Abstract
Whether there are increased rates of chronic diseases associated with the combination of chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) overlap syndrome (OVS) has not been determined. The purpose of this study was to assess the prevalence of five comorbidities in COPD and OVS patients. A total of 968 patients with confirmed COPD were included in this study. Participants were requested to fill out a questionnaire involving their basic information and medical history. All subjects underwent one overnight polysomnography and were then divided into an OVS group or a COPD only group according to their apnea–hypopnea index. The prevalence of hypertension, diabetes, cardiovascular disease, arrhythmia and cerebrovascular disease were compared and risk factors for comorbidities in COPD patients were identified. Compared with the COPD only group, the prevalence of hypertension was significantly higher in the OVS group, however, the prevalence rates of the other four kinds of diseases were not statistically different between the two groups. In COPD patients, the prevalence of hypertension increased with the severity of OSA and the prevalence of arrhythmia increased with airflow limitation severity. Risk factors for OSA in patients with COPD included BMI, FEV1%, Epworth Sleepiness Scale score and the Sleep Apnea Clinical Score. OSA was an independent risk factor for hypertension. The other risk factors for hypertension in COPD patients included age, BMI, CAT score and alcohol consumption. Age, lower FEV1% may be risk factors for arrhythmia. OVS patients were associated with a high prevalence rate of hypertension, while OSA was an independent risk factor for hypertension.
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent condition, which has an estimated prevalence in adults in the USA of 13.9% [Citation1,Citation2] and in adults in China aged 40 years or older of 13.7% [Citation3]. COPD often coexists with other diseases that may have a significant impact on disease course [Citation4–9], especially cardiovascular diseases [Citation10]. Furthermore, comorbidities are common with any severity of COPD [Citation11]. On the other hand, due to the sleep disordered-breathing and the repeated episodes of upper airway closing, obstructive sleep apnea (OSA) not only affects 9–26% of adults in the USA [Citation12], but its effect on adverse cardiovascular consequences is increasingly being recognized [Citation13] as well. OSA seems to be more common in patients with COPD and it has bidirectional interactions with COPD [Citation14]. The term “overlap syndrome” (OVS) has been used to describe both conditions in a single patient [Citation15]. Compared with either COPD only or with OSA, patients with OVS may suffer more frequent episodes of nocturnal oxygen desaturation and have more total time with profound hypoxemia during sleep [Citation16,Citation17], since patients with COPD do not typically return to normal baseline oxygen saturation levels [Citation18]. Additionally, OVS patients are more likely to have a worse prognosis [Citation19].
Since the association between the comorbidities of OSA or COPD is well known, the exact relationship of COPD overlapping OSA associated comorbidities should be further elucidated. However, whether the positive effect on comorbidities is more pronounced in OVS patients than in patients with COPD only is unclear because there have been few studies on this issue. The purpose of this study was to assess the prevalence of five separate comorbidities in COPD only and OVS patients.
Subjects and study protocol
Design
Since January 2016, we have been participating in a project entitled “Study on the diagnosis and treatment of complications and comorbidities in patients with chronic obstructive pulmonary diseases” supported by the National Key Research and Development Program of China (project number: 2016YFC1304403). As part of one of its five sub-projects, this observational study analyzed baseline data for eligible consecutive participants in order to examine differences in the prevalence rates of hypertension, diabetes, cardiovascular disease, arrhythmia and cerebrovascular diseases between patients with OVS and those with COPD only.
Subjects
From January 2016 to December 2018, 968 patients with confirmed COPD were consecutively recruited for overnight polysomnography (PSG) examination in a sleep laboratory from the Outpatient Department of Respiratory and Critical Care Medicine, Renmin Hospital of Wuhan University. Inclusion criteria for the study were as follows [Citation20]: (1) age between 40 to 80 years; (2) having typical symptoms of COPD (chronic cough, breathlessness or sputum production) and/or a history of exposure to risk factors such as tobacco use; (3) the presence of a post-bronchodilator ratio of forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) <0.70, and the FEV1/FVC being less than the lower limit of normal (LLN). Exclusion criteria included: (1) patients who had been hospitalized for cardiac failure or respiratory exacerbations within the previous 6 weeks and those who could not undergo PSG for various reasons; (2) needing oxygen supplementation or having other major causes of airway limitation such as tuberculosis or other lung diseases; (3) those with renal insufficiency (blood creatinine level ≥1.5 mg/dL); (4) regular use of sedative drugs such as benzodiazepines, opiates, antipsychotics, etc.; (5) having evidence of narcolepsy, insomnia or any other comorbid sleep disorder and (6) those who were unwilling to participate in the study.
This study procedure was approved by the Scientific Research and Technology Ethics Committee of Renmin Hospital of Wuhan University and is registered in ClinicalTrials.gov (Clinical Trials ID: NCT 03182309). All participants recruited in this study provided their written consent.
Study protocol
Patients were initially eligible to take part in the study if they had suspected COPD.
Patients were asked whether they had been previously diagnosed with hypertension, diabetes mellitus, heart attack, angina pectoris, stroke or other comorbidities. We ascertained their history of using tobacco (current or past use of any type of tobacco product) and/or alcohol (>2 drinks per day), pharmacologic therapies of COPD. The Epworth Sleepiness Scale (ESS), the Sleep Apnea Clinical Score (SACS), the Modified British Medical Research Council (mMRC) and the COPD Assessment Test (CATTM) were used to evaluate daytime sleepiness, sleep complaints and COPD symptoms, respectively. A nurse and/or doctor revised the questionnaire during the consultation to make sure the answers were reliable. Changes were made if necessary.
After the first interview, each patient was scheduled to undergo PSG. Blood glucose measurement and blood pressure (BP) screening were taken before and after the PSG. According to the apnea–hypopnea index (AHI), each patient was categorized into a COPD only group (AHI < 5 events/hour) or an OVS group (COPD patients had an AHI of ≥5 events/hour).
shows a flowchart of the study design and process; 1105 patients with suspected COPD received a spirometry test and were the study candidates. Based on the principles mentioned above, 137 subjects were excluded, 43 of them due to an FEV1/FVC ≥ 0.70 and 94 because of their unwillingness to undergo a PSG (n = 36) or the lack of an adequate PSG record for severe breathlessness (n = 58). In total, 968 patients were recruited for the study.
Spirometry
The FVC and the FEV1 post-bronchodilator were measured using a body plethysmograph (MasterScreen Body, Jaeger, Germany) and the FEV1/FVC ratio was calculated. The classification of severity of airflow limitation was based on post-bronchodilator FEV1%.
PSG monitoring
All patients underwent an overnight PSG (SOMNOscreen Plus Tele PSG, SOMNOmedics GmbH, Randersacker, Germany). Sleep recordings included EEG, EOG and chin EMG. Respiratory parameters were recorded with a nasal cannula, thoracic and abdominal strains and pulse oxyhemoglobin saturation (SpO2). All tracings were scored manually by trained personnel according to the AASM guidelines [Citation21]. The following sleep variables were calculated: total sleep time, average pulse oxygen saturation (mean SpO2), lowest pulse oxygen saturation (Min SpO2), oxygen desaturation index (ODI), percentage of sleep time with SpO2 <90% (T90) and AHI. A cutoff point of AHI ≥ 5 events/hour was used to classify patients with or without OSA.
BP measurements and judgment
The BP of each patient was measured using a pneumoelectric microprocessor - controlled instrument with appropriate-sized cuffs (YE 660D, Yuyue Ltd, Nanjing, China) at three time points: at the first interview, in the evening about half an hour before the PSG and in the next morning after the PSG but while still in bed. The recorded BP was calculated as the average of three awake consecutive measurements during a 5-minute period after resting for ≥10 min. Hypertension was defined as a systolic BP (SBP) ≥140 mm Hg or a diastolic BP (DBP) ≥90 mmHg [Citation22]; or the nocturnal mean systolic pressure ≥120 mmHg and/or nocturnal mean diastolic BP ≥70 mmHg [Citation23]. In this study, four conditions were judged as hypertension: (1) having a diagnosis of hypertension by a physician as per clinical history; (2) having orally taken anti-hypertensive medications; (3) SBP ≥140 mmHg and/or DBP ≥90 mmHg at either the evening or morning measurement and (4) PSG diagnosed nocturnal hypertension, which was measured by the Somnotouch-NIBP noninvasive continuous BP monitor. Beat-to-beat BP was monitored continuously during PSG examination via the pulse transit time (PTT)-based method [Citation24,Citation25]. Calibration of PTT was implemented when measurement by cuff realized three consecutive stable results after a supine resting period of 10 min. All systolic and diastolic BP values, as well as pulse oximetry values were synchronized with PSG and analyzed by “DOMINO” software (version 2.7.0, GmbH, Randersacker, Germany).
Evaluation for diabetes mellitus and cardio-cerebrovascular comorbidities
Diabetes mellitus was diagnosed according to a previous diagnosis or from examination for fasting blood glucose ≥7.0 mmol/l or random blood glucose ≥11.1 mmol/l or 2 h value in the 75 g oral glucose tolerance test ≥11.1 mmol/l [Citation26] tested by a portable blood glucose meter (Type GA-3, SANNUO, Shuangxu Electronics Ltd, Shanghai, China) during the first interview or before and after PSG.
The judgment for cardio-cerebrovascular diseases was based on each patient's previous diagnosis of cardiovascular disease, myocardial infarction, a history of angina or cardiac dysfunction caused by cardiovascular disease or a history of cerebral infarction or cerebral hemorrhage. Twelve-lead electrocardiograph (ECG) was conducted for all participants. Holter ECG was performed on whom the ECG had an indeterminate diagnosis or had a history of arrhythmia. Arrhythmia was diagnosed according to the comprehensive results of ECG traces, Holter ECG, ECG on PSG or past medical history.
Other key measurements
ESS was used to evaluate daytime sleepiness and SACS was used as a screening tool for OSA. In non-COPD populations, a cutoff point of ESS ≥10 was defined as subjective excessive daytime sleepiness [Citation27] and those with SACS scores ≥15 points are considered as having a high likelihood of OSA [Citation28].
Main outcome measures
The primary end point in this study was to examine differences in the prevalence rate of specific types of comorbidities between patients with COPD only and those with OVS. The secondary end point was to analyze major risk factors for comorbidities.
Statistical analysis
Continuous variables are expressed as means ± standard deviation and categorical variables are expressed as absolute numbers and percentages. The normality of the distribution of variables was tested using the Kolmogorov–Smirnov test and the COPD only group was statistically compared with the OVS group using a t-test. The χ 2 test was used to compare dichotomous variables. Interrelations among variables were examined using Pearson's correlation coefficient. Binary logistic regression was used to assess the effects of OSA severity and the severity of airflow limitation on the co-existence of morbidities, and was also used to explore the risk factors for comorbidity. Independent variables without multicollinearity were included in univariate regression analysis. Further multivariate analysis involved regression of variables that were showed a univariate p value <0.05. A p value <0.05 was considered significant. All statistical analyses were performed using SAS 9.4 software (SAS Institute Inc, Cary, NC).
Results
Coexisting rate of OSA in COPD patients and characteristics
The participants had a ratio of FEV1/FVC < 0.70 and their FEV1/FVC were less than LLN. Furthermore, all the patients aged 75–80 years had FEV1/FVC < 65% and the mean FEV1/FVC in patients >70 years was 48.0 ± 13.9%. Among the 968 patients with confirmed COPD, 660 (68.18%) of those patients coexisted with OSA (AHI ≥ 5) and 308 (31.82%) patients had COPD only. 30.48% of the COPD population had a AHI of at least ≥15 events/hour. Compared with the COPD only group, the OVS group (AHI ≥ 5) had a significantly higher BMI, AHI and a larger neck circumference, however, there were no significant differences in sex, age and tobacco and/or alcohol use between the two groups ().
Table 1. Patients’ baseline characteristics, sleep data, scores and comorbidities of the COPD only group and the OVS group.
All patients in this study were surveyed with questionnaires and were monitored by overnight PSG. The ODI, mean SpO2, Min SpO2, T90, FVC%, FEV1%, FEV1/FVC, ESS score and SACS score in the OVS group were significantly different from those in the COPD only group. Nevertheless, there were no statistical differences in mMRC, CAT and CCI scores between the two groups ().
Prevalence of comorbidities
In terms of comorbidities, we found that the prevalence of five comorbidities in the 968 COPD patients were hypertension (38.02%), diabetes (10.64%), cardiovascular disease (23.45%), arrhythmia (35.54%) and cerebrovascular disease (9.40%). The prevalence of hypertension in the OVS group was significantly higher than in the COPD only group (40.91% vs. 31.81%, p = 0.007). However, we did not find that there were significant statistical differences in the prevalence of diabetes, cardiovascular disease, arrhythmia or cerebrovascular disease between the two groups, neither in several types of arrhythmia including atrial fibrillation, premature atrial contraction, ventricular premature contraction, intraventricular block, nodal tachycardia and sinus bradycardia (). In terms of pharmacologic therapies of COPD, there was no significant difference between patients with COPD only and OVS groups ().
According to their AHI, patients with COPD were classified into four groups: non-OSA (AHI 0–4.9), mild OSA (AHI 5–14.9), moderate OSA (AHI 15–29.9) and severe OSA (AHI ≥30). Statistical analysis showed that the prevalence of hypertension had obvious differences among those four groups (), which increased with the increasing severity of OSA (). However, the prevalence rates of the other four complications in COPD patients were not significantly different.
Figure 2. The prevalence of hypertension increased with the increasing severity of OSA. A Chi-square test indicated that the prevalence of hypertension increased with the OSA severity (χ 2 = 19.364, p < 0.0001). There were no significant relationships between OSA severity and diabetes (χ 2 = 2.292, p = 0.541), coronary heart disease (χ 2 = 1.743, p = 0.628), arrhythmia (χ 2 = 3.398, p = 0.334) or cerebrovascular disease (χ 2 = 4.895, p = 0.182).
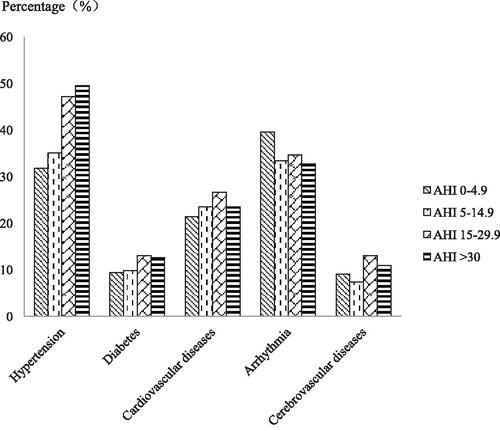
Table 2. Prevalence rates of five comorbidities in COPD patients according to AHI (events/hour).
COPD patients were stratified into four groups based on the GOLD classification of the severity of airflow limitation. The prevalence of the five comorbidities in COPD patients is shown in . We found that the prevalence of arrhythmia significantly increased with the increasing severity of airflow limitation (). There was statistical significant differences among the four groups in nodal tachycardia (p < 0.0001); no statistical difference in atrial fibrillation, premature atrial contraction, ventricular premature contraction, intraventricular block and sinus bradycardia ().
Figure 3. The prevalence of arrhythmia increased with the increasing severity of airflow limitation. A χ 2 test indicated that the prevalence of arrhythmia increased with the airway limitation severity (χ 2 = 30.835, p < 0.0001). There were no significant relationships between the varying degrees of airway limitation and hypertension (χ 2 = 5.271, p = 0.153), diabetes (χ 2 = 3.022, p = 0.388), coronary heart disease (χ 2 = 5.297, p = 0.151) or cerebrovascular disease (χ 2 = 1.245, p = 0.182).
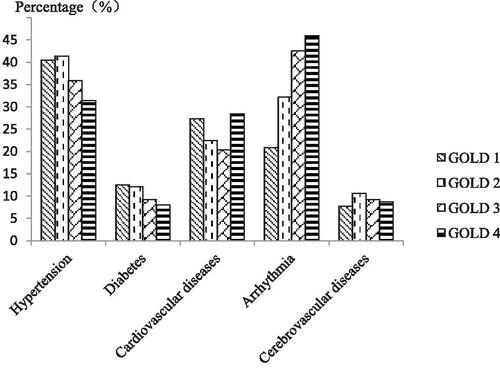
Table 3. Prevalence rates of five comorbidities in COPD patients according to the severity of airflow limitation.
Risk factors for comorbidities
When hypertension, diabetes, cardiovascular diseases, arrhythmia and cerebrovascular diseases were recognized as the dependent variables, OSA severity was recognized as the independent variable [0 = AHI (0–4.9), 1 = AHI (5–14.9), 2 = AHI (15–29.9), 3 = AHI (≥30)], binary logistic regression analysis showed that the prevalence of hypertension increased with OSA severity (non-OSA OR = 1, mild OSA OR = 1.15, 95% CI 0.839–1.597; moderate OSA OR = 1.912, 95% CI 1.307–2.799; severe OSA OR = 2.07, 95% CI 1.368–3.246). After adjusting for sex, age, BMI and neck circumference, the prevalence of hypertension was still positively correlated with the severity of OSA (mild OSA OR = 1.112, 95% CI 0.794–1.557; moderate OSA OR = 1.531, 95% CI 1.025–2.287; severe OSA OR = 1.621,95% CI 1.018–2.581), which implied that OSA was an independent risk factor for hypertension. However, logistic regression analysis did not show a significant relationship between the other four comorbidities and OSA severity ().
Table 4. Results of logistic regression in analyzing the effect of OSA severity on comorbidities (Crude OR, 95% CI).
When the five comorbidities were recognized as the dependent variables and the severity of airflow limitation (GOLD grade) was the independent variable, logistic regression analysis found that the prevalence of arrhythmia increased with the severity of airflow limitation (GOLD 1 OR = 1, GOLD 2 OR = 1.803, 95% CI 1.167–2.786; GOLD 3 OR = 2.813, 95% CI 1.822–4.343; GOLD 4 OR = 3.235, 95% CI 1.959–5.342). After adjusting for confounding factors, the prevalence of arrhythmia was still positively correlated with the severity of airflow limitation (GOLD 2 OR = 1.531, 95% CI 0.976 − 2.400; GOLD 3 OR = 2.166, 95% CI 1.375–3.412; GOLD 4 OR = 2.733, 95% CI 1.606–4.649) ().
Table 5. Results of logistic regression in analyzing the effect of the severity of airflow limitation on comorbidities.
Logistic regression was used to explore the risk factors for OSA in COPD patients. Univariate regression analysis showed that the BMI, neck circumference, FEV1%, ESS score, SACS score were positively correlated with the prevalence of OSA in COPD patients; multivariate logistic regression showed that the BMI, FEV1%, ESS score and SACS score were positively correlated with OSA prevalence ().
Table 6. Results of logistic regression used to explore risk factors for OSA in COPD patients.
Multicollinearity diagnosis was performed on all independent variables except SACS score and CCI, for which were directly related to the prevalence of hypertension. The results showed that there was no collinearity among the respective variables. Univariate logistic regression analysis demonstrated that age, BMI, neck circumference, AHI, T90, FEV1%, CAT score, ESS score and alcohol consumption had correlation with the prevalence rate of hypertension. Independent variables related to hypertension were included in the multivariate logistic regression analysis, which revealed that age, AHI, BMI and alcohol consumption were positive correlated with the prevalence of hypertension, CAT score was negative correlated with the prevalence of hypertension (). The same approach was used to explore risk factors for arrhythmias in COPD patients. Univariate logistic regression analysis indicated that sex, age, BMI, T90, FEV1%, mMRC score, CAT score and using tobacco were significantly associated with prevalence rate of arrhythmia, multivariate logistic regression analysis showed that age was positive correlated with the prevalence of arrhythmia, FEV1% was negative correlated with the prevalence of arrhythmia ().
Table 7. Results of logistic regression used to explore risk factors for hypertension.
Table 8. Results of logistic regression used to explore risk factors for arrhythmia.
Discussion
In present study, we found that the OVS patients were associated with a high prevalence rate of hypertension, OSA was an independent risk factor for hypertension. These results indicated that COPD patients with hypertension should be screened for OSA, and OVS patients should also be screened for hypertension.
An important factor involving in the prognosis and health-related outcomes of COPD patients is the comorbidity [Citation4]. Both COPD and OSA are highly prevalent and have similar physiological consequences, which may contribute to cardiovascular and other comorbidities [Citation29,Citation30]. However, the prevalence of comorbidities in patients with OVS has been less investigated. Papachatzakis et al. [Citation31] found that comorbidities in patients with OVS were at least as prevalent as those in OSA only, however, their study was conducted on a small group of 38 patients with coexisting COPD and OSA.
In this hospital population-based study, we selected five main harmful chronic complications that are closely related to both COPD and OSA [Citation32] as the primary factors. We found that COPD patients had a high prevalence of hypertension, diabetes, cardiovascular disease, arrhythmia and cerebrovascular disease, which was similar to the results published by Mannino et al. [Citation6]. The OVS group had a higher prevalence of hypertension than the COPD only group, but did not have significant differences in the prevalence of the other four chronic diseases. An observational study in a Swiss cohort of COPD patients also showed that the cardiovascular disease was not more frequent in the patients with OVS compared with patients with COPD alone [Citation33], although 61% (20/33) of arterial hypertension in OVA patients were found in their study, however, which was a small sample, unattended sleep studying at home, and with a different cutoff value (AHI >10) for OSA. The results in a retrospective study [Citation34] showed that the prevalence of arterial hypertension was as high as 82% in OVS patients. In which, however, all the patients were referred to the sleep center for sleep diagnostic testing, with the BMI of 33.32 ± 7.23 kg/m2 and an AHI of 42.69 ± 23.74 events/hour. Du et al. [Citation35] investigated the role of OSA on all-cause mortality in patients with COPD, they found that there was no significant difference in overall mortality between OVS patients and patients with COPD alone, which implied that OSA did not significantly increase the risk of death in patients with COPD.
Our data also showed that COPD patients had a high prevalence of OSA. Compared with patients with COPD only, the OVS patients had significantly higher BMI, neck circumference, AHI, ODI, T90, ESS and SACS scores, lower mean SpO2 and Min SpO2 and longer SpO2 <90% during sleep, which were also consistent with the results of other previous studies [Citation36,Citation37]. Although male and older age are generally considered as risk factors for OSA [Citation38], we did not find that the COPD only group and the OVS group had obvious differences in sex and age, perhaps the older, male-dominated Chinese COPD patients in our study may contribute to this lack of difference. In the present study, the COPD patients had an average age of (65.68 ± 9.546) years and males accounted for 84.3% of the patients, other studies had the same results [Citation3,Citation39]. Tobacco [Citation37] and alcohol use [Citation40] were also suggested as risk factors for OSA, but the results are conflicting [Citation41]. We did not find significant differences in tobacco or alcohol use between patients with OVS or with COPD only.
We also found that the prevalence of arrhythmia increased with the severity of airflow limitation. Increased arrhythmogenicity in COPD patients has been recognized for decades [Citation41], especially during acute exacerbation [Citation42]. The oxidative stress, low oxygenation, pulmonary hypertension and the use of β-agonistic bronchodilators are speculated to be liable causative factors [Citation43].
The interaction between COPD and OSA has been studied for a long time and the results are controversial. Earlier studies showed higher prevalence of OVS in patients with more severe grade of COPD, however, recent studies demonstrated that there was no or an inverse correlation between the AHI and the severity of emphysema [Citation44,Citation45]. The investigators proposed that lung volume is an important “protective” determinant for upper airway stability. Potential mechanisms are (1) mediastinal caudal traction generated by the diaphragm; and (2) an increase in the transmural distending pressure in the upper airway induced by thoracic expansion during inspiration [Citation45].
Multivariate logistic regression analysis showed that the prevalence rate of hypertension in COPD patients had positive correlation with age, BMI and alcohol consumption, which had already been believed as the risk factors for hypertension by other researchers [Citation46]. Logistic regression analysis also found that the risk factors for arrhythmia included older age and lower FEV1%. The high prevalence of arrhythmia in COPD patients was speculated to be ultimately caused by hypoxia, hypercapnia, pulmonary arterial hypertension [Citation47].
It was reported that LLN of FEV1/FVC was superior to FEV1/FVC < 0.7 in the diagnosis of COPD [Citation48], for fixed ratio defined airflow limitation may lead to over-diagnosis in the elderly. However, the results from Bhatt et al. [Citation49] showed that the diagnostic value of LLN was equivalent to a fixed value of 0.7. We used the reference equations provided by the Global Lung Initiative for North East Asia to calculated LLN [Citation48], and the results showed that all participants had FEV1/FVC < LLN, which indicated no over-diagnosis of COPD in this cohort.
The major strength of our study was that all diagnoses of OSA in COPD patients were established by overnight PSG, which is the gold standard for diagnosing OSA. Questionnaires are of great value for screening simple OSA, however, there is controversy about their significance in screening OVS [Citation50].
We also acknowledge several limitations of this study. First, although this study had a large sample size, only baseline results have been evaluated so far and follow-up studies are being conducted. Second, the enrolled patients were from hospitals rather than the community population and therefore, there is likely a population selective bias in this study. Third, we did not rule out the effect of the “first night” of PSG on results. Forth, for baseline data evaluation, only five major comorbidity diseases were selected for observation, and other comorbidities that required special examination or subjective diseases were not included. Last, this study did not observe comorbidities in the OSA only group at the same time.
Conclusions
Our study revealed that: (a) OSA was associated with 68.18% of patients with confirmed COPD; and (b) in patients with coexisting OSA, the frequency of hypertension was high and increased with the severity of OSA. OSA was an independent risk factor for hypertension; (c) the prevalence of arrhythmia increased with the severity of COPD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jemal A , Ward E , Hao Y , et al. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294(10):1255–1259. DOI:10.1001/jama.294.10.1255
- Mannino DM , Gagnon RC , Petty TL , et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160(11):1683–1689. DOI:10.1001/archinte.160.11.1683
- Wang C , Xu JY , Yang L , et al. China pulmonary health study group. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross- sectional study. Lancet. 2018;391(10131):1706–1717. DOI:10.1016/S0140-6736(18)30841-9
- Barnes PJ , Celli BR . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. DOI:10.1183/09031936.00128008
- Soriano JB , Visick GT , Muellerova H , et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. DOI:10.1378/chest.128.4.2099
- Mannino DM , Thorn D , Swensen A , et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. DOI:10.1183/09031936.00012408
- Sin DD , Anthonisen NR , Soriano JB , et al. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245–1257. DOI:10.1183/09031936.00133805
- Miller J , Edwards LD , Agusti A , et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. DOI:10.1016/j.rmed.2013.05.001
- Divo M , Cote C , de Torres JP , et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. DOI:10.1164/rccm.201201-0034OC
- Fisk M , McEniery CM , Gale N , et al. Surrogate markers of cardiovascular risk and chronic obstructive pulmonary disease: A large case-controlled study. Hypertension. 2018;71(3):499–506. DOI:10.1161/HYPERTENSIONAHA.117.10151
- Agusti A , Calverley PM , Celli B , et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. DOI:10.1186/1465-9921-11-122
- Young T , Palta M , Dempsey J , et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. DOI:10.1056/NEJM199304293281704
- Dewan NA , Nieto FJ , Somers VK . Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147(1):266–274. DOI:10.1378/chest.14-0500
- McNicholas WT . COPD-OSA Overlap Syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest. 2017;152(6):1318–1326. DOI:10.1016/j.chest.2017.04.160
- Malhotra A , Schwartz AR , Schneider H , et al. Research priorities in pathophysiology for sleep-disordered breathing in patients with chronic obstructive pulmonary disease. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;197(3):289–299. DOI:10.1164/rccm.201712-2510ST
- Chaouat A , Weitzenblum E , Krieger J , et al. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. DOI:10.1164/ajrccm.151.1.7812577
- Shepard JW Jr , Garrison MW , Grither DA , et al. Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Am J Med. 1985;78(1):28–34. DOI:10.1016/0002-9343(85)90457-7
- Ioachimescu OC , Teodorescu M . Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology. 2013;18(3):421–431. DOI:10.1111/resp.12062
- Marin JM , Soriano JB , Carrizo SJ , et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. DOI:10.1164/rccm.200912-1869OC
- Vogelmeier CF , Criner GJ , Martínez FJ , et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology. 2017;22(3):575–601. DOI:10.1111/resp.13012
- Berry RB , Budhiraja R , Gottlieb DJ , et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. DOI:10.5664/jcsm.2172
- Pickering TG , Hall JE , Appel LJ , et al . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. DOI:10.1161/01.HYP.0000150859.47929.8e
- Li Y , Staessen JA , Lu L , et al. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50(2):333–339. DOI:10.1161/HYPERTENSIONAHA.107.087767
- Gesche H , Grosskurth D , Küchler G , et al. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112(1):309–315. DOI:10.1007/s00421-011-1983-3
- Bilo G , Zorzi C , Ochoa Munera JE , et al. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20(5):291–294. DOI:10.1097/MBP.0000000000000124
- American Diabetes Association . Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–S66.
- Johns MW . A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. DOI:10.1093/sleep/14.6.540
- Flemons WW , Whitelaw WA , Brant R , et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1279–1285. DOI:10.1164/ajrccm.150.5.7952553
- McNicholas WT . Comorbid obstructive sleep apnoea and chronic obstructive pulmonary disease and the risk of cardiovascular disease. J Thorac Dis. 2018;10(Suppl 34):S4253–S4261. DOI:10.21037/jtd.2018.10.117
- McNicholas WT . Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med. 2009;180(8):692–700. DOI:10.1164/rccm.200903-0347PP
- Papachatzakis I , Velentza L , Zarogoulidis P , et al. Comorbidities in coexisting chronic obstructive pulmonary disease and obstructive sleep apnea-overlap syndrome. Eur Rev Med Pharmacol Sci. 2018; 22(13):4325–4331.
- Gonzaga C , Bertolami A , Bertolami M , et al. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum Hypertens. 2015;29(12):705–712. DOI:10.1038/jhh.2015.15
- Steveling EH , Clarenbach CF , Miedinger D , et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2014;88(6):451–457. DOI:10.1159/000368615
- Lacedonia D , Carpagnano GE , Patricelli G , et al. Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome. Clin Respir J. 2018;12(5):1905–1911. DOI:10.1111/crj.12754
- Du W , Liu J , Zhou J , et al. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005-2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2018;13:665–674. DOI:10.2147/COPD.S148735
- Shawon MS , Perret JL , Senaratna CV , et al. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Med Rev. 2017;32:58–68. DOI:10.1016/j.smrv.2016.02.007
- Lacedonia D , Carpagnano GE , Aliani M , et al. Daytime PaO2 in OSAS, COPD and the combination of the two (overlap syndrome). Respir Med. 2013;107(2):310–316. DOI:10.1016/j.rmed.2012.10.012
- Choi KM , Thomas RJ , Kim J , et al. Overlap syndrome of COPD and OSA in Koreans. Medicine (Baltimore). 2017;96(27):e7241.
- Sweed RA , Hassan S , ElWahab NHA , et al. Comorbidities associated with obstructive sleep apnea: a retrospective Egyptian study on 244 patients. Sleep Breath. 2019;23(4):1079–1085. 01783-w. DOI:10.1007/s11325-019-
- Franklin KA , Lindberg E . Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. DOI:10.3978/j.issn.2072-1439.2015.06.11
- McCord J , Borzak S . Multifocal atrial tachycardia. Chest. 1998;113(1):203–209. DOI:10.1378/chest.113.1.203
- Rusinowicz T , Zielonka TM , Zycinska K . Cardiac arrhythmias in patients with exacerbation of COPD. Adv Exp Med Biol. 2017;1022:53–62. DOI:10.1007/5584_2017_41
- Caglar IM , Dasli T , Turhan Caglar FN , et al. Evaluation of atrial conduction features with tissue Doppler imaging in patients with chronic obstructive pulmonary disease. Clin Res Cardiol. 2012;101(8):599–606. DOI:10.1007/s00392-012-0431-7
- Sharma B , Feinsilver S , Owens RL , et al. Obstructive airway disease and obstructive sleep apnea: effect of pulmonary function. Lung. 2011;189(1):37–41. DOI:10.1007/s00408-010-9270-3
- Krachman SL , Tiwari R , Vega ME , et al. Effect of emphysema severity on the apnea-hypopnea index in smokers with obstructive sleep apnea. Ann Am Thorac Soc. 2016;13(7):1129–1135. DOI:10.1513/AnnalsATS.201511-765OC
- Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. 2019;16(3):182–241.
- Goudis CA , Konstantinidis AK , Ntalas IV , Korantzopoulos P . Electrocardiographic abnormalities and cardiac arrhythmias in chronic obstructive pulmonary disease. Int J Cardiol. 2015;199:264–273. DOI:10.1016/j.ijcard.2015.06.096
- Quanjer PH , Stanojevic S , Cole TJ , et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. DOI:10.1183/09031936.00080312
- Bhatt SP , Balte PP , Schwartz JE , et al. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA. 2019;321(24):2438–2447. DOI:10.1001/jama.2019.7233
- Soler X , Liao SY , Marin JM , et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): The challenge to predict OSA in advanced COPD. PLoS One. 2017;12(5):e0177289. DOI:10.1371/journal.pone.0177289