Abstract
Chronic obstructive pulmonary disease (COPD) is the leading cause of hospitalization for chronic respiratory illness in Spain. In recent years hospital admissions due to bronchiectasis have been increasing, although it is not known whether this is in proportion to COPD hospitalizations. Our main objective was to analyze the temporal evolution of discharges due to COPD, bronchiectasis, and their combination, and secondly, to assess their impact on in-hospital mortality and healthcare costs. We performed a retrospective study, based on the analysis of the Minimum Basic Data Set (MBDS) of hospital discharges using data from Spanish Ministry of Health with diagnostic codes of COPD or bronchiectasis between 2004 and 2015. We found 3 356 186 discharges with a diagnosis of COPD or bronchiectasis. After exclusions, 1 386 430 episodes were analyzed: 85.2% with COPD, 8.4% bronchiectasis, and 6.4% with both pathologies. Mean age of patients was 74.8 (10.9) years and with a male predominance of 80.1%. The increase in the annual number of discharges was greater in the two groups with bronchiectasis: 48.8% in the bronchiectasis group and 55.4% in the mixed group, compared to 6.6% in the COPD group. The mean length of stay was greater in both groups with bronchiectasis (p < 0.001), while in-hospital mortality was higher in the COPD group (p < 0.001). Similarly, the annual increase of costs was more evident in the two groups with bronchiectasis. Conclusions: Hospitalizations and health costs for bronchiectasis have increased in recent years significantly more than for COPD.
Supplemental data for this aricle can be accessed here.
Introduction
Bronchiectasis is defined as a chronic, progressive, and abnormal bronchial dilation due to multiple causes [Citation1]. Its usual clinical presentation includes recurrent respiratory infections, conditioning a progressive clinical deterioration, and increased mortality [Citation2].
Its prevalence varies widely between countries, and it is higher in women and in the older population, ranging from about 52 cases/100 000 inhabitants to 500 cases/100 000 inhabitants, while the annual rate of admissions ranges between 9.4/100 000 inhabitants in Germany and 16.5/100 000 in the US and Spain [Citation3–5].
Chronic obstructive pulmonary disease (COPD) is one of the main causes of morbidity, mortality, and healthcare expenses worldwide [Citation6]. Its prevalence varies among countries and populations [Citation7–9], and it is growing continuously despite the reduction in smoking rates, probably due to the progressive aging of the population [Citation10]. Thus, unlike what is seen with other diseases, COPD-related hospitalizations have not decreased, and in countries like Canada they already top cardiac diseases as the main cause of hospital admissions [Citation11].
Multiple studies have documented the high prevalence of bronchiectasis in patients with COPD, especially those with more severe disease leading to higher rate of readmissions and mortality [Citation12,Citation13]. The previous lack of suspicion about the existence of bronchiectasis has contributed to classically mislabeling smokers with bronchiectasis as having only COPD without performing a chest high-resolution computed tomography (HRCT), or even having evidence of an obstructive spirometric disorder [Citation14]. Nowadays, increased diagnoses of bronchiectasis in hospital discharges have raised awareness of the impact of the disease as well as the high healthcare costs involved, although current evidence remains scarce [Citation15].
Improving the epidemiological knowledge of a disease requires that a large number of patients be analyzed, in order to permit drawing countrywide conclusions. The population databases created to improve the management, planning, and evaluation of healthcare has contributed to increasing this knowledge. Since the beginning of the 1990s, Spanish hospitals have recorded the Minimum Basic Data Set (MBDS) for each patient treated at the health centers of the National Health System [Citation16].
Our hypothesis is that there is a greater presence of bronchiectasis in patients with COPD, and that the availability of HRCT has generated a progressive increase in the number of hospital discharges coded as bronchiectasis. This increment may be even greater during the time period analyzed than the discharge codes for COPD. Therefore, we carried out a study at the national level to analyze the temporal evolution of discharges due to COPD, bronchiectasis, and both pathologies simultaneously, and secondly, to assess their impact on in-hospital mortality, and healthcare costs.
Material and methods
We performed a retrospective study based on analysis of the MBDS database. We requested data from hospital discharges with diagnostic codes of bronchiectasis (494, 494.0, 494.1) and/or COPD (491.20, 491.21, 491.22, 492.8, 493.20, 493.21, 493.22, 496) from 2004 to 2015. The diagnoses collected are coded according to the International Classification of Diseases (ICD-9-CM), which also makes it possible to sort the hospitalization files into groups of patients with similar clinical characteristics and use of healthcare resources.
Patients under 40 years of age (because COPD is rarely diagnosed before this age, we used this cutoff point as have other studies) [Citation8], those with a diagnostic code of cystic fibrosis (277.0 − 277.09; V18.19; V77.6; V83.81), patients discharged for services not related to an admission due to exacerbation of COPD and/or bronchiectasis (pediatric surgery, dietetics, pharmacy, neonatal intensive medicine, pediatric intensive medicine, nuclear medicine, neonatology, ophthalmology, pediatrics, psychology, spinal cord injury unit, transplant extraction units), and patients coded as ‘indeterminate sex’ were excluded.
The MBDS includes three types of data. First, there are demographic data: age, date of birth, sex, and province of residence. Second, identification data for the episode: date of admission, financing, type of admission (urgent, scheduled, other), whether the current hospitalization is due to readmission of previous hospitalization (readmission before 30 days after a previous hospital discharge), type of hospital (small regional hospital with fewer than 150 beds, basic general hospital with 200 beds, area hospital with about 500 beds, large hospital of medium complexity, large hospital of high complexity), responsible service, discharge date, and type of discharge (home, transfer to another hospital, voluntary discharge, death or transfer to a nursing home, among others). Finally, clinical data such as the main diagnosis code and secondary diagnosis codes are collected (up to a total of 13, which include some procedures).
To calculate the annual admission rate, the population of the country was taken into account for each of the years studied, according to data from the National Institute of Statistics [Citation17].
Using the secondary diagnostic codes, the age-adjusted Charlson index was calculated, using the methodology described by Deyo et al. [Citation18]. The comorbidity data obtained from an administrative database are similar to data in a patient's medical history, although with a certain degree of underdiagnosis, so residual confusion in this regard is inevitable. The Charlson index includes 15 chronic diseases with different scores depending on their severity. The diagnostic codes for COPD and bronchiectasis were included in ‘chronic respiratory disease’. Correction for age was carried out by adding one point for each decade above 50 years of age [Citation19].
To carry out the comparative analyses, three groups of patients were created according to their codes at discharge: patients with bronchiectasis without COPD, patients with COPD without bronchiectasis, and patients with both diseases. In addition, ‘related diagnoses’ considered were those that could be associated with hospitalization for bronchiectasis or COPD, such as pneumonia, influenza, acute and chronic bronchitis, emphysema, acute/chronic respiratory failure, dyspnea/respiratory failure, stridor, cough, and hemoptysis.
In the bronchiectasis group, patients with a primary diagnosis of bronchiectasis, and those with a related primary diagnosis and a secondary diagnosis of bronchiectasis, were included. The approach was the same for the diagnosis of COPD. In the mixed group, patients with a primary diagnosis of COPD and a secondary diagnosis of bronchiectasis (or vice versa), and those with a primary diagnosis related to COPD and bronchiectasis as a secondary diagnosis, were included.
Ethics statement
The study was carried out using public data provided to the researchers by the Ministry of Health and Consumer Affairs, without the possibility of identifying the patients.
Statistical analysis
In order to describe the qualitative variables, absolute frequencies and percentages were used. The description of quantitative variables was performed using the mean, standard deviation (SD), median, and quartiles.
In the case of quantitative variables, comparison of the characteristics of the patients depending on the study group was carried out using the ANOVA test. The chi-squared test was employed for the comparison of categorical variables.
Before inclusion in the multivariate models for the possibility of there being a readmission, mortality and length of hospital stay, age, and Charlson score were transformed into binary variables using the best predictive cutoff obtained from ROC curve (receiver operating characteristics) analysis, and the area under the curve (AUC).
A final model for mortality was developed using back stepwise logistic regression analysis including sociodemographic and clinical variables as independent factors. Variables with a significance < 0.2 in the bivariate analysis were included as independent variables. The results were described with odds ratios (OR) with a 95% confidence interval (CI) and p-values. The Hosmer-Lemeshow goodness-of-fit test was performed to assess the overall fit of the model.
Finally, a Poisson model was used to analyze factors related to length of hospital stay. For all the tests p-values <0.05 were considered statistically significant. The statistical package R Studio (V2.5.1) was used for the statistical analyses.
Results
Between 2004 and 2015, a total of 43 806 699 hospital discharges were recorded in Spain. Of these, 3 356 186 had at least one primary and/or secondary diagnostic code of COPD or bronchiectasis. After removing patients who met the exclusion criteria (), a total of 1 386 430 episodes were analyzed, representing 3.2% of hospitalizations during the period reviewed.
Figure 1. Flow chart of patient selection.
BE: bronchiectasis; COPD: chronic obstructive pulmonary disease.
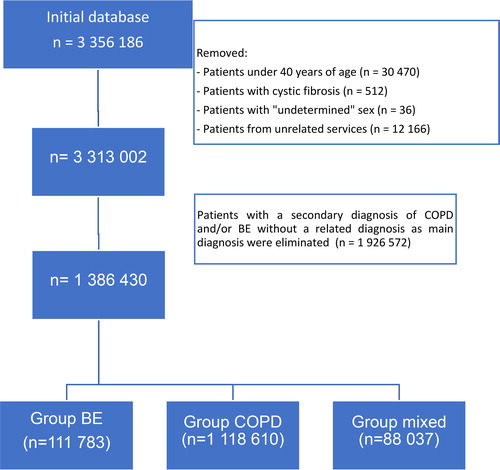
The distribution of discharges by province can be seen in (supplementary material). Out of these, 1 181 610 (85.2%) had a diagnosis of COPD, 116 783 (8.4%) of bronchiectasis, and 88 037 (6.4%) of both. Of the 1 269 647 patients who had COPD (COPD group + mixed group), 6.9% had associated bronchiectasis, while of the 204 820 discharges with a bronchiectasis code (bronchiectasis group + mixed group), 42.9% had a diagnosis of associated COPD. The proportion of patients with bronchiectasis was higher in more complex hospitals (p < 0.001).
Table 1. General characteristics of patients and hospitalizations.
shows the characteristics of patients and differences between the groups. The patients in COPD and mixed groups were mostly men (82.9 and 81.9% respectively), compared to 49.7% in the bronchiectasis group (p < 0.001). Most admissions were made through the emergency department, although the proportion of scheduled admissions was significantly higher in the bronchiectasis and mixed groups. Hospitalization in the previous 30 days was more frequent in the mixed group (21.9%) compared to the COPD (16.8%) and bronchiectasis groups (16.6%). The mean length of hospital stay was longer for the two groups with bronchiectasis: 9.9 days in the mixed group and 9.7 days in the bronchiectasis group vs. 8.7 in the COPD group (p < 0.001).
shows the total number of discharges in each group and year studied, as well the annual rate of admission and inter-annual variation. The annual number of discharges increased progressively in all groups, being more notable in the two groups with the presence of bronchiectasis (48.8% in the bronchiectasis group and 55.4% in the mixed group vs. 6.6% in COPD). The trend toward progressive increase and differences between groups was maintained even when the calculation was performed using annual rates per 100 000 inhabitants. Thus, between 2004 and 2015, the increase in the bronchiectasis group was 37.9% (from 18.3 to 25.2 admissions/100 000 inhabitants/year) and in the mixed group it was 43.7% (from 13.6 to 19. 6 admissions/100 000 inhabitants/year), compared to a decrease of 1.2% in the COPD group (from 216.7 to 214.1 admissions/100 000 inhabitants/year). This increase in the number of discharges was significantly higher in patients over 80 years of age, mainly in those with bronchiectasis (p < 0.001) ().
Figure 2. Temporal evolution of hospitalizations in each group, by sex and age ranges.
BE: bronchiectasis; COPD: chronic obstructive pulmonary disease.
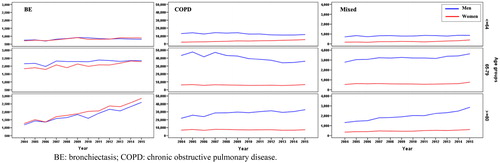
Table 2. Temporal evolution of discharges between 2004 and 2015. Total number of hospitalizations each year, annual rate of admissions per 100,000 inhabitants, and percentage of interannual variation of the rate of hospitalizations for each group are indicated.
Comorbidities
The mean Charlson score was similar for all groups (4.5 vs. 4.4 and 4.4, respectively). A detailed analysis of comorbidities showed that chronic cardiovascular diseases (myocardial infarction, congestive heart failure, and peripheral vascular disease) and tumors predominated in the COPD group and in the male sex, while in the bronchiectasis group connective tissue diseases predominated (, Supplementary material).
Temporal evolution of length of hospital stay and mortality
The mean length of stay was significantly greater in the two groups of patients with bronchiectasis (p < 0.001) (). However, between 2004 and 2015, it was progressively reduced in all groups () and for all age ranges (≤64, 65-79 and ≥ 80) (, Supplementary material).
Figure 3. Evolution of the mean length of stay by groups.
BE: bronchiectasis; COPD: chronic obstructive pulmonary disease.
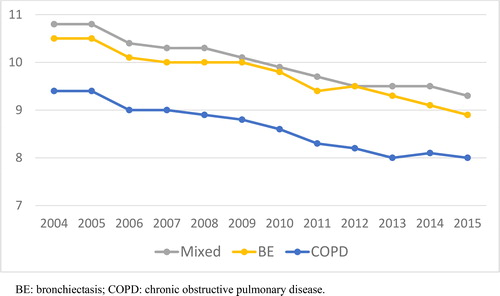
Table 3. Bivariate and multivariate logistic regression analysis for mortality.
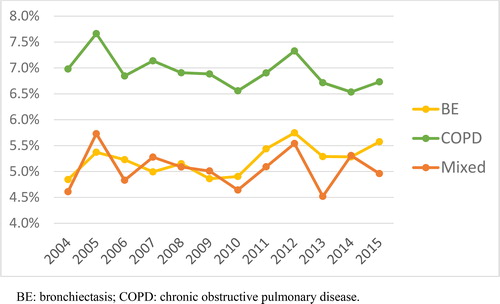
In-hospital mortality was higher in the group with COPD without bronchiectasis than in the two groups in which bronchiectasis was recorded as a discharge code: 6.9% vs. 5.2% in the bronchiectasis group and 5.1% in the mixed group (p < 0.001). During the 11 years assessed, mortality remained relatively stable in the COPD group, but increased significantly in the two groups with bronchiectasis (). Mortality was also higher in older patients () and in men (p < 0.001). Best cutoff values obtained for mortality were age ≥75 years (AUC= 0.759, Sensitivity 87%, and Specificity 65%), and Charlson score ≥3 (AUC= 0.721, Sensitivity 98%, and Specificity 37%).
Figure 4. Annual mortality rate between 2004 and 2015.
BE: bronchiectasis; COPD: chronic obstructive pulmonary disease.
In the multivariate analysis of readmission, age ≥75 years (OR = 1.890; 95% CI = 1.802-1.875), a ≥ 3 score in the Charlson index (OR = 2.121; 95% CI = 2.100-2.183), and chronic domiciliary oxygen treatment (OR = 1.521; 95% CI = 1.499-1.602) were independently related to the possibility that current admission was a readmission following a hospitalization in the previous 30 days. The readmission probabilities combining different variables are shown in (Supplementary material).
Table 4. Bivariate and multivariate analysis for hospital length of stay. Poisson model.
The bivariate and multivariate variables of in-hospital mortality with their respective OR and 95% CI are detailed in . In the multivariate analysis, patients with COPD without bronchiectasis had higher mortality, as did those who were older, those with pneumococcal pneumonia, and those with a greater comorbidity. The multivariate model of length of stay is shown in .
shows the global annual cost of admissions for each group, as well as the inter-annual evolution of costs in the period studied. Similarly to the number of hospitalizations, the increase in expenses was more evident in the two groups with bronchiectasis, with an annual increase of 4.9% in the mixed group and 4.6% in the bronchiectasis group, compared to 1.5% in the COPD group.
Table 5. Cost of hospitalizations: Global annual cost of admissions in each group and interannual cost variation.
Discussion
Our study shows a progressive increase in hospital discharges for bronchiectasis at the national level between 2004 and 2015. Although the number of hospitalizations for COPD continues to be proportionally higher, the increase was lower in percentage terms than that observed in discharges for bronchiectasis. The increment in hospitalizations in both bronchiectasis groups was associated with higher healthcare costs. These results may be related to the increased use of imaging tests, such as HRCT, although the lack of data in our analysis does not allow us to confirm this. It should be taken into account that the procedures considered were those performed during the analyzed admission; therefore, if the patient had prior radiological confirmation, this was not gleaned from the hospitalization under consideration. Neither the cause of the exacerbation nor the severity of the underlying disease was considered since we could not obtain this information from the database. Similar studies, carried out using the same database, have reported the performance of HRCT during admission in 14% of patients with a diagnosis code of bronchiectasis and 1.7% in patients with COPD without bronchiectasis [Citation20].
The mean length of stay was longer in patients with bronchiectasis, probably due to the fact that these patients more frequently require longer treatment with antibiotics, as previously observed [Citation4, Citation21]. In contrast, in-hospital mortality was higher in the COPD group, presumably due to the older age of the patients and greater frequency and severity of comorbidities, especially cardiovascular and neoplasms, compared to the groups with bronchiectasis. Our data are consistent with results of previous smaller studies series conducted with the same database [Citation5, Citation20].
To our knowledge, this is the first study to compare the temporal evolution and characteristics of hospitalizations for bronchiectasis and COPD in a population database over a long period of time. In accordance with previous studies the rate of admission for COPD has decreased slightly in recent years. On the contrary, the number of hospitalizations for bronchiectasis has increased around 2-3% annually [Citation2, Citation5, Citation22]. In the last decade, a high prevalence of bronchiectasis has been evidenced in patients with COPD, especially in advanced stages of the disease, which are associated with an increase in symptoms, exacerbations, and mortality [Citation12, Citation23,Citation24]. However, few series have compared the characteristics of the hospitalizations of patients with COPD and concomitant bronchiectasis. In our study, the hospitalizations of the mixed group had different characteristics from those of the COPD group, with a longer length of stay and an increase of previous hospitalizations in the last 30 days. While in more than 40% of the patients with bronchiectasis the presence of COPD was recorded, in only 6.9% of the patients with COPD the associated code of bronchiectasis was registered. Although the literature reveals a higher presence of bronchiectasis on HRCT in patients hospitalized for COPD, in many cases these are basal cylindrical bronchiectasis, with less clinical impact that perhaps went unnoticed or were not reflected in the codes at discharge, which would explain the low percentage of COPD with an associated diagnosis of bronchiectasis [Citation25–27].
In our cohort, we observed a progressive reduction in the mean hospital length of stay in all groups, reflecting the increasing use of other healthcare resources such as day hospitals, and also outpatient and home follow-up units that facilitate early discharge from hospital [Citation5, Citation28–30]. Despite this, patients with bronchiectasis had an average of one more day of hospitalization.
Hospitalizations that had required admission in the previous 30 days also presented a greater hospital length of stay, which probably reflects a more advanced respiratory disease, with a greater need for chronic home oxygen therapy and a greater comorbidity.
Finally, our study shows a progressive increase in annual healthcare costs, associated with a progressive increase in the number of discharges due to bronchiectasis, higher in patients with COPD and concomitant bronchiectasis [Citation31]. It is possible that our study underestimates these costs since the average cost of each hospitalization for bronchiectasis estimated by the Ministry of Health and Consumption (€3834.5) [Citation32] is lower than that calculated in a previous study (€5284.7) [Citation33].
Regarding limitations of our study, we should highlight the potential bias of underreporting of the main diagnostic codes used to identify hospitalized patients with COPD, bronchiectasis, or both conditions. This limitation is inherent to the methodology, but at least in part was compensated for by the large number of discharges analyzed. Also, to reduce the potential bias of underreported diagnoses and to improve the sensitivity of our search, those cases in which the main disease was a related diagnosis were also included.
Spain is a country with a public health system that allows free medical care for the entire population; therefore, patients present great socioeconomic variety, which improves the external validity of our results and represents an advantage over studies carried out in other countries [Citation14, Citation34–Citation36]. Due to the large number of admissions analyzed, statistical significance by means of the p-value was achieved in practically all comparisons, so we decided to assess the differences that we considered to be clinically relevant. Also, more recent data were not included, due to the change, in place since 2016, of the diagnostic codes to ICD-10.
Information was sought through the diagnostic codes of procedures such as spirometry and thoracic HRCT in the current episode without success. The absence of spirometric data or radiological extension of the bronchiectasis prevented us from directly establishing disease severity, but an approximation could be made from other available data, such as mortality, the use of chronic home oxygen therapy, and the comorbidities. Finally, as mortality in the 30 days following hospital discharge could not be analyzed, the characteristics of hospitalizations that had required prior admission in the previous 30 days could be subject to an immortality bias.
Conclusions
Hospitalizations for bronchiectasis, associated or not with COPD, have increased in recent years, contributing to an increase in overall healthcare spending. Our study reaffirms the relevance of bronchiectasis and the need to develop strategies aimed at improving its diagnosis and treatment, and increasing understanding of its relationship with COPD.
Acknowledgements
We thank Tom Yohannan for his editorial assistance.
Disclosure statement
Annie Navarro has received speaker fees from Boehringer Ingelheim, Chiesi, Zambon, Mundipharma, and AstraZeneca. David de la Rosa has received speaker fees from PARI, Praxis, TEVA, and Zambon, and research grants from Praxis. Cristina Esquinas has received speaker fees from CSL Behring, Bayer, and Grifols. Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols, and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi, and Grifols, and research grants from GlaxoSmithKline and Grifols. Miguel Angel Martinez-Garcia has received speaker fees from Boehringer Ingelheim, Chiesi, Menarini, Rovi, Bial, Zambon, Grifols, TEVA, and Novartis, consulting fees from Chiesi, TEVA, Novartis, and Grifols, and research grants from Vitalaire and TEVA. Pedro Almagro has received speaker fees from Boehringer Ingelheim, Chiesi, Menarini, Rovi, Esteve, and Novartis, consulting fees from Chiesi and GlaxoSmithKline, and research grants from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Chiesi, and Menarini.
References
- Barker AF. Bronchiectasis. N Engl J Med. 2002;346(18):1383–1393. DOI:https://doi.org/10.1056/NEJMra012519
- King PT, Holdsworth SR, Freezer NJ, et al. Outcome in adult bronchiectasis. COPD. 2005;2(1):27–34. DOI:https://doi.org/10.1081/copd-200050685
- Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest. 2010;138(4):944–949. DOI:https://doi.org/10.1378/chest.10-0099
- Ringshausen FC, de Roux A, Pletz MW, et al. Bronchiectasis-associated hospitalizations in Germany, 2005-2011: a population-based study of disease burden and trends. PLoS One. 2013;8(8):e71109. DOI:https://doi.org/10.1371/journal.pone.0071109
- Sánchez-Muñoz G, López de Andrés A, Jiménez-García R, et al. Time trends in hospital admissions for bronchiectasis: analysis of the Spanish national hospital discharge data (2004 to 2013). PLoS One. 2016;11(9):e0162282. DOI:https://doi.org/10.1371/journal.pone.0162282
- Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, et al. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20(1):11–23. DOI:https://doi.org/10.5588/ijtld.15.0472
- Diaz-Guzman E, Mannino DM. Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):7–16. DOI:https://doi.org/10.1016/j.ccm.2013.10.002
- Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. DOI:https://doi.org/10.1183/09031936.06.00124605
- Soriano JB, Alfageme I, Miravitlles M, et al. Prevalence and determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. 2021;57(1):61–69. DOI:https://doi.org/10.1016/j.arbr.2020.07.017
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. DOI:https://doi.org/10.1111/resp.12660
- Dang-Tan T, Ismaila A, Zhang S, et al. Clinical, humanistic, and economic burden of chronic obstructive pulmonary disease (COPD) in Canada: a systematic review. BMC Res Notes. 2015;8:464. DOI:https://doi.org/10.1186/s13104-015-1427-y
- Ni Y, Shi G, Yu Y, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465–1475. DOI:https://doi.org/10.2147/COPD.S83910
- Du Q, Jin J, Liu X, et al. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150532. DOI:https://doi.org/10.1371/journal.pone.0150532
- O’Brien C, Guest PJ, Hill SL, et al. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000; 55(8):635–642.
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis - known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. DOI:https://doi.org/10.1186/s12890-019-0818-6
- Explotación estadística del Conjunto Mínimo Básico de Datos Hospitalarios Nota Metodológica - NORMA ESTATAL [Internet]. [cited 2019 apr 14]. available at http://www.mscbs.gob.es/estadEstudios/estadisticas/docs/NORMAGRD2011/norma_estatal_2011_notas_metod.pdf.
- Instituto Nacional de Estadística (INE). [Internet]. [cited 2019 April 14]. available at http://www.ine.es.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. DOI:https://doi.org/10.1016/0895-4356(92)90133-8
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. DOI:https://doi.org/10.1016/0021-9681(87)90171-8
- Sánchez-Muñoz G, Lopez-de-Andrés A, Hernández-Barrera V, et al. Bronchiectasis in patients hospitalized with acute exacerbation of COPD in Spain: Influence on mortality, hospital stay, and hospital costs (2006-2014) according to gender. PLoS One. 2019;14(1):e0211222. DOI:https://doi.org/10.1371/journal.pone.0211222
- Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J. 2016;47(1):186–193. DOI:https://doi.org/10.1183/13993003.01033-2015
- De la Rosa D, Martínez-Garcia MA, Olveira C, et al. Annual direct medical costs of bronchiectasis treatment: Impact of severity, exacerbations, chronic bronchial colonization and chronic obstructive pulmonary disease coexistence. Chron Respir Dis. 2016;13(4):361–371. DOI:https://doi.org/10.1177/1479972316643698
- Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. DOI:https://doi.org/10.1164/rccm.201208-1518OC
- Gatheral T, Kumar N, Sansom B, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD. 2014;11(6):605–614. DOI:https://doi.org/10.3109/15412555.2014.922174
- Garcia Vidal C, Almagro P, Romani V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbations: a prospective study. Eur Respir J. 2009;34(5):1072–1078. DOI:https://doi.org/10.1183/09031936.00003309
- Chung WS, Lin CL. Acute respiratory events in patients with bronchiectasis-COPD overlap syndrome: A population-based cohort study. Respir Med. 2018;140:6–10. DOI:https://doi.org/10.1016/j.rmed.2018.05.008
- Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–1411. DOI:https://doi.org/10.2147/COPD.S132961
- Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989–998. DOI:https://doi.org/10.1378/chest.14-2146
- Jinjuvadia C, Jinjuvadia R, Mandapakala C, et al. Trends in outcomes, financial burden, and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD) in the United States from 2002 to 2010. COPD. 2017;14(1):72–79. DOI:https://doi.org/10.1080/15412555.2016.1199669
- De Miguel-Díez J, Jiménez-García R, Hernández-Barrera V, et al. Trends in hospital admissions for acute exacerbation of COPD in Spain from 2006 to 2010. Respir Med. 2013;107(5):717–723. DOI:https://doi.org/10.1016/j.rmed.2013.01.007
- Seifer FD, Hansen G, Weycker D. Health-care utilization and expenditures among patients with comorbid bronchiectasis and chronic obstructive pulmonary disease in US clinical practice. Chron Respir Dis. 2019;16:1479973119839961. DOI:https://doi.org/10.1177/1479973119839961
- Portal Estadístico. Área de inteligencia de gestión [internet]. [cited 2019. April 14]. available at https://pestadistico.inteligenciadegestion.mscbs.es/publicoSNS/S.
- De la Rosa Carrillo D, Navarro Rolon A, Girón Moreno RM, et al. Cost of hospitalizations due to exacerbation in patients with non-cystic fibrosis bronchiectasis. Respiration. 2018;96(5):406–416. DOI:https://doi.org/10.1159/000489935
- Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432–439. DOI:https://doi.org/10.1378/chest.11-2209
- Ringshausen FC, de Roux A, Diel R, et al. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J. 2015;46(6):1805–1807. DOI:https://doi.org/10.1183/13993003.00954-2015
- Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12(4):205–209. DOI:https://doi.org/10.1097/01.cpm.0000171422.98696.ed
