as much as they wanted (O = 3, E = 9.8, SR=-3.0; ; ).
For recreational activities, there were more individuals than expected in the moderate airflow obstruction group (Q2) and fewer in both the severe (Q3) and very severe airflow obstruction groups (Q4) who participated as much as they wanted (O = 18, E = 10.1, SR = 3.4; O = 6, E = 10.6, SR=–2.0; O = 5, E = 10.6, SR=–2.4, respectively). For breathlessness burden and exacerbation risk, more individuals than expected in the low exacerbation groups (groups A and C; O = 15, E = 7.8, SR = 3.5; O = 9, E = 4.4, SR = 2.8, respectively) and fewer in the high exacerbation groups (groups B and D; O = 10, E = 15.3, SR=–2.1; O = 6, E = 12.5, SR=-2.7, respectively) participated as much as they wanted in recreational activities ().
Age, sex and PR differences
Only a few activities differed by age, sex, and PR participation status. For age and sex, the overall sample (n = 200) was used for analysis, while PR participation looked at those with confirmed COPD using FEV1/FVC ratio <0.70 (n = 148). For age, there were differences in Activity 9: carrying-out low intensity physical activity related to your home, Activity 19: maintained personal relationships with family and friends, and Activity 25: carrying-out activities you were paid for. Sex differences were also found for Activity 25, as well as Activity 2: moving from one place to another using motorised transportation, Activity 7: carrying light objects on a flat surface and Activity 20: maintaining or forming an intimate physical relationship with a current or new spouse/partner. Lastly for PR participation status, the only activity that had significant differences was Activity 5: Participating in regular exercise requiring physical effort, to maintain or improve health or fitness. Details of the significant findings can be found in the supplemental documents (Supplementary material Appendix C).
Barriers and facilitators
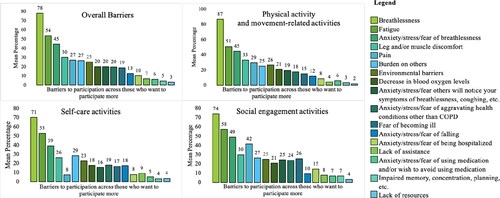
Regardless of the degree of airflow obstruction (Q1–4), most individuals (78%) selected breathlessness as a barrier at least once, and it was selected significantly more often than all other barriers (F (4.687,112.485)=150.62, p<.001, η 2 p=.86, using Greenhouse–Geisser correction [Citation24]; ). The second most common barrier was fatigue (53%), followed by anxiety/stress/fear of breathlessness (44%). For physical activity and movement-related activities, breathlessness and fatigue were selected as barriers by 87% and 51% of individuals who wanted to increase with their participation in at least one activity, respectively. Breathlessness and fatigue were selected as barriers at a similar rate across the other categories ().
Figure 5. Barriers to participation, overall and by each category of activity*. *Activity 16: Taking your medication as prescribed by your healthcare provider has different barriers and facilitators and was not included in this analysis. The top barrier for taking your medication was my COPD medication(s) is/are used too often; however, it was only selected by 2 responders.
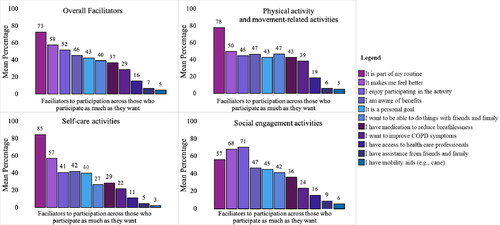
The most common facilitator for participation in the activities was being part of their routine (73% selected the facilitator at least once), followed by participating makes them feel better (58%) and they enjoy participating in the activity (52%; ). Participating in an activity because it was part of their routine was selected significantly more often than all other facilitators (F (3.064,73.545)=70.40, p<.001, η2 p=.75, using Greenhouse–Geisser correction [Citation24]; ). Being part of their routine (78 and 85%) and making them feel better (50 and 57%) were the top two facilitators in physical activity and movement-related activities and self-care activities, respectively. However, the top two facilitators were different in the social engagement category ().
Figure 6. Facilitators to participation, overall and by each category of activity*. *Activity 16: Taking your medication as prescribed by your healthcare provider had different barriers and facilitators and was not included in this analysis. The top facilitator was part of my routine selected by 84% of respondents, followed by my prescribed COPD medication(s) relieve my COPD symptoms (76%).
Discussion
This study provided new insights into the daily and social activities that individuals with COPD participate in and want to participate in more regularly. Individuals with COPD primarily wanted to increase their participation in physical activity and movement-related activities, as only 26% were participating as much as they wanted. Individuals were satisfied with their participation in self-care activities and wanted to increase their participation in social engagement activities. Further, individuals indicated that the most common facilitator for participation was having activities be part of their routine; however, individuals were most often limited in their participation by breathlessness and fatigue.
Although all participants were recruited from COPD outpatient clinics in a secondary or tertiary care centre, 52 individuals (26%) were either missing FEV1/FVC ratio data from their medical chart or had a FEV1/FVC ratio >0.70. These individuals likely represent a portion of the COPD population who does not fit under the spirometry diagnostic criteria guidelines. In fact, debates on spirometry as the sole diagnostic tool for COPD are emerging [Citation25–27]. Thus, our participants were likely typical of what is seen in real-world clinics. A portion of our sample was also living with both asthma and COPD, often referred to as the asthma–COPD overlap syndrome (ACOS). Researchers have estimated that individuals with ACOS represent 15-25% of the COPD population [Citation28,Citation29]. In this study, 32% of participants indicated they also had asthma. However, there was no difference in the representativeness of these individuals across the quartiles of airflow obstruction. Individuals living with asthma experience similar symptoms as those with COPD (e.g., breathlessness, chest tightness) [Citation30]. While clinical management of ACOS may differ from that of COPD or asthma alone, the individuals in our study with both COPD and asthma were equally limited in their participation of daily and social activities. These individuals represent a rather prevalent and clinically relevant group of adults regularly seen by respiratory specialists in COPD outpatient clinics in secondary or tertiary care centres. By keeping individuals with self-reported asthma, as well as those who did not fit under the spirometric diagnostic criteria of COPD, in our study, we obtained a real-world picture of participation for all adults who live with symptoms associated with COPD.
Most individuals in this study indicated wanting to increase their participation in physical and movement-related activities. These results align with the ICF core set for obstructive pulmonary disease as activities that include walking, moving around, dressing, doing housework and carrying-out one’s daily routine are outlined as the most commonly affected in individuals living with COPD and present the highest level of burden [Citation2]. There is value in identifying ways to increase physical activity participation to meet the needs of individuals with COPD and because physical activity has been shown to decrease breathlessness in individuals living with COPD [Citation31,Citation32]. However, adhering to physical activity is challenging as highlighted by individuals who report having difficulty maintaining their physical activity levels, even after a PR programme [Citation33–36]. Although people with COPD struggle to maintain physical activity as part of their day life, individuals in this study were motivated to increase their participation.
Individuals in this study also reported a desire for increased participation in social engagement activities. Social isolation is often cited as a negative consequence of living with COPD [Citation33,Citation37]; however, behavioural interventions tend to target other behaviours (e.g., improvement in health status or physical activity participation), rather than social isolation specifically. For example, group-based physical activity interventions have been shown to reduce social isolation during the programme, but the group often dissolves after the intervention [Citation35,Citation36]. Future research should focus on how social interactions among individuals living with COPD can be fostered and maintained over time. For example, the Quebec Lung Association hosts regular support group meeting for individuals living with COPD [Citation38]. These support groups are ongoing, and individuals learn about COPD while interacting with others. These community-based programmes have the potential to decrease social isolation (and perhaps also improve physical activity participation), while being sustainable over the long-term.
The ICF core set also fails to capture all social activities [Citation7]. For instance, maintaining personal relationships with friends and family, and participating in holiday/travelling activities are not captured in the ICF core set. The brief ICF obstructive pulmonary disease core set may consider adding such social activities to capture the apparent needs of individuals with COPD. From a practical standpoint, identifying that individuals with COPD want to increase their participation in such social activities can help future programmes, such as community support groups, target the behaviours that are important to individuals living with COPD.
Furthermore, intimate and sexual relationships are known to be important for individuals living with COPD, but difficult to engage in due to breathlessness [Citation39,Citation40]. In this study, men were not satisfied with their participation in this activity (Supplementary material Appendix C). The distribution of responses may reflect a lack of sexual education or knowledge on how to best engage in intimate relationships when living with COPD [Citation39]. However, it may also be that individuals with COPD are not able to manage their breathlessness while being intimate. A better understanding of educational programmes, such as the Living Well with COPD PR curriculum [Citation41], which explains how to maintain an intimate relationship while minimising breathlessness, is needed to ensure that healthcare providers are meeting the needs of their patients.
In this study, individuals’ participation in daily and social activities was impacted primarily by the degree of airflow obstruction and the level of breathlessness burden and exacerbation risk. Of the 26 activities assessed, only four (15%) differed by sex, three (11.5%) by age, and 1 (3.8%) by PR participation status. It follows that the results of our study support current national [Citation42] and international clinical practice guidelines [Citation23] for COPD management that focus on an individual patient’s disease severity and/or breathlessness burden and exacerbation risk when trying to increase participation in daily and social activities.
Regardless of how the data were examined (i.e., Q1–4, group A–D, etc.), breathlessness was identified as the most common barrier for individuals’ participation across most activities, followed by fatigue and anxiety/stress/fear of breathlessness. Sustained breathlessness is the primary symptomatic manifestation of COPD [Citation1] and is the primary or co-primary factor that limits most individuals’ exercise tolerance (i.e., peak capacity for exercise) [Citation43]. While breathlessness prevents people with COPD from participating in daily and social activities, there are both pharmacologic and non-pharmacologic interventions - beyond inhaled bronchodilators, supplemental oxygen and PR—that can be used to minimise the impact of breathlessness on individuals’ daily lives [Citation44–46]. Another barrier that is amenable to intervention is fatigue [Citation47], which was selected by over 50% of our respondents. Continued efforts are needed to identify new and/or more effective approaches to alleviate breathlessness and reduce fatigue to help people with COPD to participate more regularly in daily and social activities.
Individuals in this study indicated that the most common facilitator to participating in daily and social activities was that they were part of their daily routine. However, changing one’s routine or incorporating new behavioural elements takes time and can be difficult depending on one’s environment and resources. For example, most PR programmes are not successful at getting individuals to maintain their exercise participation at follow up [Citation48]. This study found that those who completed a PR programme reported not participating in regular exercise as much as they wanted. Self-management or educational interventions may be a possible modality for examining how to best motivate individuals to take up new behaviours and incorporate them into their lives. For instance, self-management strategies have been shown to help individuals living with COPD continue their participation in activities over time [Citation49]. As well, interventions that used behaviour change techniques, such as implementation intentions [Citation50,Citation51] action planning [Citation52,Citation53], or cognitive behavioural therapy [Citation54] have been shown to help individuals with COPD as well as other clinical conditions (e.g., mental illness) improve their routine physical activity participation [Citation55]. Including behaviour change techniques in exercise and/or pharmacological interventions may help long-term participation in daily and social activities among individuals living with COPD [Citation56,Citation57]. Yet, little research exists on the best suited and most effective behaviour change techniques for this population.
Methodological considerations
This descriptive study included a relatively large sample (n = 200) of elderly and symptomatic COPD outpatients, with a roughly equal distribution of men and women, who were recruited from secondary and tertiary care clinics in the Greater Montreal Area. Participants were also living with a broad range of airflow obstruction, breathlessness burden and exacerbation risk. These types of patients are most often managed by respiratory specialists and/or are under the care of a family physician. Their input highlights the need for more effective management of troublesome symptoms (most notably breathlessness and fatigue) and the identification of novel ways of incorporating healthy behaviours into the daily routine of these patients. However, the findings may only be generalisable to this population of patients and not necessarily to younger and/or less symptomatic adults being managed exclusively in primary care. Similarly, 45% of the participants had completed PR, which is much higher than national averages (i.e., 0.4% of Canadians have access to PR and 3.7% of Americans have participated) [Citation58,Citation59]. For those who consented to participate, 86% completed the study. However, as these individuals were recruited primarily in clinics linked to university affiliated research hospitals, the final sample of 200 individuals may be biased towards those who are more informed about their condition and/or those who have participated in clinical research in the past. This recruitment strategy may contribute to the higher percentage of individuals who had completed PR, compared to the national averages. Yet, there was only a one activity in which having participated in PR made a difference in their participation levels.
Disease severity was not calculated based off of the GOLD I-IV classification system [Citation23], as it could not be ensured that all spirometric pulmonary function tests were done post-bronchodilator. Therefore, quartiles of airflow obstruction were used, which limits direct comparisons to other samples, even though mean values of FEV1%predicted in Q1–4 were consistent with those used in the GOLD I-IV staging criteria, respectively. For breathlessness burden and exacerbation risk, we used individuals mMRC ratings and 12-month exacerbation history to divide individuals into groups that followed the GOLD ABCD criteria [Citation23]. When looking at our breathlessness burden and exacerbation risk groups, there were fewer individuals in the high breathlessness + low exacerbation risk group (group C, n = 14) than each of the other three groups. However, this pattern is similar to that of other, larger studies wherein relatively few adults meet GOLD criteria for being classified as having more symptoms and low exacerbation risk, i.e., GOLD group C [Citation60]. Therefore, it mimics what healthcare providers often encounter in clinical practice.
In terms of comorbidities present in our sample, 32% had asthma, 12.5% had cancer (unspecified) and 9% had diabetes mellitus, among other comorbidities. It is impossible to determine the impact living with these comorbidities had on our individual’s reported levels of participation above and beyond that associated with COPD alone. However, the distribution of comorbidities was similar to that of other studies [Citation61,Citation62] and did not differ across the quartiles of airflow obstruction or breathlessness burden and exacerbation risk groups. Individuals with COPD often live with comorbidities [Citation23]; therefore, by having these individuals represented in our sample, we better reflect the current demographics of individuals living with COPD.
Lastly, although individuals were asked if they wanted to participate in various daily and social activities, those who did not want to participate were not asked to identify their reason(s) for not wanting to participate, nor did we ask how long it took our participants to perform each activity. It would be important for future research to understand individuals’ reason for disengagement and how long it takes to perform the daily and social activities.
Conclusion
This study is unique in that it provides empirical evidence substantiating observations made by healthcare providers and qualitative studies that individuals living with COPD would like to engage in more physical activity but are limited by their symptoms of breathlessness and fatigue. By expanding the measurement of daily and social activities from the ICF obstructive pulmonary disease core set to the list generated by healthcare professionals and researchers in close collaboration with individuals living with COPD, we were able to highlight additional activities important for individuals living with COPD that are not only related to mobility but social activities as well. From a clinical management perspective, our results highlight the importance of healthcare providers effectively managing symptoms of breathlessness and fatigue as well as discussing strategies for physical activity participation. Research identifying key behavioural strategies and interventions to incorporate health behaviours into individuals’ daily routine may be one avenue (based on our results) to enhance participation and satisfaction in daily and social activities (with associated improvements in health status and quality of life) for individuals living with COPD.
Conflict of interest
In accordance with Taylor & Francis policy and our ethical obligation as researchers, we are reporting that Dr. Jensen received Investigator Initiated Funding from AstraZeneca Canada and Dr. Smith received research salary support from Quebec Health Research Fund, which may have affected the research reported in the enclosed paper. We have disclosed those interests fully to Taylor & Francis, and we have in place an approved plan for managing any potential conflicts arising from the funding and research salary support. No potential conflict of interest was reported by the other authors.
Supplemental Material
Download PDF (313.8 KB)Acknowledgments
The authors would like to thank Alexandra Schram, Frank Niro and Evan Bishop for their help throughout this project, particularly as it related to recruiting participants. As well, the authors would like to thank the two individuals living with COPD who actively participated in the working group and shared their experiences to ensure the AHRPS represented the needs of individuals living with COPD.
Additional information
Funding
References
- Disler RT , Green A , Luckett T , et al. Experience of advanced chronic obstructive pulmonary disease: metasynthesis of qualitative research. J Pain Symptom Manage. 2014;48(6):1182–1199. DOI:10.1016/j.jpainsymman.2014.03.009
- Stucki A , Stoll T , Cieza A , et al. ICF core sets for obstructive pulmonary diseases. J Rehabil Med. 2004;36(0):114–120. DOI:10.1080/16501960410016794
- World Health Organization . International classification of functioning, disability and health: ICF. WHO Library Cataloging-in-Publication Data. 2001; p. 1–299.
- Joshi M , Joshi A , Bartter T . Symptom burden in chronic obstructive pulmonary disease and cancer. Curr Opin Pulm Med. 2012;18(2):97–103. DOI:10.1097/MCP.0b013e32834fa84c
- Halding A-G , Wahl A , Heggdal K , et al . 'Belonging'. 'Patients' experiences of social relationships during pulmonary rehabilitation. Disabil Rehabil. 2010;32(15):1272–1280. DOI:10.3109/09638280903464471
- Nakamura Y , Tanaka K , Shigematsu R , et al. Effects of aerobic training and recreational activities in patients with chronic obstructive pulmonary disease. Int J Rehabil Res. 2008;31(4):275–283.
- Marques A , Jácome C , Gabriel R , et al . Comprehensive ICF core set for obstructive pulmonary diseases: validation of the activities and participation component through the patient's perspective. Disabil Rehabil. 2013;35(20):1686–1691. DOI:10.3109/09638288.2012.750691
- Jácome C , Marques A , Gabriel R , et al. Chronic obstructive pulmonary disease and functioning: implications for rehabilitation based on the ICF framework. Disabil Rehabil. 2013;35(18):1534–1545. DOI:10.3109/09638288.2012.745625
- Annegarn J , Meijer K , Passos VL , et al. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J Am Med Dir Assoc. 2012;13(3):284–290. DOI:10.1016/j.jamda.2011.01.002
- Barnett M . Chronic obstructive pulmonary disease: a phenomenological study of patients' experiences. J Clin Nurs. 2005;14(7):805–812. DOI:10.1111/j.1365-2702.2005.01125.x
- Gysels M , Higginson IJ . Access to services for patients with chronic obstructive pulmonary disease: the invisibility of breathlessness. J Pain Symptom Manage. 2008;36(5):451–460. DOI:10.1016/j.jpainsymman.2007.11.008
- McCabe C , Dinsmore J , Brady AM , et al. Using action research and peer perspectives to develop technology that facilitates behavioral change and self-management in COPD. Int J Telemed Appl. 2014;2014:380919. DOI:10.1155/2014/380919
- Svedsater H , Roberts J , Patel C , et al. Life impact and treatment preferences of individuals with asthma and chronic obstructive pulmonary disease: results from qualitative interviews and focus groups. Adv Ther. 2017;34(6):1466–1481. DOI:10.1007/s12325-017-0557-0
- Martinez CH , Diaz AA , Parulekar AD , et al. Age-related differences in health-related quality of life in COPD: an analysis of the COPDGene and SPIROMICS cohorts. Chest. 2016;149(4):927–935. DOI:10.1016/j.chest.2015.11.025
- Gruenberger JB , Vietri J , Keininger DL , et al. Greater dyspnea is associated with lower health-related quality of life among European patients with COPD. COPD. 2017;12:937–944. DOI:10.2147/COPD.S123744
- Kokturk N , Kilic H , Baha A , et al. Sex difference in chronic obstructive lung disease. Does it matter? A concise review. J Chronic Obstruct Pulmon Dis. 2016;13(6):799–806. DOI:10.1080/15412555.2016.1199666
- Reardon J , Awad E , Normandin E , et al. The effect of comprehensive outpatient pulmonary rehabilitation on dyspnea. Chest. 1994;105(4):1046–1052. DOI:10.1378/chest.105.4.1046
- Noreau L , Cobb J , Be LM , et al. Development and assessment of a community follow-up questionnaire for the rick Hansen spinal cord injury registry. Arch Phys Med Rehabil. 2013;94(9):1753–1765. DOI:10.1016/j.apmr.2013.03.006
- Jones PW , Harding G , Berry P , et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. DOI:10.1183/09031936.00102509
- Vogelmeier C , Criner G , Martinez F , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017:0–22.
- Charlson M , Szatrowski TP , Peterson J , et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. DOI:10.1016/0895-4356(94)90129-5
- Tan WC , Bourbeau J , Hernandez P , et al. Canadian prediction equations of spirometric lung function for Caucasian adults 20 to 90 years of age: results from the Canadian Obstructive Lung Disease (COLD) study and the Lung Health Canadian Environment (LHCE) study. Can Respir J. 2011;18(6):321–326. DOI:10.1155/2011/540396
- GOLD . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2018 report. Global Initiative for Chronic Obstuctive Pulmonary Disease; 2018.
- Field A . In: Carmichael M , editors. Discovering statistics using IBM SPSS Statistics. 3rd ed. London (UK): Sage; 2013. p. 1–915.
- Keener A . Redefining a disease: a proposal to expand the diagnostic criteria for chronic obstructive pulmonary disease puts overlooked groups of patients in the spotlight. Nature. 2020;581(7807):S4–S6. DOI:10.1038/d41586-020-01373-x
- Lowe KE , Regan EA , Anzueto A , et al. COPDGene® 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstruct Pulmon Dis. 2019;6(5):384–399. DOI:10.15326/jcopdf.6.5.2019.0149
- Young KA , Strand MJ , Ragland MF , COPDGene® Investigators, et al. Pulmonary subtypes exhibit differential global initiative for chronic obstructive lung disease spirometry stage progression: the COPDGene® study. Chronic Obstruct Pulmon Dis. 2019;6(5):414–429. DOI:10.15326/jcopdf.6.5.2019.0155
- Cosio BG , Soriano JB , López-Campos JL , et al. Defining the asthma–COPD overlap syndrome in a COPD cohort. Chest. 2016;149(1):45–52. DOI:10.1378/chest.15-1055
- Papaiwannou A , Zarogoulidis P , Porpodis K , et al. Asthma–chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis. 2014;6(Suppl 1):3–8.
- Postma DS , Rabe KF . The asthma–COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. DOI:10.1056/NEJMra1411863
- McCarthy B , Casey D , Devane D , et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2015(2):1–209.
- Robinson H , Williams V , Curtis F , et al. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):1–12.
- Hogg L , Grant A , Garrod R , et al. People with COPD perceive ongoing, structured and socially supportive exercise opportunities to be important for maintaining an active lifestyle following pulmonary rehabilitation: A qualitative study. J Physiother. 2012;58(3):189–195. DOI:10.1016/S1836-9553(12)70110-8
- Thorpe O , Kumar S , Johnston K . Barriers to and enablers of physical activity in patients with COPD following a hospital admission: a qualitative study. Int J Chronic Obstruct Pulmon Dis. 2014;9:115–128.
- Thorpe O , Johnston K , Kumar S . Barriers and enablers to physical activity participation in patients with COPD: a systematic review. J Cardiopulm Rehabil Prev. 2012;32(6):359–369. DOI:10.1097/HCR.0b013e318262d7df
- Poureslami I , Camp P , Shum J , et al . Using exploratory focus groups to inform the development of a peer-supported pulmonary rehabilitation program: directions for further research. J Cardiopulm Rehabil Prev. 2017;37(1):57–64. DOI:10.1097/HCR.0000000000000213
- Skingley A , Clift S , Hurley S , et al. Community singing groups for people with chronic obstructive pulmonary disease: participant perspectives. Perspect Public Health. 2018;138(1):66–75. DOI:10.1177/1757913917740930
- Pulmonaire Du Québec A, Pulmonaire Du Québec G. d A. 2019. [cited 2019 May 27]. Available from: https://pq.poumon.ca/programmes-et-services/groupes-entraide/.
- Hahn K . Sexuality and COPD. Rehabil Nurs. 1989;14(4):191–195. DOI:10.1002/j.2048-7940.1989.tb01094.x
- Ekström M , Johnson MJ , Taylor B , et al. Breathlessness and sexual activity in older adults: the Australian Longitudinal Study of Ageing. NPJ Prim Care Respir Med. 2018;28(1):20.
- Bourbeau J , Nault D , Sedeno M , et al. Living well with COPD: keeping a healthy and fulfilling lifestyle. 2005; [cited 2019 May 27]. Available from: https://www.livingwellwithcopd.com/DATA/DOCUMENT/59_en∼v∼keeping-a-healthy-and-fulfilling-lifestyle.pdf.
- Marciniuk DD , Goodridge D , Hernandez P , Canadian Thoracic Society COPD Committee Dyspnea Expert Working Group, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18(2):69–78. DOI:10.1155/2011/745047
- O’Donnell DE , Aaron S , Bourbeau J , et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Can Respir J. 2007;14(Suppl b):5B–32B. DOI:10.1155/2007/926421
- Hanania NA , O'Donnell DE . Activity-related dyspnea in chronic obstructive pulmonary disease: physical and psychological consequences, unmet needs, and future directions. Int J Chron Obstruct Pulmon Dis. 2019;14:1127–1138. DOI:10.2147/COPD.S188141
- Booth S , Moffat C , Burkin J , et al. Nonpharmacological interventions for breathlessness. Curr Opin Support Palliat Care. 2011;5(2):77–86. DOI:10.1097/SPC.0b013e3283460c93
- Vogelmeier CF , Román-Rodríguez M , Singh D , et al. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938.
- Şahin ZA , Dayapoğlu N . Effect of progressive relaxation exercises on fatigue and sleep quality in patients with chronic obstructive lung disease (COPD). Complement Ther Clin Pract. 2015;21(4):277–281. DOI:10.1016/j.ctcp.2015.10.002
- Lacasse Y , Wong E , Guyatt GH , et al. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet. 1996;348(9035):1115–1119. 3 DOI:10.1016/S0140-6736(96)04201-8
- Zielhuis G , van der Palen J , van der Valk P , et al. Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med. 2011;105(3):418–426. DOI:10.1016/j.rmed.2010.09.017
- De Vet E , Oenema A , Brug J . More or better: do the number and specificity of implementation intentions matter in increasing physical activity? Psychol Sport Exerc. 2011;12(4):471–477. DOI:10.1016/j.psychsport.2011.02.008
- Michie S , Richardson M , Johnston M , et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. DOI:10.1007/s12160-013-9486-6
- Carraro N , Gaudreau P . Spontaneous and experimentally induced action planning and coping planning for physical activity: a meta-analysis. Psychol Sport Exerc. 2013;14(2):228–248. DOI:10.1016/j.psychsport.2012.10.004
- Wiedemann AU , Lippke S , Reuter T , et al. The more the better? the number of plans predicts health behaviour change. Appl Psychol Heal Well-Being. 2011;3(1):87–106. DOI:10.1111/j.1758-0854.2010.01042.x
- Williams MT , Johnston KN , Paquet C . Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. COPD. 2020;15:903–919. DOI:10.2147/COPD.S178049
- Göhner W , Dietsche C , Fuchs R . Increasing physical activity in patients with mental illness: a randomized controlled trial. Patient Educ Counsel. 2015;98(11):1385–1392. DOI:10.1016/j.pec.2015.06.006
- Troosters T , Maltais F , Leidy N , et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(8):1021–1032. DOI:10.1164/rccm.201706-1288OC
- Lavoie KL , Sedeno M , Hamilton A , et al. Behavioural interventions targeting physical activity improve psychocognitive outcomes in COPD. ERJ Open Res. 2019;5(4):00013–2019. DOI:10.1183/23120541.00013-2019
- Camp PG , Hernandez P , Bourbeau J , et al. Pulmonary rehabilitation in Canada. A report from the Canadian Thoracic Society/Canadian Respiratory Health Professionals COPD Clinical Assembly. Can Respir J. 2015; 22(3):147–153. DOI:10.1155/2015/369851
- Nishi SPE , Zhang W , Kuo Y-F , et al. Pulmonary rehabilitation use in older adults with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2003–2012;36(5):375–382.
- Radovanovic D , Contoli M , Marco FD , et al. Clinical and functional characteristics of COPD patients across GOLD classifications: results of a multicenter observational study. COPD J Chronic Obstruct Pulmon Dis. 2019;16(3–4):215–226. DOI:10.1080/15412555.2019.1659760
- Divo M , Cote C , De Torres JP , et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. DOI:10.1164/rccm.201201-0034OC
- Mantoani LC , Dell'Era S , MacNee W , et al. Physical activity in patients with COPD: the impact of comorbidities. Expert Rev Respir Med. 2017;11(9):685–698. DOI:10.1080/17476348.2017.1354699
