 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The usefulness of the oscillometry, known as forced oscillation technique, for predicting exercise tolerance in subjects with COPD is unknown. To test the hypothesis, we investigated whether oscillometry could predict a 6-minute walking distance (6MWD) <350 m in the 6-minute walk test (6MWT).
This was a prospective, observational study. Fifty-seven subjects with COPD who attended outpatient clinics for routine checkups at Shizuoka General Hospital between April 2015 and April 2016 (54 males; median age, 70 years; and %FEV1, 61.0%). Modified MRC dyspnea scale (mMRC), COPD Assessment Test (CAT), oscillometry, spirometry, and 6MWT were performed in a stable condition. The participants were classified into subjects with 6MWD ≥350 m or 6MWD <350 m, and the predictor of 6MWD <350 m was assessed.
Of the 57 total subjects, 43 (75.4%) had a 6MWD ≥350 m, and 14 (24.6%) had a 6MWD <350 m. Between the two groups, there were significant differences in mMRC scale, GOLD stages, CAT scores, FEV1, IC, 6MWD, lowest SpO2, maximum Borg scale, respiratory resistance (Rrs), and reactance (Xrs). In multivariate regression analysis, a 6MWD <350 m was independently predicted by CAT scores (OR 1.15, 95% CI: 1.01–1.30) and inspiratory R5 (OR 6.01, 95% CI: 1.09–33.30). In receiver operating characteristic curves, the area under the curve was 0.76, 0.78, and 0.85 for CAT scores, R5, and CAT scores + R5, respectively, with the best cutoff value of 17 and 2.82 cmH20/L/s. In conclusion, the oscillatory parameter, inspiratory R5, predicted low exercise tolerance in COPD subjects.
Introduction
Chronic and progressive dyspnea is the most characteristic symptom and a major cause of the disability that is associated with chronic obstructive pulmonary disease (COPD) [Citation1]. COPD is a heterogeneous condition characterized by small and large airway dysfunction with some of its core pathophysiological mechanisms originating from the small airways [Citation2, Citation3]. The small airway disease progressively traps gas during expiration and results in expiratory flow limitation (EFL), leading to hyperinflation as the main mechanism for exertional dyspnea [Citation3, Citation4]. In this aspect, the 6-minute walk test (6MWT) is a valid and reliable measure of exercise capacity. There is a strong association between shorter 6-minute walking distance (6MWD) and an increased risk of mortality for subjects with COPD [Citation5, Citation6].
Previous studies found correlations between spirometric parameters, including FEV1 and IC, symptoms, health-related quality of life, physical activity, and 6MWD [Citation6–8]. In an official systematic review of ERS/ATS, a wide variety of independent predictors of 6MWD were identified; however, there was no consistency in COPD subjects. It suggests that there could be other contributors that have not yet been found [Citation6]. Oscillometry, also known as the forced oscillation technique, is a noninvasive method to measure lung mechanics [Citation9]. Recently, the clinical application of broadband frequency oscillometry as a measurement of lung function is widely used in the evaluation or management of obstructive lung diseases, including asthma and COPD [Citation10]. There are some reports stating that within-breath changes in reactance indices reflect the EFL [Citation4, Citation11, Citation12] and that the reactance indices correlate with the change in 6MWD during and after pulmonary rehabilitation [Citation13]. However, the relationship between oscillatory parameters and 6MWD is not fully understood. We hypothesized that oscillometry, instead of spirometry, would have some role in the 6MWT and be useful in clinical practice of COPD. In this prospective, observational study, we assessed whether oscillatory indices could predict a poor performance of 6MWD in subjects with COPD.
Materials & methods
Subjects
Outpatients with COPD at Shizuoka General Hospital, seen between April 2015 and April 2016, were enrolled in this study. All participants fulfilled the definition of GOLD, and the COPD grading was based on the GOLD classification [Citation1]. The inclusion criteria were as follows: (1) age over 40 years and (2) receiving medications, including long-acting antimuscarinic agents, long-acting β2 agonists, inhaled corticosteroids, or sustained-release theophylline. Exclusion criteria were as follows: (1) a history of lung cancer, (2) receiving home oxygen therapy, (3) angina in the four weeks preceding the study, (4) exacerbation of COPD in the four weeks preceding the study, (5) gait disturbance, and (6) lowest arterial oxygen saturation of pulse oximetry (SpO2) of less than 80% in 6MWT.
Study design
This is a prospective, observational study. On the same examination day, eligible subjects underwent the assessment of symptoms, measurement of oscillometry, and spirometry in that order. Then, the 6MWT was performed within one month in a stable condition. This study was conducted in accordance with the principles of the Declaration of Helsinki, and this protocol was approved by the Institutional Review Board of Shizuoka General Hospital (SGH 15-01-56). Written informed consent was obtained from all study participants prior to the study.
Assessment of symptoms
The modified Medical Research Council (mMRC) was used to evaluate dyspnea in daily living, grading from 0 (only get breathless with strenuous exercise) to 4 (too breathless to leave the house or breathless when dressing) [Citation14]. The COPD assessment test (CAT) (Japanese version, supplied by GlaxoSmithKline, Japan) consists of eight items (cough, phlegm, chest tightness, breathlessness going up hills/stairs, activity limitations at home, confidence leaving home, sleep, and energy) that assess and quantify the symptoms and the impact of COPD [Citation15]. Each item is scored from 0 to 5, giving a total score range from 0 to 40, corresponding to the best and worst health statuses, respectively.
Oscillometry and spirometry
The measurements of oscillometry and spirometry were performed in that order to avoid any influence of forced breathing. Broadband frequency oscillometry was performed using the MostGraph-01 (CHEST M.I. Co. Ltd., Tokyo, Japan) in accordance with standard recommendations [Citation16]. We used Rrs at 5 and 20 Hz (R5 and R20, respectively) and the difference between R5 and R20 (R5 R20) as an indicator of the frequency dependence of Rrs. We also used Xrs at 5 Hz (X5), which reflects the elastic and inertial properties of the lung; ΔXrs at 5 Hz (ΔX5), an index of expiratory flow limitation (EFL), calculated as the difference between inspiratory and expiratory phases of Xrs; resonant frequency (Fres) where Xrs crosses zero, and the elastic and inertial forces are opposite and equal in magnitude; and a low-frequency reactance area (ALX), which is an integral of Xrs at 5 Hz to Fres [Citation12]. Oscillatory indices were expressed as the values of the inspiratory phase except for ΔX5 since they were less affected by flow limitation during an expiration phase [Citation17]. All measured data were expressed as postbronchodilator values. Spirometry was performed using computerized equipment (model CHESTAC-8800; CHEST M.I. Co. Ltd., Tokyo, Japan) in accordance with the American Thoracic Society (ATS)/European Respiratory Society (ERS) documents for spirometry [Citation18] while subjects were on daily medications. All measured data were expressed as postbronchodilator values. Predicted values for pulmonary function tests were obtained from the Japanese Respiratory Society guidelines [Citation19].
Six-Minute walk test (6MWT)
The 6MWT was performed indoors, along a flat, straight, 30 m walking course supervised by a well-trained researcher. The 6MWT was performed twice upon their consent, and the best 6MWD was recorded according to the ERS/ATS documents for field walking tests [Citation20]. The study participants were encouraged every minute of the 6MWT using two phrases: ‘‘You are doing well’’ or ‘‘Keep up the good work’’. The participants were allowed to stop and rest during the test but were instructed to resume walking as soon as they felt they were able to do so. The participants were asked to grade their shortness of breath using a modified Borg scale specific for each at the beginning and the end of the test. Continuous pulse oximetry was obtained during the 6MWT to ensure that the lowest SpO2 was recorded. The participants were classified into two groups based on a 6MWD of 350 m, because a walking distance <350 m in this test was associated with a significantly poor prognosis [Citation5, Citation6].
Statistical analyses
The quantitative data were summarized as medians with range and were compared using a Mann–Whitney U test. The categorical data were summarized as counts and compared using a Fisher’s exact test. Univariate and multivariate logistic regression analyses were performed to predict a group of subjects with a poor walking distance (<350 m) using clinical parameters. Model selection was made by the best subset selection procedure using Akaike’s information criteria (AIC). Spearman's correlation coefficients were calculated to determine the association between 6MWD and oscillometry. Receiver operator characteristic (ROC) analysis was performed, and the area under the curve (AUC) was calculated to estimate the predictive capability of the poor 6MWD. The statistical significance for all analyses was set at p < 0.05 (two-tailed). All analyses were performed using EZR version 1.27 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [Citation21].
Results
The study flow and the clinical characteristics of the subjects are shown in , and Citation2. Of the 71 participants assessed for eligibility, seven were excluded because they either had lung cancer or did not perform CAT or spirometry. Seven could not complete 6MWT due to severe desaturation or gait disturbance during the test. The 6MWT was done twice in 45 participants and once in 12 participants because of refusal to perform a second 6MWT. Of the 57 total subjects, 43 (75.4%) had a 6MWD ≥350 m, and 14 (24.6%) had a 6MWD <350 m. Subjects with a 6MWD <350 m had significantly higher mMRC scale, CAT scores, Rrs, Fres, ALX, maximum Borg scale, and advanced GOLD stages and had also lower FEV1, IC, 6MWD, lowest SpO2, and Xrs than subjects with further 6MWD. The representative oscillatory patterns of both groups are shown in .
Figure 1. The flow of the subjects. Abbreviations: CAT, COPD assessment test; 6MWD, 6-minute walking distance.
Figure 2. Colored 3 D images of Rrs and Xrs in each from an individual subject in each category. These images show the 30-second time course of Rrs and Xrs during tidal breathing. Oscillometric values, including R5 and X5, were output automatically by the computer.
Abbreviations: Rrs, respiratory system resistance; R5, Rrs at 5Hz; Xrs, respiratory system reactance; X5, Xrs at 5Hz; 6MWD, 6-minute walking distance.
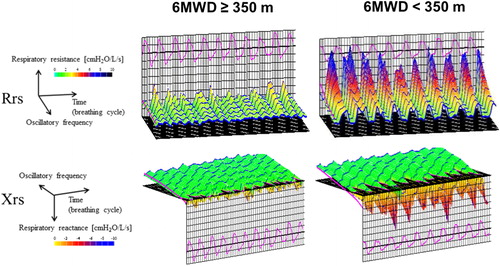
Table 1. Characteristics of the study subjects.
Table 2. Whole-breath and within-breath analyses of respiratory impedance.
There were significant associations between 6MWD and mMRC scale, CAT scores, FEV1, FVC, IC, R5, R20, R5 − R20, X5, Fres, and ALX in the univariate analysis (). The correlation between 6MWD and R5, R20, R5 − R20, X5, Fres, and ALX are shown in . However, there were no correlations between 6MWD and ΔX5. Multivariate logistic regression analysis for predicting poor 6MWD (<350 m) was performed using the selected model, including six variables (AIC = 46.3). Age, BMI, FVC (%), R20, R5-R20, ΔX5, Fres, ALX were not selected in the final model with the best AIC. Subjects with a 6MWD <350 m was independently predicted by CAT scores and R5 (). These odds ratios are odds of being in the low 6MWD (thus the higher the CAT, the greater the odds (odds ratio >1); the greater the FEV1, the lower the odds (odds ratio <1)).
Figure 3. Correlation between 6-minute walking distance and oscillometry. Abbreviations: ALX, low-frequency reactance area; Fres, resonant frequency; R5 and R20, respiratory system resistance at 5 Hz and 20 Hz; R5 − R20, the difference between R5 and R20; X5, respiratory system reactance at 5 Hz.
Table 3. Univariate and multivariate logistic regression analyses of 6MWD and predictor variables.
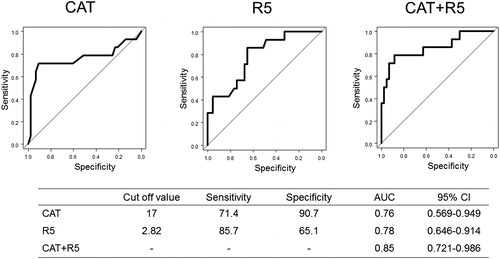
The ROC curve analysis of the poor 6MWD is shown in . The AUC for CAT scores plus R5 was superior to each CAT score or R5 with the best cutoff value of 17 points and 2.82 cmH2O/L/s, respectively. In whole-breath and expiratory phase data, multivariate analysis revealed CAT scores and FEV1, but not any oscillometric parameters, were predictors for 6MWD <350 m.
Figure 4. The ROC curve analysis predicting a 6-minute walking distance <350 m in the inspiratory phase.
Abbreviations: CAT, COPD assessment test; R5, respiratory system resistance at 5 Hz.
The results of 54 COPD subjects diagnosed based on the lower limit of normal are shown in supplemental materials. We found similar results when using LLN to define underlying obstructive airflow limitation compared to a fixed ratio of FEV1/FVC.
Discussion
We assessed whether broadband frequency oscillometry would predict the poor exercise tolerance measured by 6MWT in 57 study participants with COPD. It was found that subjects with a 6MWD <350 m had significantly higher Rrs and lower Xrs than subjects with a 6MWD ≥350 m; however, ΔX5 was not significant. In multivariate logistic regression analysis using the inspiratory data, we found that poor 6MWD (<350 m) was independently predicted by CAT scores and R5. The ROC curve analysis revealed that CAT scores plus R5 best predicted poor 6MWD. These results support our hypothesis that oscillatory indices are useful for predicting poor exercise tolerance.
R5 was higher in subjects with a 6MWD <350 m than that in subjects with a 6MWD ≥350 m and independent predictor of the poor 6MWD (<350 m). There is substantial evidence that airflow limitation and dynamic hyperinflation are predominant factors defining exertional dyspnea and disease severity in COPD. 6MWT can be used for assessing exercise tolerance and the risk of mortality [Citation1]. Weak-to-moderate correlations between 6MWD and a measure of disease severity such as FEV1, were shown in some studies [Citation6, Citation7]. IC, as a parameter of dynamic hyperinflation, and 6MWD also found significant correlations [Citation8]. There were some reports stating that severe airflow limitation was a determinant of 6MWD; however, a meta-analysis by Singh et al. showed that independent predictors of 6MWD were variable and inconsistent [Citation6, Citation22, Citation23]. The description of the relationship between oscillometry and 6MWT was scarce [Citation13, Citation24], and we could not find any related articles using MostGraph. A study by Zeng et al. using Impulse oscillometry showed that only R20 and X5 correlated with 6MWD, but both were not independent predictors [Citation24]. Although R5 is thought to be a measure of airway caliber and correlates weakly to moderately with FEV1, oscillometry and spirometry are not the same modalities [Citation10, Citation25–27]. A case-control study of the lung function in the World Trade Center Health Registry found that cases with persistent respiratory symptoms were more likely to have elevated Rrs despite normal spirometry than control subjects [Citation28]. It suggests that R5 may predict poor exercise tolerance due to not only airflow limitation but also dyspnea or early detection of lung function change induced by smoking [Citation10]. The univariate results using whole breath and expiratory breath were consistent with using the inspiratory breath parameters. However, if only whole breath and expiratory phase were adopted, multivariate analysis revealed CAT scores and FEV1, but not any oscillometric parameters, were predictors for 6MWD <350 m. The reason for the disappearance of oscillometric parameters in whole-breath and expiratory phase data could be excessive averaging (whole-breath) or noise (expiratory phase) to distinguish sensitivity of these parameters. In another previous report using IOS [Citation29], R5 values in the inspiratory phase were stable, whereas abrupt changes occurred in the expiratory phase. It suggested that the distinction between inspiratory and expiratory phases must be taken into accounts, since Rrs and Xrs may vary significantly during the respiratory cycle in subjects with COPD. This finding may explain some of the variability in findings of studies comparing oscillatory parameters to spirometry and other outcomes. This finding may also justify the use of only inspiratory parameters when exploring associations with clinical outcomes.
AlthoughΔX5 was reported as the EFL index [Citation30], there was no significant difference in ΔX5 between the two groups, nor was it a predictive value of poor 6MWD in our study. The median values of ΔX5 in all subjects and in subjects with 6MWD <350 m were 0.19 and 0.45 cmH2O/L/s, respectively. These were lower values compared to previous studies using MostGraph, 1.26, and 0.55 cmH2O/L/s, respectively [Citation11, Citation25]. Di Mango et al. found that reactance indices would be useful to assess the severity of airflow limitation, mainly in advanced subjects with COPD [Citation31]. The lack of significance can be explained by the mild case dominance or small sample size in this study. A recent study by Zimmermann et al. found that the change in 6MWD with pulmonary rehabilitation correlated with the baseline of ΔX5 [Citation13]. The relationship between ΔX5 and baseline 6MWD is still inconclusive. Further study with more cases and more severe subjects may be helpful; however, the rate of 6MWT completion would be reduced in severe cases. In this regard, our study was feasible and closer to the clinical setting.
In our previous report [Citation11], we showed that a high ΔX5 (EFL index) was independently predicted by the degree of emphysema evaluated by high-resolution CT (adjusted odds ratio: 1.296), peripheral airway obstruction (forced expiratory flow between 25% and 75% of FVC) (adjusted odds ratio: 0.752), hyperinflation (functional residual capacity) (adjusted odds ratio: 1.108), and airway caliber (R5) (adjusted odds ratio: 3.426), indicating that EFL measured by oscillometry is a comprehensive measure of COPD that has separable etiologies and R5 has a strong effect on EFL. Rrs indicates the total resistance of the respiratory system, including airway (Raw), tissue, and chest wall resistance. Since Raw is a major part of Rrs, Rrs is a sensitive parameter for Raw, and Rrs at lower frequencies, especially R5, is frequently interpreted as an index of the airway caliber [Citation10]. Thus, we think it is acceptable that R5, instead of ΔX5 (EFL index), was a major predictor of 6MWD in this study.
In our study, CAT scores were the independent predictive values of poor 6MWD with high specificity in ROC analysis. There was a weak-to-moderate correlation between Saint George Respiratory Questionnaire (SGRQ) and 6MWD [Citation6, Citation32–34], and also CAT scores and SGRQ scores have a strong correlation [Citation15]. Recently, some reports mentioned the correlation between 6MWT and CAT scores [Citation24, Citation35], and they support our results. CAT scores are a convenient and reliable predictive value of 6MWT. We hypothesized that oscillometry would have some role in the 6MWT and be useful in clinical practice of COPD. As a result, this study found CAT alone predicted exercise capacity with a reasonable AUC, but oscillometry had comparable reliability in predicting 6MWD which spirometry could not do so. One may consider that CAT is subjective, but R5 is objective, or that the combined use of CAT + R5 was better than either alone (higher AUC), so R5 adds some utility to CAT. In general, oscillometry is useful in older subjects who have difficulty in performing spirometry and who cannot cooperate with the maneuver. We can differentiate between oscillometry and spirometry in this aspect. If an oscillometry device is available, we can predict the 6MWD beforehand. This may be useful in clinical practice of COPD.
Medication may be a possible influencing factor for the 6MWD. However, most participants were on LABA/LAMA combinations and there was no difference between subjects with 6MWD ≥350 m and <350 m. Since the almost entirely male study cohort (94.7%) is a limitation, attention has to be paid for the generalizability. However, a previous Japanese epidemiological survey found 1319 of the total 1438 COPD subjects (91.7%) were male, indicating the characteristics of Japanese COPD subjects [Citation36].
In conclusion, only the inspiratory phase (not multiple) of R5, and the CAT score, predicted a 6MWD <350 m; the others did not in multivariate analysis in COPD subjects. Further studies are warranted to explore the usefulness of oscillometry measures for future evaluation of COPD.
Declaration of interest
The authors report no conflicts of interest.
Supplemental Material
Download PDF (384.5 KB)References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. Available from: https://goldcopd.org/.
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001 Sep 1;164(5):770–777.
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004 Jun 24; 350(26):2645–2653.
- Koulouris NG, Hardavella G. Physiological techniques for detecting expiratory flow limitation during tidal breathing. Eur Respir Rev. 2011 Sep 1; 20(121):147–155. 55. DOI:10.1183/09059180.00001911
- Cote CG, Casanova C, Marin JM, et al. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008 Mar; 31(3):571–578.
- Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. DOI:10.1183/09031936.00150414
- Rambod M, Porszasz J, Make BJ, et al. Six-minute walk distance predictors, including CT scan measures, in the COPDGene cohort. Chest. 2012 Apr; 141(4):867–875.
- Bell M, Fotheringham I, Punekar YS, et al. Systematic review of the association between laboratory- and field-based exercise tests and lung function in patients with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2015 Jul 8; 2(4):321–342. DOI:10.15326/jcopdf.2.4.2014.0157
- King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55(2):1900753. Nov 26.
- Shirai T, Kurosawa H. Clinical application of the forced oscillation technique. Intern Med. 2016;55(6):559–566. DOI:10.2169/internalmedicine.55.5876
- Mikamo M, Shirai T, Mori K, et al. Predictors of expiratory flow limitation measured by forced oscillation technique in COPD. BMC Pulm Med. 2014 Feb 19; 14:23. DOI:10.1186/1471-2466-14-23
- Dellaca RL, Santus P, Aliverti A, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004 Feb; 23(2):232–240. DOI:10.1183/09031936.04.00046804
- Zimmermann SC, Thamrin C, Chan AS, et al. Relationships between forced oscillatory impedance and 6-minute walk distance after pulmonary rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:157–166. DOI:10.2147/COPD.S225543
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999 Jul; 54(7):581–586. DOI:10.1136/thx.54.7.581
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009 Sep; 34(3):648–654.
- Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. DecDOI:10.1183/09031936.03.00089403
- Kubota M, Shirai G, Nakamori T, et al. Low frequency oscillometry parameters in COPD patients are less variable during inspiration than during expiration. Respir Physiol Neurobiol. 2009; 2009/04/30/166(2):73–79. DOI:10.1016/j.resp.2009.01.007
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. DOI:10.1183/09031936.05.00034805
- Japanese Respiratory Society. Guideline of respiratory function tests–spirometry, flow-volume curve, diffusion capacity of the lung. Nihon Kokyuki Gakkai Zasshi. 2004:1–56.
- Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014 Dec; 44(6):1428–1446. DOI:10.1183/09031936.00150314
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013 Mar; 48(3):452–458. DOI:10.1038/bmt.2012.244
- Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010 Jun; 104(6):849–857.
- Perez T, Deslee G, Burgel PR, et al. Predictors in routine practice of 6-min walking distance and oxygen desaturation in patients with COPD: impact of comorbidities. COPD. 2019;14:1399–1410.
- Zeng GS, Chen LC, Fan HZ, et al. The relationship between steps of 6MWT and COPD severity: a cross-sectional study. COPD. 2018;14:141–148.
- Mori K, Shirai T, Mikamo M, et al. Colored 3-dimensional analyses of respiratory resistance and reactance in COPD and asthma. COPD. 2011 Dec; 8(6):456–463. DOI:10.3109/15412555.2011.626818
- Kanda S, Fujimoto K, Komatsu Y, et al. Evaluation of respiratory impedance in asthma and COPD by an impulse oscillation system. Intern Med. 2010;49(1):23–30. DOI:10.2169/internalmedicine.49.2191
- Karayama M, Inui N, Mori K, et al. Respiratory impedance is correlated with morphological changes in the lungs on three-dimensional CT in patients with COPD. Sci Rep. 2017 Feb 08; 7:41709.
- Friedman SM, Maslow CB, Reibman J, et al. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med. 2011 Sep 1; 184(5):582–589.
- Ohishi J, Kurosawa H, Ogawa H, et al. Application of impulse oscillometry for within-breath analysis in patients with chronic obstructive pulmonary disease: pilot study. BMJ Open. 2011 Jan 1; 1(2):e000184. DOI:10.1136/bmjopen-2011-000184
- Dellaca RL, Pompilio PP, Walker PP, et al. Effect of bronchodilation on expiratory flow limitation and resting lung mechanics in COPD. Eur Respir J. 2009 Jun; 33(6):1329–1337.
- Di Mango AM, Lopes AJ, Jansen JM, et al. Changes in respiratory mechanics with increasing degrees of airway obstruction in COPD: detection by forced oscillation technique. Respir Med. 2006 Mar; 100(3):399–410.
- Punekar YS, Riley JH, Lloyd E, et al. Systematic review of the association between exercise tests and patient-reported outcomes in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2487–2506. DOI:10.2147/COPD.S100204
- Oga T, Nishimura K, Tsukino M, et al. Relationship between different indices of exercise capacity and clinical measures in patients with chronic obstructive pulmonary disease. Heart Lung. 2002;31(5):374–381. DOI:10.1067/mhl.2002.127941
- Brown CD, Benditt JO, Sciurba FC, et al. Exercise testing in severe emphysema: association with quality of life and lung function. COPD: J Chron Obs Pulm Dis. 2008;5(2):117–124.
- Kerti M, Balogh Z, Kelemen K, et al. The relationship between exercise capacity and different functional markers in pulmonary rehabilitation for COPD. COPD. 2018;13:717–724.
- Tatsumi K, Kasahara Y, Kurosu K, et al. Clinical phenotypes of COPD: results of a Japanese epidemiological survey. Respirology. 2004;9(3):331–336. Aug
