Abstract
The ZZ genotype of alpha-1 antitrypsin deficiency (AATD) is strongly associated with COPD, even in never-smokers. Moderate AATD genotypes (MZ and SZ) have been shown to increase the severity of COPD in smokers. In this comparative study, we examine the association between AATD, genotypes, and smoking cessation. Two hundred and ninety-three Irish people with AATD [MZ (n = 91), SZ (n = 72), and ZZ/rare (n = 130)] completed a custom questionnaire assessing their social and smoking histories. The primary outcomes analyzed were the predictors of ever-smoking and effect of genotype on awareness of AATD and maintained smoking cessation, using logistic regression analyses. Parental smoking exposure was associated with ever-smoking status (OR 1.84 vs. no parental smoking, p = 0.018), higher cumulative tobacco consumption (23.47 vs. 14.87 pack-years, p = 0.005) and more quit attempts required to achieve cessation among former-smokers (2.97 vs. 5.60, p = 0.007). Awareness of genotype was 67.7% versus 56.3% versus 33% for ZZ, SZ, and MZ, respectively (p < 0.001). Among ever-smokers, current-smoking was uncommon (2.5% vs. 17% vs. 16% for ZZ, SZ, and MZ, respectively, p = 0.009) with ZZs significantly less likely to be current-smokers (OR 0.15 relative to MZ, p = 0.025). These results suggest that the genetic risk of COPD in AATD families is compounded by transmission of social risk factors (via parental smoking). Increasing severity of genotype is associated with lower current-smoking rates among ever-smokers. Whether this is attributable to greater awareness of risk is an area of interest. Achieving a change in smoking habits may also result in positive health behavior in subsequent generations.
Introduction
Tobacco smoking remains the predominant cause of chronic obstructive pulmonary disease (COPD) in the western world. Alpha-1 antitrypsin deficiency (AATD) is the only commonly identifiable monogenic risk factor for COPD. The rare ZZ genotype of AATD results in severe deficiency of the serine protease inhibitor AAT, with plasma levels approximately 10–20% of those seen in the normal non-AATD population (MM genotype) [Citation1]. The severity of the resulting imbalance in plasma protease:antiprotease activity is such that ZZ AATD is associated with the development of airflow obstruction even in never-smokers [Citation2] and severe, early onset lung disease in smokers. The SZ genotype results in a less severe deficiency than ZZ, with a plasma AAT level that is 30–40% that of MM individuals (wild-type homozygotes). The heterozygous MZ genotype of AATD is as common as 1/25 − 1/75 people of European descent [Citation3, Citation4] and also results in moderate deficiency. For decades, the MZ genotype was considered to represent a “carrier” state. Recently however, an interaction between the MZ genotype and smoking has been shown to significantly increase the risk of COPD [Citation5] and the severity of airflow obstruction relative to usual (MM) smokers [Citation6, Citation7]. Consequently, MZ AATD smokers represent a common, high-risk subgroup, with a genetically predetermined course toward greater morbidity. Similar findings have been demonstrated in SZ individuals who smoke [Citation8–10].
Severity of COPD is associated with higher healthcare use and greater cost [Citation11, Citation12]. While promotion of smoking cessation should remain a key strategy for all smokers, targeting smoking cessation in AAT deficient individuals may result in substantially greater individual reduction of disease progression. Evidence suggests that genetic testing for the purpose of encouraging lifestyle modification generally lacks efficacy [Citation13]. Whether a diagnosis of AATD in particular promotes smoking cessation is unclear. Nevertheless, there is evidence to suggest a cessation effect seen in AATD, with one prospective study demonstrating an increase in quit attempts among ZZ individuals three months post-diagnosis [Citation14] and registry data suggesting that active smoking in AATD cohorts is relatively uncommon and may be affected by genotype [Citation15].
We examined the relationship between demographic factors and smoking in an Irish AATD population and to assess what impact, if any, a diagnosis of AATD has on smoking cessation. Hereafter, we present the results of a cross-sectional, comparative study in an Irish AATD cohort.
Methods
Individuals enrolled in the National AATD Registry consent to collection of clinical data and voluntary participation in ongoing research projects (Beaumont Hospital Ethics REC No. 05-03). A custom questionnaire was designed to capture responses relating to demographics, parental smoking history, personal smoking history, AATD awareness, and personal medical history. Data were collected using surveying software (© 2019 QuestionPro Survey software) with participation limited to the three main genotypes populating the registry: MZ, SZ and ZZ (with ZZ-equivalent rare genotypes (e.g. Null/Null, Z/Null, Z/Mmalton) included as ZZ). Registry participants were contacted by phone offering participation. A total of three contact attempts were made for each individual at least one day apart and at different times of the day. Responses were defined as per the standard definitions set out by The American Association for Public Opinion Research [Citation16]. Consent to inclusion was recorded and responses were linked to anonymized study-specific IDs. Actual genotypes (vs patient reported) and measurements of airflow obstruction were collected from the registry database.
To better assess maintained smoking cessation, only individuals diagnosed with AATD for more than 2 years were included. Smokers were defined as any person with a lifetime consumption of greater than 20 packs of cigarettes (where one pack = 20 cigarettes). Severity of lung disease was defined by GOLD classification, where GOLD 0 represented those with no airflow obstruction or airflow obstruction (FEV1/FVC ratio <0.7) with an FEV1% predicted (pp) of >100%, and GOLD 1-4 representing airflow obstruction with an FEV1pp 80–100%, 50–79%, 30–49%, and <30%, respectively. Educational achievement was measured on a 6-point scale with 1 representing primary level education and 6 representing attainment of post-graduate degree. Insight was assessed by an individual’s ability to state their genotype or “type” of AATD (where the response “carrier” for MZ was taken as incorrect).
Statistical analysis was performed in RStudio Version 1.1.463 running R v3.5.2 (www.cran.r-project.com). Continuous data was validated for normality of distribution using the Shapiro-Wilk test. Normally and non-normally distributed data were analyzed by Student t test or ANOVA and Mann-Whitney U test or Kruskal-Wallis tests, respectively. Given the explorative, hypothesis-forming nature of the study, univariate analyses were not corrected for multiplicity. Categorical dichotomous variables were coded with the supposed lower risk variable as the reference and the proposed higher risk variable as the comparator. The MZ genotype was coded as the reference genotype with SZ and ZZ as comparators. Odd ratios (OR) of outcomes were calculated using binomial logistic regression models (glm in R) with confounders (such as age, sex, and pack-years smoked, GOLD classification) and predictors modeled as fixed effects. The primary outcomes analyzed were (a) association of patient and social factors with ever-smoker status and (b) the effect of genotype on insight and successful/maintained smoking cessation. Covariates for logistic regression were selected based on preliminary univariate associations. Sequential testing of logistic regression models was performed with removal of covariates with statistically non-significant effects at each new iteration. The final model covariates were selected on the basis of best fit as determined by comparison of the Bayesian Information Criterion.
Results
In total, 477 individuals enrolled in the National AATD Registry were identified as being eligible for inclusion. One hundred and fifty one (31.6%) were uncontactable. Of the remaining 326 individuals, the cooperation rate (100% question completion among contactable individuals) was 92.2% for ZZ/rare AATD (where non-ZZ rare genotypes n = 5), 87.8% for SZ, and 89.8% for MZ registry participants totaling 293 responders (89.9%, [MZ (n = 91), SZ (n = 72), and ZZ/rare (n = 125/5)]). No difference in age was detected between responders and non-responders or responders and uncontactable individuals (p = 0.6). The mean study population age was 55.29 years, with 48.8% females, and 58% ever-smokers. Airflow obstruction differed significantly between genotypes (p < 0.001, ) but was more similar between MZs and SZs than ZZs, with the latter more likely to have been diagnosed due to lung disease.
Table 1. Characteristics of respondents, presented by AATD genotype.
Awareness of genotype
Awareness of AAT type differed significantly between genotypes with increased awareness associated with genotype (67.7% vs. 58.3% vs. 33% for ZZ, SZ, and MZ, respectively, p < 0.001). Moreover, when assessing awareness in MZs only, 34 individuals (37%) reported being a “carrier” but could not specify genotype. When assessed by multivariable analysis, awareness of AAT type was associated with severity of genotype (OR 3.34 for SZ vs. MZ, 95% CI; 1.70 to 6.56, p < 0.001 and OR 6.06 for ZZ vs. MZ, 95% CI; 3.23 to 11.38, p < 0.001), sex (OR 0.44 for males, 95% CI; 0.27 to 0.75, p = 0.002), and increasing educational attainment (OR 1.47 per educational grade increase, 95% CI; 1.18 to 1.82, p = 0.005).
Predictors of ever-smoking
Multivariable analysis examining predictors of ever-smoking status revealed a strong association with parental smoking (OR 1.84, 95% CI; 1.09 to 3.06, p = 0.018), a trend toward inverse association with educational attainment (OR 0.85 per educational grade increase, 95% CI; 0.69 to 1.027, p = 0.09) and no association with sex (p = 0.5; ). Furthermore, among former-smokers parental smoking was found to be associated with higher pack-years smoked (23.47 vs. 14.87, p = 0.005 for “parental-smoking” vs. “no parental-smoking”) and greater quit attempts to achieve cessation (5.60 vs. 2.97, p = 0.007 for “parental-smoking” vs. “no parental-smoking”; ).
Figure 1. Univariate analysis of the association of parental smoking with cumulative tobacco consumption (pack-years; top) and quit attempts (bottom) prior to successful cessation among former-smokers (n = 153). Parental smoking was associated with significantly greater tobacco consumption (+8.7 pack-year, p = 0.005) and quit attempts (+2.63 quit attempts, p = 0.007).
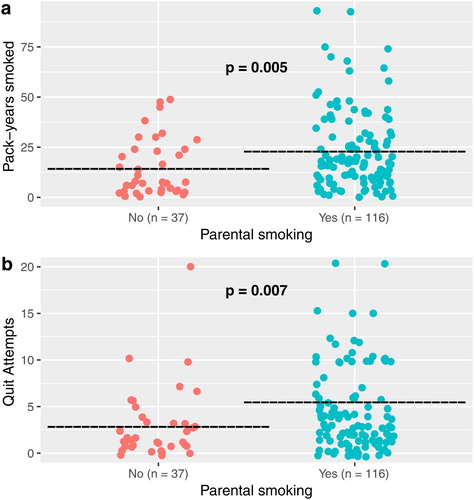
Table 2. Adjusted odds of ever-smoker status for demographic and social factors.
Smoking cessation
Among ever-smokers across all genotypes (, n = 170), the ZZ cohort reported significantly greater maintained abstinence from tobacco smoking than the SZ or MZ genotypes (current-smoker 2.5% vs. 17.1% vs. 16.3%, respectively, p = 0.009). Ninety-six percent of former-smokers reported more than 2 years abstinence and 85% reported more than 5 years abstinence. Furthermore, 58 respondents reported being smokers at the time of AATD diagnosis, with a subsequent reported quit rate of 70.7% (41/58), rising to 92% when stratifying for the ZZ genotype (23/25). Current-smokers were younger at diagnosis and at the time of the study, more commonly MZ or SZ genotype, had less airflow obstruction and were more likely to have used E-cigarettes or Vaping as cessation aids in quit attempts ().
Table 3. Characteristics of ever-smoking respondents presented by former- vs. current-smokers.
Among former-smokers (n = 153), 49 quit after a diagnosis of lung disease and 41 quit after their diagnosis of AATD, with complete cessation achieved in under 12 months in 46.9% (23/49) and 60% (25/41) of these groups, respectively. Among those who quit smoking following a diagnosis of both AATD and lung disease (n = 23), 16 (69.5%) reported their diagnosis of AATD to have been the greater motivator to quit.
In binomial logistic regression analysis, the factors significantly associated with current-smoking status were ZZ genotype (OR 0.15 vs. MZ, 95% CI; 0.03 to 0.78, p = 0.025), higher pack-years smoked (OR increased 1.04 per pack-year, 95% CI; 1.01 to 1.08, p = 0.018), and older age (OR decreased 0.91 per year, 95% CI; 0.86 to 0.96, p < 0.001). No significant effect was seen for the SZ genotype (OR 0.58 vs. MZ, p = 0.421), sex (OR 0.65 for males, p = 0.447), or parental smoking (OR 0.95, p = 0.939) ().
Table 4. Adjusted odds of current-smoker (vs. former-smoker) status for patient factors.
Discussion
Increasing smoking cessation remains a major challenge to public health. In general, cessation rates remain disappointing with one large longitudinal study identifying a 39% cessation rate in the general population at 11 years of follow up [Citation17], as well as similar cessation rates demonstrated in COPD [Citation18] and cancer cohorts [Citation19], even in those with early stage lung cancer [Citation20]. Our cohort reported a genotype-dependent 83–97.5% maintained smoking cessation rate and a 70–92% cessation rate among those who were smoking at the time of their diagnosis of AATD. It is worth acknowledging that our study participants may not be wholly representative of the wider AATD population, especially considering the fact that they are consenting participants in the National AATD Registry and may be biased as a cohort toward greater health-seeking behavior. Nevertheless, the low rates of smoking in our cohort replicate findings previously reported in AATD.
AATD remains the most commonly identifiable and best understood monogenic risk factor for development of COPD. When associated with smoking, the risk and severity of COPD increase in both moderate and severe AATD genotypes. In spite of this, the evidence suggests that AATD remains underdiagnosed [Citation21, Citation22]. The increased risk of COPD in heterozygote smokers has only been demonstrated in recent years [Citation5, Citation6, Citation8, Citation23] and this risk may not yet be sufficiently recognized among clinicians. Lower perception of risk has previously been associated with reduced smoking cessation [Citation24] and our data suggests that severity of deficiency correlates with awareness of diagnosis and former-smoker (vs current-smoker) status. When comparing the adjusted OR of persistent smoking among individuals in our cohort by genotype, the highest risk (ZZ/rare) cohort was strongly associated with greater cessation. While awareness of genotype did not associate with a lower odds of being a former smoker in our multivariable model, it is possible that this may have been due to confounding by genotype, as the two are inextricably linked. Awareness of increased risk may stimulate a change in behavior and though the retrospective and self-reported nature of our study does not allow for this hypothesis to be tested sufficiently, this should be an area of focus going forward. A previous prospective study suggested that a diagnosis of ZZ AATD resulted in greater quit attempts and reduced tobacco consumption compared to non-AATD smokers three months after diagnosis [Citation14]. In the same study such an effect was not seen in those diagnosed with MZ AATD although the increased risk of COPD in MZ smokers had not yet been demonstrated at that time, as evidenced by the fact the authors classified participants with the MZ genotype as “carriers.” Similarly, many of our registry participants with MZ AATD were diagnosed at a time when the true effect of smoking in MZ AATD was not known and in our study 37% identified as “carriers.”
The MZ genotype of AATD has a prevalence of 1/25 in Ireland, equivalent to 250,000 people on the island [Citation3] and is common in much of the western world. In Ireland, each of the estimated 40–45,000 current-smokers who are MZ [Citation25] are at a tenfold increased risk of developing COPD compared to usual-smokers [Citation5]. Informing these individuals of their increased risk should be viewed as standard of care, given the potential for significant reduction of harm, with our data suggesting that in particular men and individuals with lower educational attainment may be at greater risk of poor awareness. The outstanding question is whether improving awareness of the implications of smoking among newly diagnosed MZ and SZ individuals can be shown to improve smoking cessation rates.
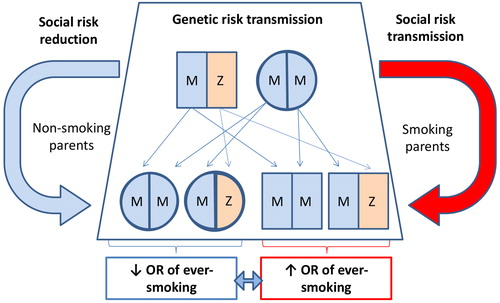
We have identified parental smoking as a strong predictor of ever-smoking status in our cohort, a finding that has been described in previous large cross-sectional population studies [Citation26, Citation27]. In our data, parental smoking was also associated with greater tobacco consumption (pack-years) and quit attempts required prior to successful cessation, a similar effect to that observed in a previous study examining passive smoke exposure in childhood in a ZZ registry cohort [Citation28]. In this regard, AATD individuals are primed for a hereditary “double-hit” with regards to risk of COPD: inheritance of both genetic and social risk factors (). Despite this, such a finding could be viewed as an opportunity. Achievement of successful smoking cessation in an index individual may not only halt the accelerated development of airflow obstruction in that individual but also reduce the chances of their AAT-deficient children ever becoming smokers.
Figure 2. Representation of the double-hit hypothesis for COPD risk in AATD families. A single AAT deficient parent (in this example one heterozygous MZ individual) is sufficient to pass on a deficient Z allele in 50% of children. This inheritance of MZ AATD is not associated with an increase in the risk of COPD without the addition of tobacco smoking. The presence of smoking at a parental level (red arrow) will increase the likelihood of smoking in progeny compared to those born to nonsmoking parents (blue arrow). With the addition of cigarette smoking, MZ progeny will have a significantly increased risk of COPD compared to smoking MM siblings.
Conclusion
A diagnosis of AATD should be seen as an opportunity for a teachable moment, regardless of the severity of the genotype in question. Moderate AAT deficiency (genotypes MZ and SZ) has been clearly shown to result in disproportionately severe COPD in smokers, yet these individuals (in particular males and those with lower academic education) appear to be poorly informed of this risk. While identifying rare, severe (e.g. ZZ) AATD offers the greatest opportunity for therapeutic intervention at an individual level [Citation29, Citation30], a greater benefit may be achieved at population level by identifying and educating the far larger heterozygote group at risk due to smoking, with a view to halting accelerated lung function decline and the intergenerational transmission of smoking habits demonstrated in our cohort. Additional prospective studies of the impact of AATD diagnosis on smoking cessation would be helpful in further investigating and validating these hypotheses.
| Abbreviations | ||
| AAT | = | Alpha-1 antitrypsin |
| AATD | = | Alpha-1 antitrypsin deficiency |
| COPD | = | chronic obstructive pulmonary disease |
| FEV1 | = | forced expiratory volume in 1 s |
| glm | = | generalized linear model |
| GOLD | = | global initiative for obstructive lung disease |
| MZ | = | Pi*M/Pi*Z allele heterozygous genotype for AAT |
| OR | = | odds ratio |
| SZ | = | Pi*S/Pi*Z allele heterozygous genotype for AAT |
| ZZ | = | Pi*Z allele homozygous genotype for AAT |
Acknowledgment
The authors would like to thank all participants who generously gave of their time to take part in our study and RCSI undergraduate medical student research programs.
Disclosure statement
The authors report no conflict of interests.
Data availability
Data from this study will not be publicly available due to the terms of the patient consent to participation.
Additional information
Funding
References
- Brantly ML, Wittes JT, Vogelmeier CF, et al. Use of a highly purified Alpha 1-antitrypsin standard to establish ranges for the common normal and deficient Alpha 1-antitrypsin phenotypes. Chest. 1991;100(3):703–708. DOI:10.1378/chest.100.3.703
- Piitulainen E, Tornling G, Eriksson S. Effect of age and occupational exposure to airway irritants on lung function in non-smoking individuals with Alpha 1-antitrypsin deficiency (PiZZ). Thorax. 1997;52(3):244–248. DOI:10.1136/thx.52.3.244
- Carroll TP, O'Connor CA, Floyd O, et al. The prevalence of Alpha-1 antitrypsin deficiency in Ireland. Respir Res. 2011;12:91. DOI:10.1186/1465-9921-12-91
- Blanco I, de Serres FJ, Carcaba V, et al. Alpha-1 antitrypsin deficiency PI*Z and PI*S gene frequency distribution using on maps of the world by an inverse distance weighting (IDW) multivariate interpolation method. Hepat Mon. 2012;12(10 HCC):e7434. DOI:10.5812/hepatmon.7434
- Molloy K, Hersh CP, Morris VB, et al. Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189(4):419–427. DOI:10.1164/rccm.201311-1984OC
- Foreman MG, Wilson C, DeMeo DL, et al. Alpha-1 antitrypsin PiMZ genotype is associated with chronic obstructive pulmonary disease in two racial groups. Ann Am Thorac Soc. 2017;14(8):1280–1287. DOI:10.1513/AnnalsATS.201611-838OC
- Ortega VE, Li X, O’Neal WK, et al. The effects of rare SERPINA1 variants on lung function and emphysema in SPIROMICS. Am J Respir Crit Care Med. 2020;201(5):540–554. DOI:10.1164/rccm.201904-0769OC
- Franciosi AN, Hobbs BD, McElvaney OJ, et al. Clarifying the risk of lung disease in SZ Alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2020;202(1):73–82. DOI:10.1164/rccm.202002-0262OC
- Franciosi AN, Carroll TP, McElvaney NG. SZ alpha-1 antitrypsin deficiency and pulmonary disease: more like MZ, not like ZZ. Thorax. 2020 Sep 11:thoraxjnl-2020-215250. doi: 10.1136/thoraxjnl-2020-215250.
- Turino GM, Barker AF, Brantly ML, et al. Clinical features of individuals with PI*SZ phenotype of Alpha 1-antitrypsin deficiency. Alpha 1-Antitrypsin Deficiency Registry Study Group. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1718–1725. DOI:10.1164/ajrccm.154.6.8970361
- Byng D, Lutter JI, Wacker ME, et al. Determinants of healthcare utilization and costs in COPD patients: first longitudinal results from the German COPD cohort COSYCONET. COPD. 2019;14:1423–1439. DOI:10.2147/COPD.S201899
- Wacker ME, Jorres RA, Schulz H, et al. Direct and indirect costs of COPD and its comorbidities: results from the German COSYCONET study. Respir Med. 2016;111:39–46. DOI:10.1016/j.rmed.2015.12.001
- Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. DOI:10.1136/bmj.i1102
- Carpenter MJ, Strange C, Jones Y, et al. Does genetic testing result in behavioral health change? Changes in smoking behavior following testing for Alpha-1 antitrypsin deficiency. Ann Behav Med. 2007;33(1):22–28. DOI:10.1207/s15324796abm3301_3
- Holm KE, Mannino DM, Choate R, et al. Genotype is associated with smoking and other key health behaviors among individuals with Alpha-1 antitrypsin deficiency-associated lung disease. Respir Med. 2018;143:48–55. DOI:10.1016/j.rmed.2018.08.016
- AAPOR. Standard definitions: Final dispositions of case codes and outcome rates for surveys. 9th ed. Washington DC, AAPOR; 2016.
- Holm M, Schioler L, Andersson E, et al. Predictors of smoking cessation: a longitudinal study in a large cohort of smokers. Respir Med. 2017;132:164–169. DOI:10.1016/j.rmed.2017.10.013
- Tonnesen P. Smoking cessation and COPD. Eur Respir Rev. 2013;22(127):37–43. DOI:10.1183/09059180.00007212
- Burke L, Miller LA, Saad A, et al. Smoking behaviors among cancer survivors: an observational clinical study. J Oncol Pract. 2009;5(1):6–9. DOI:10.1200/JOP.0912001
- Hopenhayn C, Christian WJ, Christian A, et al. Factors associated with smoking abstinence after diagnosis of early stage lung cancer. Lung Cancer. 2013;80(1):55–61. DOI:10.1016/j.lungcan.2012.12.013
- Stoller JK, Sandhaus RA, Turino G, et al. Delay in diagnosis of Alpha1-antitrypsin deficiency: a continuing problem. Chest. 2005;128(4):1989–1994. DOI:10.1378/chest.128.4.1989
- Greulich T, Ottaviani S, Bals R, et al. Alpha1-antitrypsin deficiency - diagnostic testing and disease awareness in Germany and Italy. Respir Med. 2013;107(9):1400–1408. DOI:10.1016/j.rmed.2013.04.023
- Silverman EK. Risk of lung disease in PI MZ heterozygotes. Current status and future research directions. Ann Am Thorac Soc. 2016;13(Suppl 4):S341–S345. DOI:10.1513/AnnalsATS.201507-437KV
- Schnoll RA, James C, Malstrom M, et al. Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med. 2003;25(3):214–222. DOI:10.1207/S15324796ABM2503_07
- Summary SiI. Available from: https://www.hse.ie/eng/about/who/tobaccocontrol/research/smoking-in-ireland-2019.pdf; Health Service Executive of Ireland (HSE), Dublin. 2019.
- Epstein JA, Williams C, Botvin GJ, et al. Psychosocial predictors of cigarette smoking among adolescents living in public housing developments. Tob Control. 1999;8(1):45–52. DOI:10.1136/tc.8.1.45
- de Vries H, Engels R, Kremers S, et al. Parents' and friends' smoking status as predictors of smoking onset: findings from six European countries. Health Educ Res. 2003;18(5):627–636. DOI:10.1093/her/cyg032
- O’Brien ME, Pennycooke K, Carroll TP, et al. The impact of smoke exposure on the clinical phenotype of Alpha-1 antitrypsin deficiency in Ireland: exploiting a national registry to understand a rare disease. COPD. 2015;12(sup1):2–9. DOI:10.3109/15412555.2015.1021913
- Chapman KR, Chorostowska-Wynimko J, Koczulla AR, et al. Alpha 1 antitrypsin to treat lung disease in Alpha 1 antitrypsin deficiency: recent developments and clinical implications. Int J Chron Obstruct Pulmon Dis. 2018;13:419–432. DOI:10.2147/COPD.S149429
- McElvaney NG, Burdon J, Holmes M, et al. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med. 2017;5(1):51–60. DOI:10.1016/S2213-2600(16)30430-1
