Abstract
Chronic obstructive pulmonary disease (COPD) may increase the risk and severity of pertussis infection. Health care resource utilization (HCRU) and direct medical costs (DMC) of treating pertussis among patients with COPD are unknown. Reported incidence of pertussis among individuals aged ≥ 50 years with COPD was assessed in Clinical Practice Research Datalink and Hospital Episode Statistics databases during 2009–2018 using a retrospective cohort design. HCRU and DMC from the National Health Service perspective were compared between patients with COPD and pertussis and propensity score-matched patients with COPD without pertussis. Seventy-eight new pertussis events were identified among 387 086 patients with COPD aged ≥ 50 years (incidence rate: 4.73; 95% confidence interval 3.74–5.91 per 100 000 person-years). HCRU and DMC were assessed among 67 patients with COPD and pertussis and 267 matched controls. During the month before the pertussis diagnosis, the rates of general practitioner (GP)/nurse visits (4289 vs. 1774 per 100 patient-years) and accident and emergency visits (182 vs. 18 per 100 patient-years) were higher in the pertussis cohort; GP/nurse visits (2935 vs. 1705 per 100 patient-years) were also higher during the following 2 months (all p < 0.001). During the month before the pertussis diagnosis, annualized per-patient total DMC were £2012 higher in the pertussis cohort (£3729 vs. £1717; p < 0.001); during the following 2 months, they were £2407 higher (£5498 vs. £3091; p < 0.001). In conclusion, a pertussis episode among individuals with COPD resulted in significant increases in HCRU and DMC around the pertussis event.
Plain language summary
What is the context?
Whooping cough (also known as pertussis) is a highly contagious bacterial respiratory infection. Patients with chronic obstructive pulmonary disease (COPD) are more susceptible to infection with a potential aggravation of the disease. However, the impact of pertussis on patients diagnosed with COPD in England is unknown.
What is new?
In this retrospective study we:
Determined the rate of people with pertussis among patients with COPD aged ≥ 50 years in England from 2009 to 2018.
Compared healthcare costs among patients with COPD with and without pertussis.
We found that:
Among 100,000 patients with COPD, 4.73 of them developed pertussis in a typical year. The rate of new pertussis cases reached its maximum in 2012 and tended to decrease as patients were getting older.
More general practitioner/nurse visits, prescriptions, hospitalizations, accident and emergency visits occurred one month before the diagnosis in patients with COPD and pertussis.
During the 2 months after a pertussis diagnosis, general practitioner/nurse visits occurred more often among patients with COPD and pertussis compared to patients without pertussis.
The infection of patients with COPD and pertussis resulted in significant increase in healthcare costs 1 month before and 2 months after the diagnosis.
What is the impact?
These results quantify the burden and cost linked to pertussis infecting patients diagnosed with COPD. Ultimately, it can be used to evaluate if preventive measures such as vaccination among these patients in England have a positive value/cost balance.
Introduction
Pertussis (whooping cough) is a highly contagious bacterial respiratory infection that is most commonly caused by Bordetella pertussis [Citation1]. Key clinical features of pertussis are paroxysmal cough, inspiratory whooping, post-tussive vomiting, and absence of fever [Citation2]. However, the clinical presentation of pertussis in adults is often atypical, with less than half of adults demonstrating the inspiratory whoop [Citation3]. In adults, paroxysmal cough and absence of fever have high sensitivity but low specificity for pertussis diagnosis, while inspiratory whooping and post-tussive vomiting have high specificity but low sensitivity [Citation2]. In adults, the cough can last for around 12 weeks [Citation4]. Pertussis can also present with other symptoms (e.g. shortness of breath, post-tussive apnea, disturbed sleep, and sore ribs) [Citation4, Citation5] and complications (e.g. sinusitis, pneumonia, urinary incontinence, and rib fractures) [Citation4, Citation6] and can result in hospitalization, particularly among older adults [Citation4, Citation7–10]. Overall, pertussis can have a detrimental impact on quality of life (20–36 quality adjusted life days lost per laboratory confirmed episode) and result in time off work [Citation5]. The morbidity of pertussis in older adults is also reflected in substantial health care resource utilization (HCRU) and direct medical costs (DMC) [Citation11–13].
Although pertussis is often considered to be a childhood illness, 2707/3681 (73.5%) of laboratory-confirmed pertussis cases in England in 2019 were in people aged ≥ 15 years. The incidence in this age group is around 6 per 100 000, although this varies widely by year, from approximately 0 to 18 per 100 000 during 1998–2019 [Citation14]. Provisional data indicate that approximately 32% of all notified pertussis cases in England in 2019 were in people aged ≥ 45 years [Citation15]. Modeling studies based on seroprevalence data from different European countries indicate that the true incidence of pertussis is hundreds to thousands of times higher than notified cases [Citation16–18], showing that pertussis is severely under-recognized and mis-diagnosed. Of note, the recognition of pertussis in chronic obstructive pulmonary disease (COPD) can be complicated by overlapping symptoms (e.g. cough, shortness of breath).
Globally in 2017, COPD was the most prevalent disease-specific chronic respiratory condition, with an estimated prevalence of 3.9% [Citation19]. The prevalence of COPD in England in 2016 was estimated – using an ontological approach – to be 4.6% among adults aged ≥ 35 years [Citation20]. Based on claims data from the United States (US), individuals with COPD appear to be at increased risk of pertussis infection, as the incidence of pertussis was 1.9–3.6-fold higher among adults with COPD than matched adults without COPD or asthma [Citation21].
A recent seroprevalence study in England showed that 12/87 (13.8%) patients with COPD had evidence of Bordetella pertussis exposure, with an estimated 4/87 (4.6%) being exposed in the previous 12 months [Citation22]. Not only are patients with COPD at increased risk of pertussis [Citation21], when they contract the infection, they are more likely to require health care, including pharmaceuticals, outpatient care, and inpatient care than pertussis patients without COPD [Citation21]. Further, among adults hospitalized for pertussis in a study conducted in the US, 18.8% had COPD (26.8% of those aged ≥ 65 years) [Citation23], which is higher than the overall prevalence of COPD in the US (6.1% [11.9% of those aged ≥ 65 years]) [Citation24], suggesting that underlying COPD increases the risk of more severe pertussis clinical expressions [Citation25]. Patients with COPD who suffer pertussis could also experience an exacerbation [Citation26].
No studies have compared the impact of comorbid pertussis – in terms of HCRU and DMC – among patients with COPD. The objectives of the current study were, therefore, to estimate the reported incidence and economic burden of reported pertussis among individuals aged ≥ 50 years with a history of COPD in England between 2009 and 2018; and to compare HCRU and DMC among COPD patients with vs. without pertussis. These results could be used to help evaluate the cost/benefit impact of recommending preventive measures (e.g. pertussis vaccination) among patients with COPD in England.
Materials and methods
Study design
This retrospective, observational study: (1) assessed the reported incidence of pertussis among individuals aged ≥ 50 years with COPD using a retrospective cohort design and (2) evaluated HCRU and DMC among patients with COPD with vs. without pertussis using a propensity score-matched cohort analysis.
Ethics and policies
The study protocol received Clinical Practice Research Datalink (CPRD) ethics approval via the Independent Scientific Advisory Committee on March 4, 2020 (protocol number 20_043).
Data sources
This study used data from the CPRD GOLD and Aurum datasets, which only include primary care data. For completeness, we also used data from the following linked datasets: Hospital Episode Statistics (HES) Admitted Patient Care, HES Outpatient, HES Accident and Emergency (A&E), and Index of Multiple Deprivation (IMD). Further details on these data sources are provided in Supplementary Text SCitation1.
Study dates
Data were available until November 30, 2018, hence patients were identified during January 1, 2009, to August 31, 2018 to allow for ≥ 3 months of follow-up. For patients with pertussis, the index date was defined as the date of pertussis diagnosis; for non-pertussis comparators, the index date was defined as the date of pertussis diagnosis of a pertussis case with the same year of birth, sex and general practitioner (GP) practice area.
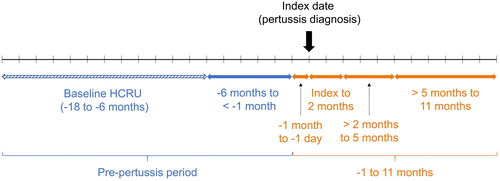
HCRU & DMC were measured at intervals from −18 months to +11 months from index date (). Baseline HCRU and DMC were assessed during −18 to −6 months. A 1-year period was chosen as pertussis and COPD exacerbations vary by season [Citation14, Citation27]. The 1-year follow-up period started at −1 month to capture HCRU and costs during the time between pertussis symptom onset and diagnosis. The buffer zone during months −6 to −1 was used to ensure that no pertussis-related HCRU was included in the HCRU and DMC baseline period. HCRU was also ascertained on a monthly basis throughout the study. The post-index period was defined as the time from the index date to the earliest of the end of the study period, disenrollment from the database (date of transfer out of the database), or death.
Patients and case definitions
The study population included patients aged ≥ 50 years with COPD (≥ 1 of the specific diagnosis codes detailed in Supplementary Tables S1–S3) who were registered with general practices in the CPRD GOLD and Aurum datasets during the study period. Patients had to have no prior history of pertussis (Supplementary Tables S4 and S5). Incident pertussis was identified using the diagnosis codes shown in Supplementary Tables S6–S8.
Table 1. Baseline demographic and clinical characteristics for the post-matched cohort.
For the evaluation of HCRU and DMC, patients were required to have continuous enrollment in CPRD for 18 months prior to the index date, and CPRD GOLD practices were required to have a recorded “up-to-standard” designation prior to 18 months before the index date.
Baseline characteristics
Baseline characteristics after propensity score matching are described: at the index date (age, sex, season, calendar year); or before the index date (smoking status, body mass index [BMI], COPD-specific measures, comorbidities, COPD exacerbations, HCRU, and DMC). COPD-specific measures were: COPD Assessment Test (CAT) scale, modified Medical Research Council (mMRC) dyspnea scale, lung function (forced expiratory volume in the first second [FEV1] % predicted), and number of previous COPD exacerbations during the 18-month baseline period (moderate or severe, as defined by Punekar et al. [Citation28]). These were combined as shown in Supplementary Figure S1 to define COPD severity as significant or non-significant [Citation29, Citation30].
Propensity score matching
For the HCRU analyses, patients with pertussis were propensity-score matched (1:4) to those without pertussis to minimize between-group differences in baseline characteristics. Variables used to calculate the propensity score were: age, sex, environmental/socioeconomic deprivation (IMD), baseline HCRU cost (−18 to −6 months), composite score for COPD severity categorization (Supplementary Figure S1), number of previous severe and moderate COPD exacerbations during the pre-index period, and presence of asthma. More details on the matching steps and the propensity score matching method can be found in Supplementary Text S2.
Endpoints
Three types of endpoints were determined in this study. Firstly, the incidence of pertussis (first record) among individuals aged ≥ 50 years with COPD was estimated during the whole 10-year study period, for each calendar year, and stratified by age group: 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, and ≥ 85 years. For the calculation of pertussis incidence rates, patients contributed to person-time at risk from the latest of: COPD diagnosis date (if that was recorded after the study start date), the study start date, the start date of current registration, the month of their 50th birthday, or the date at which the practice had an “up-to-standard” flag (CPRD GOLD only).
Secondly, HCRU was estimated among patients with COPD aged ≥ 50 years with vs. without pertussis. HCRU included: all-cause GP and nurse consultations (practice visit, home visit, or phone consultation), all-cause GP prescriptions, all-cause outpatient specialist visits, all-cause A&E visits, and all-cause and COPD-related hospitalizations. The latter were defined as hospitalizations with ≥ 1 COPD-related admission (including a primary admission diagnosis for COPD) with ≥ 1 overnight stay in HES, identified using the HES Admitted Patient Care file.
Thirdly, annualized DMC (calculated in 2019 £ accounting for inflation and variation of the unit costs/pricing over time, from the perspective of the National Health Service [NHS]) of HCRU were estimated from resource use identified in the second endpoint, multiplied by relevant unit costs. Unit costs for GP, nurse, and outpatient specialist visits were derived from the 2019 Unit Costs of Health and Social Care published by the Personal Social Services Research Unit (PSSRU) [Citation31]. Costs of the five most commonly prescribed products and clinical assessments were taken into account. Unit costs for the five most commonly prescribed products were derived from the September 2019 NHS Drug Tariff [Citation32], while those for the five most common clinical assessments were derived from the 2018/2019 National Schedule of NHS Reference Costs [Citation33]. A&E costs were also extracted from the 2018/2019 National Schedule of NHS Reference Costs [Citation33]. As costs for inpatient admissions are financed by Healthcare Resource Group (HRG) groups in the United Kingdom (UK), the 2018/2019 HRG4+ Reference Costs Grouper software [Citation34, Citation35] was used to assign a HRG code for each inpatient episode of care. Costs were then derived by matching the assigned HRG code and hospitalization type of each inpatient episode with those in the 2018/2019 National Schedule of Reference Costs [Citation36]. Further costing details can be found in Supplementary Text S3.
Statistical analysis
Descriptive analyses for baseline characteristics of the cohorts were conducted using numbers and proportions for categorical variables and means and standard deviations (SDs) for continuous variables.
The incidence of pertussis among those aged ≥ 50 years with COPD was estimated by dividing the number of incident pertussis cases by the number of person-years at risk. Incidence was estimated for the whole 10-year study period (for all patients and by 5-year age group) and per calendar year (for all patients). Ninety-five percent confidence intervals (95% CIs) of the incidence rates were calculated using the “exact” method by means of the Poisson distribution [Citation37].
GP/nurse visits, prescriptions, outpatient appointments, A&E visits, and hospitalizations (all-cause and COPD-related) are described in terms of annualized rates and number (%) of subjects with each HCRU. The rates and 95% CIs of the HCRU events were estimated using a negative binomial model. For A&E visits during month −1 to the day before the index date, a Poisson model was used as the negative binomial model did not converge.
Wilcoxon rank-sum test was used to compare HCRU between cases and controls. In total, 56 tests were performed, given the different HCRU categories and time periods, or pooled time periods. The threshold for statistical significance was set at p < 0.001, which roughly corresponds to a Bonferroni correction (p < 0.0009), with p < 0.05 (but ≥ 0.001) considered suggestive of a trend. HCRU was also measured on a monthly basis throughout the study period, but no statistical analysis was performed for these outcomes.
DMC results were also compared between cohorts using the Wilcoxon rank-sum test, with the same significance thresholds. Excess DMC during the month before to 11 months after the pertussis diagnosis code were estimated using a generalized linear model (GLM). Total DMC (primary and secondary care) was the dependent variable, with log-years as an offset. Smoking status (current/past/never) was used as an explanatory variable. The model used a log link, and three distributions were tested (normal, gamma, and Tweedie) [Citation38]. The threshold for statistical significance for the GLM result was set at p < 0.05. All statistical programming was performed using SAS software version 9.4.
Results
Incidence rate
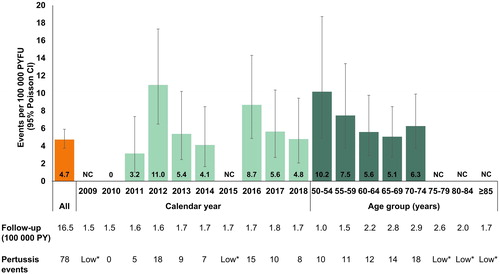
During the whole study period, there were 78 pertussis events among 387 086 patients with COPD aged ≥ 50 years (during 1.65 million person-years of follow-up [PYFU]). The incidence rate of pertussis was 4.73 (95% CI 3.74–5.91) per 100 000 PYFU (). The pertussis incidence rate, which varied by year, was highest in 2012 (10.95 [95% CI 6.49–17.31] per 100 000 PYFU) and remained higher after 2012 compared to before 2012; and incidence rates tended to decrease with increasing age ().
Figure 2. Incidence of pertussis among patients with COPD: overall, by calendar year, and by age group. CI: confidence interval; COPD: chronic obstructive pulmonary disease; CPRD: Clinical Practice Research Datalink; NC: not calculable (*due to low cell counts of 1–4, blinded as per CPRD policy); PYFU: person-years of follow-up.
Health care resource utilization (HCRU)
After matching, there were 67 patients with COPD and pertussis and 267 matched non-pertussis controls. These two cohorts were balanced after matching apart from a higher proportion of current smokers in the non-pertussis cohort ().
During −6 to −1 month, HCRU use per 100 patient-years was similar between cohorts, with a trend for increased number of A&E visits in the pertussis cohort (). During the month before the index date (i.e. before pertussis diagnosis, but likely after symptom onset), GP/nurse visits (4289 vs. 1774 per 100 patient-years; p < 0.001) and A&E visits (182 vs. 18 per 100 patient-years; p < 0.001) were significantly higher in the pertussis cohort, with a trend toward an increase in prescriptions and COPD-related inpatient care ( and ). During the 2 months after the index date, GP/nurse visits (2935 vs. 1705 per 100 patient-years; p < 0.001) were significantly higher in the pertussis cohort, with a trend toward an increase in outpatient visits ( and ). During 2–5 and 5–11 months after the index date, HCRU was similar in both cohorts, apart from a trend for lower all-cause inpatient stays in the pertussis cohort during Months 5–11.
Figure 3. Mean HCRU among patients with COPD from: a -1 month to the day before the index date (pertussis diagnosis) and b the index date to 2 months.
aRates and 95% CIs were obtained by negative binomial models, except for A&E visits for the non-pertussis cohort between -1 month and -1 day, which were obtained assuming a Poisson distribution as the negative binomial model did not converge. p values from unadjusted Wilcoxon rank sum test.
A&E: accident and emergency; CI: confidence interval; COPD: chronic obstructive pulmonary disease; GP: general practitioner; HCRU: health care resource utilization; PY: person years.
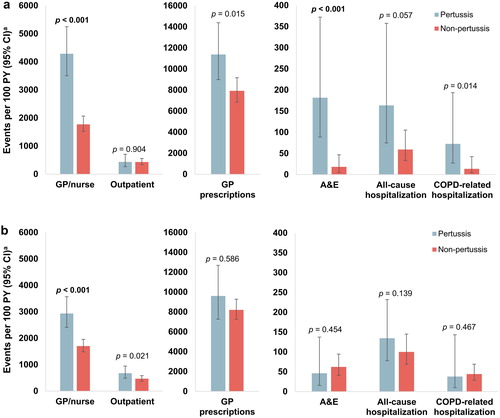
Table 2. HCRU rates per 100 patient-years during several periods from -6 months to +11 months around the index date.
Considerably more patients in the pertussis vs. non-pertussis cohort had ≥ 1 A&E visit during Months −6 to −1 (28.4% vs. 14.6%); ≥ 1 GP/nurse consultation (86.6% vs. 59.2%), ≥ 1 prescription (95.5% vs. 79.8%), or ≥ 1 A&E visit (11.9% vs. 1.5%) during the month before the index date; and ≥ 1 GP/nurse consultation (95.5% vs. 74.2%) or ≥ 1 outpatient appointment (52.2% vs. 36.7%) during the 2 months after the index date ().
Table 3. Number of patients (%) with ≥ 1 of each type of HCRU during several periods from −6 months to +11 months around the index date.
Descriptive HCRU analysis
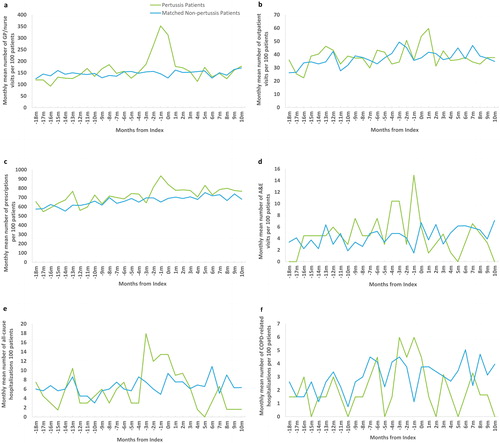
Monthly HCRU data are presented in . During the 3 months before to 1 month after the index date, pertussis patients averaged 11.2 GP/nurse consultations, compared to 5.8 in the non-pertussis cohort (). Outpatient visits generally ranged from around 30 to 50 per 100 patients per month, but peaked at 54 and 60 per 100 patients during the first and second month after the index date, respectively, in the pertussis cohort (). Patients in the non-pertussis cohort received around 600–700 prescriptions per 100 patients per month, but those in the pertussis cohort received 934 and 840 per 100 patients per month in the months before and after the index date, respectively (). There were about 2–6 A&E visits per 100 patients per month in the non-pertussis cohort, but 15 per 100 patients in the month before the index date in the pertussis cohort (). All-cause hospitalizations ranged from 3 to 11 per 100 patients per month in the non-pertussis cohort but peaked at 18 per 100 patients in Month −3 and remained elevated through to the month after the index date (). COPD-related hospitalizations were slightly higher in the pertussis cohort around the time of the index date (). Additional post-hoc analyses on the discharge diagnosis of the hospitalizations, indicated that during the −1 month and index period, a predominance of diagnosis codes for respiratory conditions was observed in the pertussis cohort whereas these codes were not present in the non-pertussis cohort (Supplementary Tables S9 and S10).
Figure 4. Monthly mean HCRUa among patients with COPD and pertussis or COPD alone from 18 months before to 11 months after the index date (pertussis diagnosis) for the following resource utilizations: a GP or nurse visits; b outpatient visits; c GP prescriptions; d A&E visits; e all-cause hospitalizations; and f COPD-related hospitalizations.
aMonths are labeled according to the start time of each interval, e.g., utilization reported at Month 0 is the average from index day (Day 0) to Day 30.
A&E: accident and emergency; COPD: chronic obstructive pulmonary disease: GP: general practitioner; HCRU: health care resource utilization.
Direct medical costs (DMC)
DMC were comparable in the pertussis and non-pertussis cohorts during Months −6 to −1 ( and Supplementary Table S11). During the month before the index date, total annualized per patient DMC were £2012 higher in the pertussis cohort (£3729 vs. £1717; p < 0.001), mainly due to a £913 increase in GP/nurse visits (£1491 vs. £578; p < 0.001) and an £800 increase in inpatient care (£1204 vs. £404; p = 0.073) ( and Supplementary Table S11). There was also a significant increase in the annualized per patient costs of A&E visits (£269 vs. £27; p < 0.001). During the 2 months after the index date, total annualized per patient DMC were £2407 higher in the pertussis cohort (£5498 vs. £3091; p < 0.001), mainly due to a £444 increase in GP/nurse visit costs (£1003 vs. £559; p < 0.001) and a £1683 increase in inpatient care (£3454 vs. £1771; p = 0.105) ( and Supplementary Table S11). Annualized per patient outpatient costs were also increased (£928 vs. £643; p = 0.021). Annualized DMC beyond 2 months after the index date were comparable between the two cohorts ( and Supplementary Table S11).
Figure 5. Annualized per-patient DMCa during the various time periods of the study.
aValues above bars show total mean ± 95% CI annualized DMC per patient including the most common five prescriptions and the most common five clinical assessments (which were added to the cost assessment in order to give a more precise estimate of the total DMC). 95% CI were obtained assuming Student’s t distribution for the annualized DMC means. GP, nurse, and outpatient specialist costs were derived from the 2019 Unit Costs of Health and Social Care published by the Personal Social Services Research Unit [Citation31]. Unit costs for the five most commonly prescribed products were derived from the September 2019 NHS Drug Tariff [Citation32]. The unit costs for the five most common clinical assessments and A&E costs were extracted from the 2018/2019 National Schedule of NHS Reference Costs [Citation33]. Inpatient hospitalization costs were derived using reference costs from the 2018/2019 National Schedule of Reference Costs [Citation36].
A&E: accident and emergency; DMC: direct medical costs; CI: confidence interval; GP: general practitioner; NHS: National Health Service.
![Figure 5. Annualized per-patient DMCa during the various time periods of the study.aValues above bars show total mean ± 95% CI annualized DMC per patient including the most common five prescriptions and the most common five clinical assessments (which were added to the cost assessment in order to give a more precise estimate of the total DMC). 95% CI were obtained assuming Student’s t distribution for the annualized DMC means. GP, nurse, and outpatient specialist costs were derived from the 2019 Unit Costs of Health and Social Care published by the Personal Social Services Research Unit [Citation31]. Unit costs for the five most commonly prescribed products were derived from the September 2019 NHS Drug Tariff [Citation32]. The unit costs for the five most common clinical assessments and A&E costs were extracted from the 2018/2019 National Schedule of NHS Reference Costs [Citation33]. Inpatient hospitalization costs were derived using reference costs from the 2018/2019 National Schedule of Reference Costs [Citation36].A&E: accident and emergency; DMC: direct medical costs; CI: confidence interval; GP: general practitioner; NHS: National Health Service.](/cms/asset/5c11445b-bb35-4d70-b09f-21194306093b/icop_a_1899155_f0005_c.jpg)
Generalized linear model
The Tweedie model was selected among the tested distributions as it was the only model to converge due to the skewness of the data and existence of zero values [Citation38]. The total modeled annualized DMC per patient with vs. without pertussis during the 1 month before to 11 months after pertussis were £3487 vs. £2839 (p = 0.175), equating to an adjusted DMC increase of £837 (95% CI −128 to 2187; 32.9% higher; p = 0.097) for the pertussis cohort using a GLM.
Discussion
Using merged CPRD Aurum and GOLD data from 2009–2018, we estimated the incidence of reported pertussis among individuals aged ≥ 50 years with COPD to be 4.73 events per 100 000 PYFU. Pertussis incidence varied by year from 0 to 10.95 per 100 000 PYFU, with peaks in 2012 and 2016, which is in line with the numbers of laboratory-confirmed cases from Public Health England [Citation15]. The estimated incidence of pertussis in the current study among people aged ≥ 50 years with COPD was similar to that of a general cohort of people aged ≥ 50 years in the same study period, as reported by Aris et al. (5.76 events per 100 000 PYFU) [Citation39]. Similar results were observed for ≥ 65 years without COPD or asthma cohort in a US claims data study (2006–2014) by Buck et al. [Citation21]. However, in this study, the incidence of pertussis was estimated to be higher among those with COPD than in those without COPD or asthma (age 19–64 years: 20.6 vs. 5.7 per 100 000 person-years; age ≥ 65 years: 11.4 vs. 6.1 per 100 000 person-years).
In 2013, the estimated incidence of diagnosed pertussis among patients with COPD in the current study was 5.38 per 100 000 PYFU. This is much lower than was found in an English seroprevalence study that used sera from people with COPD aged 40–85 years in the same year, in which 4/87 patients (4.6%) showed recent exposure to Bordetella pertussis [Citation22]. While comparisons between studies should be undertaken with caution, and only 87 patients were sampled, this implies that the actual incidence rate of pertussis infection could have been several hundred-fold higher than the reported incidence rates estimated in the current study. Similar findings have been reported in various studies, as recently reviewed by Kandeil et al. [Citation40], clearly showing that most pertussis cases among adults (with or without COPD) may be misdiagnosed or not reported.
Patients with pertussis in the current study sought more HCRU in the month before a pertussis diagnosis than matched patients without pertussis. This included more GP/nurse visits (3.5 vs. 1.5 per patient; p < 0.001), prescriptions (9.3 vs. 6.5 per patient; p = 0.015), A&E visits (14.9 vs. 1.5 per 100 patients; p < 0.001), and COPD-related hospitalizations (6.0 vs. 1.1 per 100 patients; p = 0.014), the last of which is indicative of a severe exacerbation. During the 2 months after diagnosis, HCRU continued to be elevated, including GP/nurse visits (4.9 vs. 2.9 per patient; p < 0.001) and outpatient appointments (1.1 vs. 0.8 per patient; p = 0.021), which indicates additional medical follow-up of patients with pertussis.
During the 1 month before to the 5 months after the index date, GP/nurse visits were observed to be 1.5-fold higher (2674 vs. 1803 per 100 patient-years; p < 0.001) and A&E visits were 1.3-fold higher, although not significantly (71 vs. 54 per 100 patient-years; p = 0.409) in the pertussis vs. matched non-pertussis cohorts (data not shown). In a study by Leong et al. [Citation12] that was conducted in a general population of Australian adults aged ≥ 45 years, GP visits were 1.9-fold higher (1169 vs. 611 events per 100 patient-years) and emergency department visits were 2.2-fold higher (54 vs. 24 events per 100 patient-years) during a similar time interval (30 days before to 120 days after a pertussis diagnosis) among 524 confirmed pertussis cases vs. 524 matched controls without pertussis [Citation12]. Both studies show that diagnosed adult pertussis has an HCRU burden, whether in patients with COPD (current study) or generally in older adults [Citation12].
In the current study, the mean total annual DMC of a patient with COPD without pertussis was £2839. This is similar to the mean annual management cost of treating a patient with COPD in an older UK study after allowing for inflation (£2108 in 2010–2011) [Citation28]. Although the difference was not statistically significant, probably due to the small sample size, patients with COPD and pertussis in the current study had an excess annual DMC of £648 (£837 after GLM adjustment) compared to those with COPD without pertussis. Of note, the cost of treating a case of pertussis infection among patients with COPD was higher than the cost of treating a case of acute respiratory illness during influenza seasons in 2001–2009 in another CPRD study in the UK (£116 for those aged 50–64 years; £478 for those aged ≥ 65 years) [Citation41].
Patients with COPD are more susceptible to respiratory tract infections [Citation42], and such infections can result in COPD exacerbations and disease progression [Citation43]. In the US, patients with lung diseases (including COPD) are recommended, by the Centers for Disease Control and Prevention (CDC), to receive influenza, pneumococcal, zoster, and tetanus, diphtheria, and acellular pertussis (Tdap) vaccinations [Citation44]. In fact, all adults in the US are recommended to receive a dose of Tdap if they have never received one, plus a tetanus and diphtheria (Td) or Tdap booster every 10 years [Citation45]. Uptake of Td was estimated to be 63.4% in the 10 years up to 2017, but uptake of Tdap was considerably lower, with 31.7% of adults in 2017 having received Tdap in the previous 10 years in the US [Citation46]. Similarly, in a survey of 187 patients with COPD in Belgium, while 64.7% had received influenza vaccination and 40.1%, pneumococcus, only 12.3% had received pertussis vaccination [Citation47].
In the UK, patients with COPD are only eligible for free influenza [Citation48] and pneumococcal [Citation49] vaccinations, but not pertussis vaccinations [Citation50]. However, vaccinating adults with COPD against pertussis could help to reduce morbidity and increases in pertussis-related HCRU and DMC, including those related to exacerbations [Citation26, Citation40]. General vaccination of adults against pertussis can also help to prevent its transmission to susceptible infants before they have received their primary vaccinations [Citation25, Citation40]. Indeed, the Global Initiative for Chronic Obstructive Lung Disease (GOLD), that previously recommended influenza and pneumococcal vaccines for people with COPD, recently leveraged the CDC recommendation for Tdap vaccination for COPD patients to the 2021 guideline [Citation27]. Although there are no specific studies of Tdap efficacy among patients with COPD, pertussis vaccines have been shown to be immunogenic [Citation51, Citation52] and effective [Citation53] in older adults.
The results from this study can be used to inform inputs for a cost-effectiveness analysis of Tdap vaccination among patients with COPD in England. Nine previous studies that estimated the cost-effectiveness of Tdap among US healthy adults have recently been reviewed by Leidner et al. [Citation54]. These studies included heterogeneous vaccination strategies and only three of them were specifically assessing the prevention of pertussis infections in the adult population [Citation55–57]. Results showed that the efficiency profile was dependent on the pertussis incidence rate in the population [Citation56, Citation57]. The only adult Tdap cost-effectiveness study from England examined maternal Tdap vaccination [Citation58]. They concluded that it was effective, but that cost-effectiveness depended on future incidence [Citation58]. Other European studies have concluded that adult Tdap vaccination is cost-effective in Germany [Citation59] and is “likely to be considered as cost-effective” in the Netherlands [Citation60].
Strengths and limitations
This is the first study to estimate the incremental DMC of a reported pertussis case among adults with COPD in England. This incremental approach allowed us to estimate the additional DMC of a pertussis episode among people who already require considerable HCRU. Other strengths of the current study include the use of a large observational dataset that is generalizable to the overall population with COPD of England, with demographic and longitudinal clinical information recorded from both the primary and secondary care settings.
However, individuals who did not obtain health care support for their symptoms or those in the control group with pertussis that was not reported or misdiagnosed, were therefore not assigned as pertussis cases, hence the true pertussis incidence may be considerably higher. On the other hand, pertussis events were derived from medical diagnoses only and no laboratory confirmations were requested because of incomplete recording of such data in the database.
The CPRD consists of information collected for clinical and routine use rather than for research purposes. Although extensive practice-level quality control checks are conducted, the validity and completeness of individual patient records could not be assessed. Inpatient and outpatient care can only be identified using a linkage to the HES database. Also, the CPRD contains data on drug prescriptions, but not use, and, for practical purpose, only the top five prescription medications were included (as unit costs for individual medications need to be identified by generic name, strength and formulation and prescription costs account for only a small percentage of overall DMC). Deprivation measures were included as baseline covariates, but the IMD is estimated based on average material deprivation by residence postcode rather than individual deprivation or socioeconomic status.
This analysis of patients with COPD with and without pertussis was limited in its ability to detect statistically significant differences between cohorts due to the relatively small number of patients identified with COPD and pertussis and the relative rarity of some HCRU endpoints (e.g. inpatient admissions) during time intervals of interest. Further, DMC were estimated based on unit costs, so may not reflect true DMC. We also only assessed DMC, so did not account for other costs, such as productivity losses, caregiver burden, etc.
Despite these limitations, this study provides valuable information on real-world HCRU and DMC of treating patients with COPD and diagnosed pertussis compared to a matched cohort of patients with COPD only.
Conclusions
In this study, the overall incidence rate of reported pertussis among adults with COPD was nearly 5 per 100 000 PYFU, although the true incidence of pertussis infection is likely much higher. Reported pertussis in adults with COPD can result in significant additional health care being sought, and significantly increased DMC, especially around the time of the pertussis diagnosis. Although the greatest DMC increase for patients with COPD and pertussis vs. COPD alone was within the 2 months after a pertussis diagnosis, there was also a significant increase in the month before diagnosis, likely due to health care related to pertussis symptoms before an actual diagnosis. These results could be used to help evaluate the cost-effectiveness of recommending pertussis vaccination for patients with COPD.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors were involved in the conception and/or design of the study; Emmanuel Aris, Lauriane Harrington, Amit Bhavsar, Jason C. Simeone, Anna Ramond, Kinga Meszaros, Dimitra Lambrelli, and Piyali Mukherjee acquired and analyzed the data; all authors drafted the manuscript and were members of the writing committee.
Prior presentation
ERS 2020. Aris E, Akpo EI, Bhavsar A, et al. Burden of pertussis in COPD: a retrospective database study in England [abstract]. Eur Respir J. 2020;56:2468.
ERS 2020. Aris E, Akpo EI, Bhavsar A, et al. The burden of pertussis in adults with asthma: a retrospective database study in England [abstract]. Eur Respir J. 2020;56:4926.
Supplemental Material
Download PDF (560.3 KB)Acknowledgements
The authors are grateful to Robert Donaldson and Evie Merinopoulou (Evidera Inc., London) for their contribution to the study as well as Esse Ifebi Herve Akpo, Yves Brabant and Elisa Turriani (GSK, Belgium) for their support during the early phases of the study. The authors would like to thank Business & Decision Life Sciences platform for editorial assistance, writing support and manuscript coordination, on behalf of GSK. Gauhar Masgutova (Business & Decision Life Sciences, on behalf of GSK) coordinated manuscript development and editorial support. Jenny Lloyd (Compass Medical Communications Ltd., on behalf of GSK) provided medical writing support.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available as CPRD data are accessed via a license, the terms of which do not allow for the sharing of raw data.
Disclaimer This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone.
Declaration of interest
Emmanuel Aris, Lauriane Harrington, Amit Bhavsar, Kinga Meszaros, and Piyali Mukherjee are employed by the GSK group of companies. Emmanuel Aris, Kinga Meszaros, and Piyali Mukherjee hold shares in the GSK group of companies. Jason C. Simeone, Anna Ramond, and Dimitra Lambrelli are employed by Evidera Inc., which received funding from the GSK group of companies to complete the work disclosed in this manuscript. Alberto Papi is a board member of the GSK group of companies, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Mundipharma, Novartis, Roche, Sanofi/Regeneron, TEVA and Zambon; he also declares having received consulting and lecturing fees from the GSK group of companies, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Mundipharma, Novartis, Sanofi/Regeneron, TEVA and Zambon; and consulting fees from Edmondparma and Roche; and lecturing fees from Menarini; and grant support for vaccine studies from the GSK group of companies, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Fondazione Chiesi, Fondazione Maugeri, Menarini and TEVA; and travel expenses reimbursement from the GSK group of companies, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Menarini, Mundipharma, Novartis, Roche, Sanofi/Regeneron, TEVA and Zambon. Claus F. Vogelmeier reports grants and personal fees from the GSK group of companies, AstraZeneca, Boehringer Ingelheim, Grifols and Novartis, and personal fees from Chiesi, CSL Behring, MedUpdate, Menarini and Nuvaira.
Additional information
Funding
References
- Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr. 2016;4(3): 1–21 DOI: https://doi.org/10.1128/microbiolspec.EI10-0008-2015
- Moore A, Harnden A, Grant CC, et al. Clinically diagnosing pertussis-associated cough in adults and children: CHEST guideline and expert panel report. Chest. 2019;155(1):147–154. DOI:https://doi.org/10.1016/j.chest.2018.09.027
- Lee GM, Lett S, Schauer S, Massachusetts Pertussis Study Group, et al. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis. 2004;39(11):1572–1580. DOI:https://doi.org/10.1086/425006
- De Serres G, Shadmani R, Duval B, et al. Morbidity of pertussis in adolescents and adults. J Infect Dis. 2000;182(1):174–179. DOI:https://doi.org/10.1086/315648
- van Hoek AJ, Campbell H, Andrews N, et al. The burden of disease and health care use among pertussis cases in school aged children and adults in England and Wales; a patient survey. PLoS One. 2014;9(11):e111807 DOI:https://doi.org/10.1371/journal.pone.0111807
- Zambrano JA, Herman TN. Rib fracture associated with Bordetella pertussis infection. N Engl J Med. 2018;378(3):e4. DOI:https://doi.org/10.1056/NEJMicm1701940
- Clarke MF, Rasiah K, Copland J, et al. The pertussis epidemic: informing strategies for prevention of severe disease. Epidemiol Infect. 2013;141(3):463–471. DOI:https://doi.org/10.1017/S095026881200091X
- Liu BC, McIntyre P, Kaldor JM, et al. Pertussis in older adults: prospective study of risk factors and morbidity. Clin Infect Dis. 2012;55(11):1450–1456. DOI:https://doi.org/10.1093/cid/cis627
- Skoff TH, Hadler S, Hariri S. The epidemiology of nationally reported pertussis in the United States, 2000-2016. Clin Infect Dis. 2019;68(10):1634–1640. DOI:https://doi.org/10.1093/cid/ciy757
- Karki S, McIntyre P, Newall AT, et al. Risk factors for pertussis hospitalizations in Australians aged 45 years and over: a population based nested case-control study. Vaccine. 2015;33(42):5647–5653. DOI:https://doi.org/10.1016/j.vaccine.2015.08.068
- McGuiness CB, Hill J, Fonseca E, et al. The disease burden of pertussis in adults 50 years old and older in the United States: a retrospective study. BMC Infect Dis. 2013; 13:32 DOI:https://doi.org/10.1186/1471-2334-13-32
- Leong RNF, Wood JG, Liu B, et al. High healthcare resource utilisation due to pertussis in Australian adults aged 65 years and over. Vaccine. 2020;38(19):3553–3559. DOI:https://doi.org/10.1016/j.vaccine.2020.03.021
- McGirr A, Fisman DN, Tuite AR. The health and economic burden of pertussis in Canada: a microsimulation study. Vaccine. 2019;37(49):7240–7247. DOI:https://doi.org/10.1016/j.vaccine.2019.09.070
- Public Health England [Internet]. Laboratory confirmed cases of pertussis in England: annual report for 2019 [cited 2020. Oct 21]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881380/hpr0820_PRTSSS_annual.pdf.
- Public Health England [Internet]. Laboratory confirmed cases of pertussis in England. Annual report for 2019 supplementary data tables [cited 2020 Nov 20]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881410/Laboratory_confirmed_cases_of_pertussis_in_England_2019.pdf.
- Rønn PF, Dalby T, Simonsen J, et al. Seroepidemiology of pertussis in a cross-sectional study of an adult general population in Denmark. Epidemiol Infect. 2014;142(4):729–737. DOI:https://doi.org/10.1017/S0950268813002446
- Jõgi P, Oona M, Toompere K, et al. Estimated and reported incidence of pertussis in Estonian adults: A seroepidemiological study. Vaccine. 2015;33(38):4756–4761. DOI:https://doi.org/10.1016/j.vaccine.2015.08.007
- de Melker HE, Versteegh FG, Schellekens JF, et al. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J Infect. 2006;53(2):106–113. DOI:https://doi.org/10.1016/j.jinf.2005.10.020
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596.
- Rayner L, Sherlock J, Creagh-Brown B, et al. The prevalence of COPD in England: An ontological approach to case detection in primary care. Respir Med. 2017; 132:217–225. DOI:https://doi.org/10.1016/j.rmed.2017.10.024
- Buck PO, Meyers JL, Gordon LD, et al. Economic burden of diagnosed pertussis among individuals with asthma or chronic obstructive pulmonary disease in the USA: an analysis of administrative claims. Epidemiol Infect. 2017;145(10):2109–2121. DOI:https://doi.org/10.1017/S0950268817000887
- Mukherjee P, Cheuvart B, Baudson N, et al. Seroprevalence of Bordetella pertussis in chronic obstructive pulmonary disease (COPD) patients [abstract]. Eur Respir J. 2020;56(suppl. 64):4927. DOI: https://doi.org/10.1183/13993003.congress-2020.4927.
- Mbayei SA, Faulkner A, Miner C, et al. Severe Pertussis Infections in the United States, 2011–2015. Clin Infect Dis. 2019;69(2):218–226. DOI:https://doi.org/10.1093/cid/ciy889
- Ward BW, Nugent CN, Blumberg SJ, et al. Measuring the prevalence of diagnosed chronic obstructive pulmonary disease in the United States using data from the 2012–2014 National Health Interview Survey. Public Health Rep. 2017;132(2):149–156. DOI:https://doi.org/10.1177/0033354916688197
- Jenkins VA, Savic M, Kandeil W. Pertussis in high-risk groups: an overview of the past quarter-century. Hum Vaccin Immunother. 2020;16(11):2609–2617. DOI: https://doi.org/10.1080/21645515.2020.1738168.
- Blasi F, Bonanni P, Braido F, et al. The unmet need for pertussis prevention in patients with chronic obstructive pulmonary disease in the Italian context. Hum Vaccin Immunother. 2020;16(2):340–348. DOI:https://doi.org/10.1080/21645515.2019.1652517
- Global Initiative for Chronic Obstructive Lung Disease [Internet]. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonar disease [cited 2020 Nov 20]. Available from: https://goldcopd.org/2021-gold-reports/.
- Punekar YS, Shukla A, Mullerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis. 2014; 9:65–73. DOI:https://doi.org/10.2147/COPD.S54417
- Ghobadi H, Ahari SS, Kameli A, et al. The relationship between COPD assessment test (CAT) scores and severity of airflow obstruction in stable COPD patients. Tanaffos. 2012;11(2):22–26.
- Rebordosa C, Plana E, Aguado J, et al. GOLD assessment of COPD severity in the Clinical Practice Research Datalink (CPRD). Pharmacoepidemiol Drug Saf. 2019;28(2):126–133. DOI:https://doi.org/10.1002/pds.4448
- Curtis L, Burns A. [Internet]. Unit costs of health and social care 2019 [cited 2020 Dec 18]. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/.
- National Health Service (NHS) [Internet]. Amendments to the drug tariff. September 2019 [cited 2020 Mar 26]. Available from: https://www.nhsbsa.nhs.uk/sites/default/files/2019-08/Drug%20Tariff%20September%202019.pdf.
- National Health Service (NHS) [Internet]. National cost collection for the NHS [cited 2020 Mar 26]. Available from: https://www.england.nhs.uk/national-cost-collection/#ncc1819.
- Barker J. Interactive complexity and comorbidity splits in Health Resource Group 4+. Brit J Healthcare Manage. 2015;21(9):433–439. DOI:https://doi.org/10.12968/bjhc.2015.21.9.433
- National Health Service (NHS) [Internet]. Costing grouper [cited 2020 Mar 26]. Available from: https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/costing–-hrg4-2018-19-reference-costs-grouper.
- Weir S, Samnaliev M, Kuo TC, et al. The incidence and healthcare costs of persistent postoperative pain following lumbar spine surgery in the UK: a cohort study using the Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES). BMJ Open. 2017;7(9):e017585 DOI:https://doi.org/10.1136/bmjopen-2017-017585
- Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol. 1990;131(2):373–375. DOI:https://doi.org/10.1093/oxfordjournals.aje.a115507
- Kurz CF. Tweedie distributions for fitting semicontinuous health care utilization cost data. BMC Med Res Methodol. 2017;17(1):171–178. DOI:https://doi.org/10.1186/s12874-017-0445-y
- Aris E, Akpo EI, Bhavsar A, et al. Burden of pertussis in COPD: a retrospective database study in England [abstract]. Eur Respir J. 2020;56 (suppl.64):2468. DOI:https://doi.org/10.1183/13993003.congress-2020.2468.
- Kandeil W, Atanasov P, Avramioti D, et al. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines. 2019;18(5):439–455. DOI:https://doi.org/10.1080/14760584.2019.1588727
- Meier GC, Watkins J, McEwan P, et al. Resource use and direct medical costs of acute respiratory illness in the UK based on linked primary and secondary care records from 2001 to 2009. PLoS One. 2020;15(8):e0236472 DOI:https://doi.org/10.1371/journal.pone.0236472
- Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–742. DOI:https://doi.org/10.1164/rccm.201006-0833OC
- Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35(6):1209–1215. DOI:https://doi.org/10.1183/09031936.00081409
- Centers for Disease Control and Prevention (CDC) [Internet]. Lung disease including asthma and adult vaccination [cited 2020 Nov 5]. Available from: https://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/lung-disease.html.
- Centers for Disease Control and Prevention (CDC) [Internet]. Adult immunization schedule [cited 2020 Aug 19]. Available from: https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
- Centers for Disease Control and Prevention (CDC) [Internet]. Vaccination coverage among adults in the United States, National Health Interview Survey, 2017. [cited 2020 Nov 5]. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2017.html.
- Boey L, Bosmans E, Ferreira LB, et al. Vaccination coverage of recommended vaccines and determinants of vaccination in at-risk groups. Hum Vaccin Immunother. 2020;16(9):2136–2143. DOI:https://doi.org/10.1080/21645515.2020.1763739
- National Health Service (NHS) [Internet]. Flu vaccine [cited 2020 Nov 5]. Available from: https://www.nhs.uk/conditions/vaccinations/flu-influenza-vaccine/.
- National Health Service (NHS) [Internet]. Who should have the pneumococcal vaccine? [cited 2020 Nov 5]. Available from: https://www.nhs.uk/conditions/vaccinations/when-is-pneumococcal-vaccine-needed/.
- National Health Service (NHS) [Internet]. Whooping cough [cited 2021 Jan 21]. Available from: https://www.nhs.uk/conditions/whooping-cough/.
- Van Damme P, McIntyre P, Grimprel E, et al. Immunogenicity of the reduced-antigen-content dTpa vaccine (Boostrix®) in adults 55 years of age and over: a sub-analysis of four trials. Vaccine. 2011;29(35):5932–5939. DOI:https://doi.org/10.1016/j.vaccine.2011.06.049
- Weston WM, Friedland LR, Wu X, et al. Vaccination of adults 65 years of age and older with tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Boostrix(®)): results of two randomized trials. Vaccine. 2012;30(9):1721–1728. DOI:https://doi.org/10.1016/j.vaccine.2011.12.055
- Liu BC, He WQ, Newall AT, et al. Effectiveness of acellular pertussis vaccine in older adults: Nested matched case-control study. Clin Infect Dis. 2020;71(2):340–350. DOI:https://doi.org/10.1093/cid/ciz821
- Leidner AJ, Murthy N, Chesson HW, et al. Cost-effectiveness of adult vaccinations: a systematic review. Vaccine. 2019;37(2):226–234. DOI:https://doi.org/10.1016/j.vaccine.2018.11.056
- Kamiya H, Cho BH, Messonnier ML, et al. Impact and cost-effectiveness of a second tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine dose to prevent pertussis in the United States. Vaccine. 2016;34(15):1832–1838. DOI:https://doi.org/10.1016/j.vaccine.2016.02.027
- Lee GM, Murphy TV, Lett S, et al. Cost effectiveness of pertussis vaccination in adults. Am J Prev Med. 2007;32(3):186–193. DOI:https://doi.org/10.1016/j.amepre.2006.10.016
- McGarry LJ, Krishnarajah G, Hill G, et al. Cost-effectiveness analysis of Tdap in the prevention of pertussis in the elderly. PLoS One. 2013;8(9):e67260 DOI:https://doi.org/10.1371/journal.pone.0067260
- van Hoek AJ, Campbell H, Amirthalingam G, et al. Cost-effectiveness and programmatic benefits of maternal vaccination against pertussis in England. J Infect. 2016;73(1):28–37. DOI:https://doi.org/10.1016/j.jinf.2016.04.012
- Lee GM, Riffelmann M, Wirsing von Konig CH. Cost-effectiveness of adult pertussis vaccination in Germany. Vaccine. 2008;26(29-30):3673–3679. DOI:https://doi.org/10.1016/j.vaccine.2008.04.068
- National Health Service [Internet]. 2017/18 Reference Costs [cited 26 Mar 2020]. Available from: https://improvement.nhs.uk/resources/reference-costs/
- National Health Service [Internet]. 2017/18 Reference Costs [cited 26 Mar 2020]. Available from: https://improvement.nhs.uk/resources/reference-costs/.