Two studies have been published in the last issue of the journal [Citation1,Citation2] that, with different aims and outcomes, were performed in the “sensitive population” with Asthma-COPD- overlap (ACO) where asthma and chronic obstructive pulmonary disease (COPD) share common features [Citation3].
Asthma and COPD are the most common chronic pulmonary conditions. Both asthma and COPD have considerable heterogeneity and intrinsically share clinical features, since they have in common a) the condition of being diseases of the same system and b) the pathophysiology and consequences of airflow limitation. Thus, it shouldn’t be a surprise that they have commonalities in their clinical and pathophysiological presentations. By contrast, and this is surprising, over the past decade, there has been significant discussion over this group of patients, in particular on whether an identifying term should be given to the condition of “overlapping features of both diseases”, as currently suggested by GINA [Citation3]. In fact, an artificial construct has been made (ACO) to label what is nothing more than one of the natural evolution/expression of the 2 clinical heterogeneous conditions. The trademark ACO has been created, and this has led to confusion and misled clinicians.
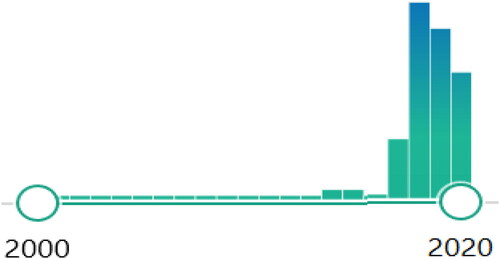
No doubts that there are traits shared by the 2 respiratory obstructive conditions, but it doesn't clinically help to identify the condition (termed ACO) on the principle that there are commonalities between the two diseases: this term is MisTaken. Hopefully, the use of this word, as we look at the of publications adopting this term, has started to decrease in recent times and expectantly this trend will continue ().
The two studies published in the last issue of the journal somehow reflect the confusion and the debate that has been ongoing since the ACO term has been generated [Citation4]. Peltola and colleagues [Citation1] in their work refer to ACO and identifies patients i) with a COPD ICD diagnosis number identified during a past hospitalization and ii) with on doctor-diagnosed asthma. Notably, in this retrospective study, only a minority of the COPD population included in the survival analyses had a lung function testing, which questions the group allocation [Citation5,Citation6]. The main message, COPD with concomitant asthma has better survival compared to COPD without concomitant asthma, is in line with the results of Burrow al et reported 33 years ago [Citation7].
The Canadian study [Citation2] embedded in CanCOLD cohort, opted for the more recent GOLD position [Citation8] and refers to asthma and coexisting COPD without mentioning ACO. However, the study equally bears shadows over the groups’ labelling as COPD subjects [including non-smokers] were identified solely on the basis of a post-bronchodilator FEV1/FVC; while concomitant asthma was defined based on self-reported physician diagnosis of asthma, atopy and bronchodilator responses. In the absence of smoking history, these latter characteristics would fulfill the clinical/functional presentation of asthma with fixed airflow limitation with no need for COPD to be involved, according to GINA listed phenotypes of asthma (Chapter 1 [Citation3]).
Clearly, there were practical reasons behind the choice of introducing this term in 2015, by GINA in conjunction with GOLD [Citation4]. Indeed, patients with features of COPD are usually excluded from asthma studies (including RCT) and this is reciprocated in COPD. However, the solution proposed to solve a practical issue had, in fact, indicated a path (i.e. ACO) leading nowhere in clarity and potentially on the verge of perilous consequences.
The cage that has been built to force “overlapping features of both asthma and COPD” into ACO, is rather loose and it includes among the others: post-bronchodilator FEV1/FVC ratio of less than 0.7 in the setting of different clinical features, patient-reported or physician’s diagnosis of asthma, significant bronchodilator reversibility, the presence of significant sputum or blood eosinophilia, documentation of wheezing and/or an atopic disease significant history of smoking or other known exposures [Citation9,Citation10].
Not unexpectedly, different aspects of the ACO multifaceted presentations are used in different studies, which recruit different populations using the same term: ACO. Although explicitly stated by GINA (Chapter 5, [Citation3]) that patients with features of both asthma and COPD do not represent a single disease, the devil lies in the term itself that, by including a variety of different conditions, brings the perils connatural to its ambiguity. Thus, as different ACO definitions have been applied in various studies (recently elegantly reviewed [Citation11]), any reliable conclusions regarding clinical severity, management, and prognosis of this condition are precluded. Clearly, the evidence base for treating ACO is very limited, due to a lack of pharmacological studies.
This confusion bears the risk of applying/extending the conclusions obtained in a study conducted in a population termed ACO (e.g. Asthma with fixed airflow limitation) to a totally different population equally termed ACO (e.g. COPD with > 300 blood eos/µL).
Indeed, one may argue that patients considered to have ACO in these studies have forms of COPD or asthma, rather than an overlapping condition. In epidemiological studies, fixed airflow limitation is “questionably” used as the only “defining” criterion for COPD. However, we know that asthma in itself, in its own evolution, can develop fixed airflow limitation. Indeed, up to 30% of patients with fixed airflow limitation have a history of asthma [Citation12] as it could develop especially in patients with long-lasting disease (as GINA underlines, Chapter 1 [Citation3]) [Citation13,Citation14]. Does asthma with fixed airflow limitation become something different from asthma and mixes up with COPD (becoming an ACO)? On the contrary, the evidence is that asthmatic airway inflammation does not change with the development of fixed airflow obstruction and it does not become similar to the airway inflammation characteristic of COPD [Citation15]. Also, lung function remains different and, most importantly, the response to treatment is different [Citation15]. Thus, no ACO has been found in this condition. So, just call it by its name: asthma with fixed airflow limitation; and treat it as such.
On the COPD site, the condition of large bronchodilator response is doubted of masking an ACO soul. Why bronchodilator response should be considered a physiologic characteristic exclusively of asthma? Indeed, substantial responses to bronchodilators have been reported in COPD particularly with the new potent bronchodilators [Citation16]. In one of the few RCT in COPD where not only current asthma but also history of asthma was excluded [Citation17], the acute bronchodilator responsiveness was greater than 12% and 200 mls in 52% of this “pure” COPD population [Citation17,Citation18]. In addition, 54% of patients had at least 12% and 200 mL improvement in FEV1, over the treatment period with a long acting bronchodilator [Citation19]. Thus, in the absence of any “smell of asthma”, there is notable reversibility to bronchodilators in a large proportion of COPD. So, call it by its name: e.g. COPD with substantial responses to bronchodilators. And you’ll find an important clinical piece of information.
Not to mention blood eosinophils. In the general population blood, eosinophils have a normal distribution [Citation20], as it happens for any biologic measure. Why should it be different in COPD? In fact, the evidence indicates that it is not different in COPD [Citation21]. So, why COPD patients with 300 blood eos/µL (within the normal range) should have acquired something closer to asthma and be blamed of any ACOish condition? Since when asthma is diagnosed based on blood eosinophils? Again, call it by its name: e.g. COPD with eosinophilic profile, and you’ll find a useful predictor of response to treatment (ICS for example).
The variability and differences of the clinical conditions described above (-and others [Citation9]) are confusing the clinicians and risk to oversimplify the diagnostic and treatment approach toward a unique black box of hybrid/chimeric clinical/biological/functional mixtures, without recognizing the underlying conditions with their specificities.
This is why I believe the term ACO should disappear, and the different conditions named after what they are. This would also clarify the most appropriate treatment approach. A simple interim and descriptive solution: name a condition after its characterizing traits. Notably, for some of these traits, effective treatments are currently available (and some others will come) and thus they are treatable traits. This is the practical piece of information that the clinician would need to exit the ACO labyrinth.
As simple as the latest GOLD statement on the topic: asthma and COPD are two different disorders and should be treated accordingly [Citation8]. Asthma and COPD may coexist in individual patients (COPD with a concurrent diagnosis of asthma), and should be treated concomitantly.
To provide clinical guidance for patient safety there is also a very simple message: ICS should be given to any asthma condition (including concomitant COPD) and to COPD conditions where the efficacy is supported by the available evidence (e.g. increased risk of exacerbations, eosinophilia, concomitant asthma, or any combination of these features) [Citation22].
COPD future RCTs should focus on patients with some questioned traits, e.g. patients with smoking-induced COPD with a past history of asthma.
Hopefully, in the future no national and international guidelines will refer to such a distorted way of approaching patients’ clinical condition, thus avoiding confusion in medical communication and the clinical consequences of recommendations (treatment in particular) in the absence of evidence.
Conflict of interest statement
Dr. Papi reports grants, personal fees, non-financial support from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini and Sanofi/Regeneron, personal fees, non-financial support from Mundipharma, Zambon, and Novartis; personal fees from Roche, grants from Fondazione Maugeri and Fondazione Chiesi, personal fees from Edmondpharma, outside the submitted work.
References
- Peltola L , Pätsi H , Harju T . COPD comorbidities predict high mortality - asthma-COPD-overlap has better prognosis. COPD. 2020;17(4):366–372. doi:10.1080/15412555.2020.1783647
- Mostafavi-Pour-Manshadi S-M-Y , Naderi N , Mancino P , et al. Fractional exhaled nitric oxide as an inflammatory biomarker in chronic obstructive pulmonary disease (COPD) with or without concurrent diagnosis of asthma: the Canadian Cohort Obstructive Lung Disease (CanCOLD). COPD. 2020;17(4):355–365. doi:10.1080/15412555.2020.1779681
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2020. Last accessed: August 21, 2020. Available from: https://ginasthma.org.
- GINA & GOLD . Diagnosis of diseases of chronic airflow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS). 2015. Available from: https://goldcopd.org/asthmacopd-asthma-copd-overlap-syndrome/.
- Diab N , Gershon AS , Sin DD , et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(9):1130–1139. doi:10.1164/rccm.201804-0621CI
- Vanfleteren LEGW , Andersson AE , Fabbri LM . COPD: what's in a name?: mismatch of diagnostic labels and required physiologic features. Chest. 2019;156(2):195–196. doi:10.1016/j.chest.2019.05.008
- Burrows B , Bloom JW , Traver GA , et al. The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. N Engl J Med. 1987;317(21):1309–1314. doi:10.1056/NEJM198711193172103
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2020. Available from: http://goldcopd.org.
- Bateman ED , Reddel HK , van Zyl-Smit RN , et al. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med. 2015;3(9):719–728. doi:10.1016/S2213-2600(15)00254-4
- Postma DS , Rabe KF . The asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. doi:10.1056/NEJMra1411863
- Halpin DMG . What is asthma chronic obstructive pulmonary disease overlap? Clin Chest Med. 2020;41(3):395–403. doi:10.1016/j.ccm.2020.06.006
- Mannino DM , Gagnon RC , Petty TL , et al . Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160(11):1683–1689. doi:10.1001/archinte.160.11.1683
- Backman KS , Greenberger PA , Patterson R . Airways obstruction in patients with long-term asthma consistent with 'irreversible asthma'. Chest. 1997;112(5):1234–1240. doi:10.1378/chest.112.5.1234
- Vonk JM , Jongepier H , Panhuysen CI , et al. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58(4):322–327. doi:10.1136/thorax.58.4.322
- Fabbri LM , Romagnoli M , Corbetta L , et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(3):418–424. doi:10.1164/rccm.200203-183OC
- Beeh KM , Westerman J , Kirsten AM , et al. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;32:53–59. doi:10.1016/j.pupt.2015.04.002
- Tashkin DP , Celli B , Senn S , et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
- Hanania NA , Sharafkhaneh A , Celli B , et al. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res. 2011;12(1):6. doi:10.1186/1465-9921-12-6
- Tashkin DP , Celli B , Decramer M , et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31(4):742–750. doi:10.1183/09031936.00129607
- Nerpin E , Jacinto T , Fonseca JA , et al. Systemic inflammatory markers in relation to lung function in NHANES. 2007–2010. Respir Med. 2018;142:94–100. doi:10.1016/j.rmed.2018.07.011
- Siddiqui SH , Guasconi A , Vestbo J , et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
- Agusti A , Fabbri LM , Singh D , et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52(6):1801219. doi:10.1183/13993003.01219-2018