Abstract
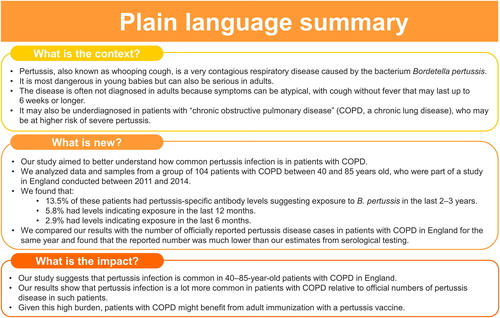
Pertussis is underdiagnosed and underreported in adults and patients with underlying conditions. Patients with chronic obstructive pulmonary disease (COPD) may be at increased risk of severe pertussis. Understanding the true prevalence of pertussis infections in such patients is important. We therefore evaluated the seroprevalence of anti-pertussis toxin (PT) antibodies in a cohort of 40–85-year-old patients diagnosed with moderate, severe or very severe COPD enrolled (between June 2011 and June 2012) in the prospective, observational “Acute Exacerbation and Respiratory InfectionS in COPD” (AERIS; NCT01360398) study, conducted in England. Serum anti-PT antibodies were measured in 104 patients using an enzyme-linked immunosorbent assay on samples collected 12 months (M12) and 24 months (M24) after enrollment. Overall, 14/104 (13.5%) patients had anti-PT concentrations ≥50 IU/mL at M12 or M24, indicative of exposure to Bordetella pertussis during the preceding 2–3 years. Of these, 6/104 (5.8%) had anti-PT ≥70 IU/mL, of whom 3/104 (2.9%) had anti-PT ≥120 IU/mL, indicative of exposure within 12 and 6 months, respectively. These results show a high circulation of B. pertussis in 40–85-year-old patients with moderate, severe or very severe COPD in England between 2012 and 2014, and call for enhanced immunization to prevent pertussis infections in such patients.
Introduction
Pertussis, or whooping cough, is a highly contagious respiratory disease caused by Bordetella pertussis. It continues to have a substantial disease burden, even after the successful implementation of effective pediatric immunization programs [Citation1–4]. While pertussis is most severe in infants, it can also be serious and lead to complications in older adults and in adults with chronic illnesses, such as asthma or chronic obstructive pulmonary disease (COPD) [Citation1,Citation5]. However, symptoms in adults are often atypical (and may overlap with those of chronic respiratory diseases): they include persistent paroxysmal coughing without fever with an average duration of 6 weeks and often lack the characteristic inspiratory whooping [Citation6,Citation7]. This leads to mis- and underdiagnosis of pertussis and an underestimation of the burden of disease in adults [Citation1,Citation4].
COPD is a progressive inflammatory lung disease that affected an estimated 174 million people worldwide in 2015 [Citation8,Citation9]. Respiratory bacterial and viral infections are known to be important triggers of acute exacerbation of COPD (AECOPD) [Citation10–12], which is characterized by a sudden worsening of respiratory symptoms and negatively affects lung function, quality of life, mortality and health care costs [Citation13–19]. Some studies have suggested an increased risk of pertussis and related hospitalizations in patients with COPD [Citation20–22]. Patients with pertussis and COPD also accrued higher health care costs compared to those with pertussis without COPD over a 6-month period after pertussis diagnosis [Citation20]. Furthermore, in a pilot study, approximately 20% of patients with acute exacerbations of chronic bronchitis showed evidence of B. pertussis infection [Citation23]. However, the link between pertussis and COPD and whether pertussis plays a role in AECOPD remains to be better characterized.
The “Acute Exacerbation and Respiratory InfectionS in COPD” (AERIS) study was a longitudinal epidemiological study that assessed the role of respiratory infections in AECOPD [Citation24,Citation25]. We used the AERIS cohort to better understand the burden of pertussis in patients with COPD. The primary purpose of the current analyses was therefore to estimate the prevalence of pertussis in this cohort by evaluating anti-pertussis toxin (PT) antibodies in serum of enrolled patients. Measurement of serum antibody levels for PT, the only known B. pertussis-specific antigen, is the standard for serological testing of B. pertussis [Citation26,Citation27] and is used (as an alternative to culture and polymerase chain reaction [PCR]) by public health authorities to confirm a pertussis diagnosis [Citation28–30]. In addition, we undertook exploratory analyses to relate the anti-PT seroprevalence to the reported incidence of clinical pertussis disease and the degree of blood eosinophilia at the time of AECOPD. provides a plain language summary of the results.
Methods
Study design and participants
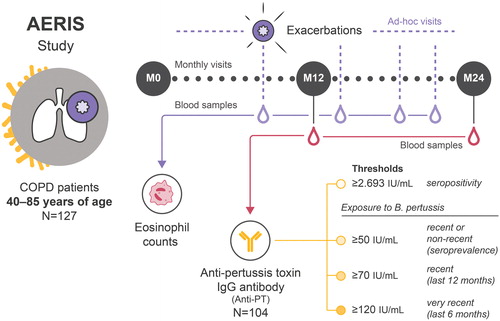
The AERIS study (ClinicalTrials.gov: NCT01360398) was a prospective, observational cohort study based at the University Hospital Southampton in England. Detailed study procedures, inclusion/exclusion criteria and primary and secondary endpoint results have been published previously [Citation24,Citation25,Citation31]. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the Southampton and South West Hampshire Research Ethics Committee. All participants provided written informed consent. The study enrolled women and men 40–85 years of age with a confirmed diagnosis of COPD based on postbronchodilator spirometry, classified as moderate, severe or very severe according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging [Citation24,Citation25,Citation32]. Participants had to be current or ex-smokers with a smoking history of ≥10 pack-years and have at least one treated exacerbation in the year preceding enrollment. Patients were recruited between June 2011 and June 2012 and followed up for 2 years in planned monthly visits during stable state and ad-hoc visits within 72 h of onset of AECOPD symptoms () [Citation24,Citation25].
Figure 2. Study design and methodology. Red droplets indicate blood samples taken at M12 and M24 analyzed for anti-PT antibodies. Purple droplets indicate blood samples at ad-hoc exacerbation visits analyzed for eosinophils. AERIS, Acute Exacerbation and Respiratory Infections in COPD; B. pertussis, Bordetella pertussis; COPD, chronic obstructive pulmonary disease; IgG, immunoglobulin G; IU, international units; M0, time of enrollment in the AERIS study (June 2011–June 2012); M12, 12 months after enrollment (June 2012–July 2013); M24, 24 months after enrollment (July 2013–June 2014); N, total number of patients in the AERIS cohort (127) or included in the analysis of seroprevalence (104); PT, pertussis toxin.
Procedures
Exacerbations were detected by use of daily electronic diary cards, and AECOPD was defined as at least two consecutive days of worsening of at least two major symptoms (dyspnea, sputum volume, sputum purulence) or of worsening of at least one major symptom and one minor symptom (wheeze, sore throat, cold symptoms, cough, fever) [Citation24]. As previously described, blood samples were collected quarterly and at each exacerbation visit [Citation24].
As part of one of the study’s tertiary objectives (i.e. to collect blood samples to describe and compare selected biomarkers in AECOPD and stable COPD), the analyses described here aimed to evaluate the seroprevalence of B. pertussis (based on anti-PT immunoglobulin G [IgG] antibodies) in the study population at two timepoints 1 year apart: 12 and 24 months after enrollment (M12 and M24). M12 was selected as the first timepoint to ensure full documentation of the patients’ history (including the recording of current medications and recommended vaccines) and records of the patients’ AECOPD episodes for at least 1 year before sampling. M24 was chosen to cover the full study period, including pertussis infections occurring in the second year of follow-up. Patients in the study cohort were unlikely to have received pertussis vaccination in the years prior to or during the study because pertussis vaccination is not recommended for adults in the United Kingdom, except in pregnant women [Citation33] and is therefore not normally prescribed in adult clinical practice. Serum anti-PT IgG antibodies were quantified at GSK using an enzyme-linked immunosorbent assay (ELISA) with an assay cutoff of 2.693 international units (IU)/mL defining seropositivity [Citation34]. Seroprevalence was defined based on three relatively conservative thresholds for anti-PT IgG antibody concentrations: concentrations of 50–<70 IU/mL were considered suggestive of a non-recent exposure to B. pertussis (between 1 and ∼2–3 years ago), and concentrations of ≥70 IU/mL and ≥120 IU/mL were considered suggestive of recent exposure to B. pertussis within the last 12 and 6 months, respectively. Different thresholds, ranging from 30 to 200 IU/mL, have been used for the serological diagnosis of pertussis based on anti-PT antibodies [Citation1,Citation26,Citation28,Citation29,Citation35]. The thresholds used in the current analyses fall within this range and were based on those recommended by the Italian Institute of Public Health (as these are among the more conservative): >120 IU/mL confirms a recent infection and 50–120 IU/mL may suggest (but does not confirm) a recent exposure to B. pertussis [Citation36]. A threshold of 70 IU/mL is used by Public Health England to define a serologically confirmed pertussis case in the absence of vaccination in the past year [Citation30].
Statistical analyses
All analyses were descriptive and were performed using the SAS software (SAS Institute Inc., Cary, NC, USA). Anti-PT geometric mean concentrations (GMCs) were calculated with 95% confidence intervals (CIs) at M12 (samples collected between June 2012 and July 2013) and M24 (samples collected between July 2013 and June 2014). This was done for the full cohort with available samples and for a subset of the full cohort with samples collected in the calendar year 2013. All patients in this subset had either their M12 or their M24 sample collected in the period covering January to December 2013. This therefore allowed for a comparison with reported pertussis incidence data for 2013 (see “Exploratory analyses” below). For GMC calculations, a concentration of half the assay cutoff was used for concentrations below the assay cutoff. The number and percentage (with 95% CIs) of participants with anti-PT antibody concentrations <50, 50–<70, 70–<120 and ≥120 IU/mL were calculated for the same timepoints and cohorts.
Exploratory analyses
An exploratory analysis was performed to compare the frequency of pertussis infections estimated based on seroprevalence with the actual clinical reporting of pertussis cases in patients with COPD in England. To this end, the proportion of patients in the AERIS cohort subset with samples taken in 2013 who had an anti-PT ≥70 IU/mL (indicative of B. pertussis infection within the last 12 months) at M12 or M24 was compared with (i.e. divided by) the reported incidence rate in 2013 in patients ≥50 years old with COPD obtained from the Clinical Practice Research Datalink (CPRD) linked to the Hospital Episode Statistics (HES) database from a separate study [Citation37].
As an initial characterization of exacerbations in patients with anti-PT ≥50 IU/mL, an exploratory analysis of eosinophilic inflammation was performed based on previously collected results of blood eosinophil counts in the AERIS study [Citation25,Citation38]. We evaluated eosinophil counts for all exacerbation visits between enrollment and M24 in patients with anti-PT ≥50 IU/mL at M12 or M24. We used the 50 IU/mL (rather than the 70 IU/mL or 120 IU/mL) threshold to make sure we included all patients with evidence of exposure to B. pertussis, even those with a less recent infection. Eosinophilic inflammation was defined as a relative blood eosinophil count of ≥2% of the total white blood cell count [Citation38]. This cutoff was shown to have a high sensitivity in identifying >3% sputum eosinophils during AECOPD, an accepted marker of airway eosinophilic inflammation [Citation38,Citation39].
Results
Pertussis serology
Characteristics of the 127 patients diagnosed with COPD comprised in the full cohort of the AERIS study were presented previously [Citation25]. Of these 127 patients, 104 had valid blood samples at the M12 and/or M24 visits and were included in the current analysis of pertussis seroprevalence; 87 had samples available at M12 and 88 at M24. A total of 87 individual patients had samples collected in the calendar year 2013 (for either M12 or M24).
In the full cohort, >86% of patients were seropositive for anti-PT antibodies (i.e. had a concentration exceeding the assay cutoff). GMCs were low (9.7 IU/mL and 12.4 IU/mL at M12 and M24, respectively; ). At M12, the seroprevalence of B. pertussis infection within the last ∼2–3 years was 8.0% (i.e. 7/87 had anti-PT concentrations ≥50 IU/mL), with 2.3% (2/87) of patients showing evidence of recent exposure to B. pertussis (within the last 12 months; anti-PT ≥70 IU/mL). Of these, no patients showed evidence of very recent exposure (within the last 6 months; anti-PT ≥120 IU/mL). At M24, the seroprevalence was 11.4% (10/88), with 5.7% (5/88) and 3.4% (3/88) of patients having anti-PT levels indicative of recent (≥70 IU/mL) and very recent exposure (≥120 IU/mL), respectively (). Overall, across the two study timepoints, among the 104 patients evaluated, seroprevalence was 13.5% (14/104), with 5.8% (6/104) and 2.9% (3/104) of patients showing evidence of exposure within the last 12 and 6 months, respectively.
Table 1. Anti-pertussis toxin antibody concentrations and seroprevalence in patients with COPD enrolled in the AERIS study.
When only considering the 87 patients with samples collected in the calendar year 2013, 89.7% were seropositive for anti-PT antibodies and the GMC was 12.4 IU/mL. Seroprevalence was 13.8% (12/87), and evidence of exposure to B. pertussis within 12 and 6 months was shown for 4.6% (4/87) and 2.3% (2/87) of patients, respectively ().
Comparison of pertussis infection with reported clinical cases
The reported pertussis disease incidence rate in a COPD cohort aged ≥50 years in the CPRD-HES database ranged between 3.15 and 10.95 per 100 000 person-years follow-up between 2011 and 2018, and was 5.38 per 100 000 person-years follow-up (95% CI, 2.46–10.21) in 2013 [37]. Pertussis is a notifiable disease in England [Citation30], and in 2013, 98.7% of confirmed pertussis cases in individuals >15 years of age were reported to Public Health England based on serology testing with a threshold of 70 IU/mL [Citation30,Citation40]. We therefore compared the reported incidence with the proportion of patients with anti-PT ≥70 IU/mL in our analysis. In 2013, 4.6% of patients with COPD in the AERIS cohort had anti-PT concentrations ≥70 IU/mL. This estimated rate of pertussis infections based on seroprevalence was approximately 850-fold higher than the incidence of 5.38 clinical pertussis cases per 100 000 person-years follow-up reported in CPRD-HES in patients with COPD in England.
Evaluation of blood eosinophils
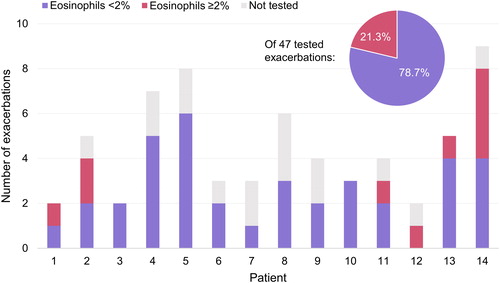
Previous studies have shown an inverse relationship between eosinophil count and bacterial infection during exacerbation in patients with COPD [Citation38,Citation41]. In the AERIS study, the prevalence of airway bacterial infection at exacerbation was greater in patients with blood eosinophils <2% [Citation38]. We therefore assessed eosinophil counts in acute exacerbations in the 14 patients that showed evidence of exposure to B. pertussis (anti-PT ≥50 IU/mL) in the full cohort (). A total of 63 exacerbations were recorded for these 14 patients between enrollment and M24, with a range of 2–9 exacerbations per patient (median: 4). Eosinophil test results were available for 47 exacerbations. Of these 47, 37 (78.7%) were associated with eosinophils <2% and the remaining 10 (21.3%) with eosinophils ≥2% (). By comparison, of all exacerbations with valid data in the AERIS cohort, 49.7% (168/338) were associated with blood eosinophils ≥2% [Citation38].
Discussion
A small number of studies suggest that patients with COPD may be at increased risk of (severe) pertussis and may experience a higher burden from pertussis than patients without COPD [Citation20–23]. Having estimates of the true prevalence of pertussis infections in patients with COPD is a crucial step toward understanding the extent of the burden of pertussis. Our exploratory analyses on a cohort of 40–85-year-old patients with moderate to very severe COPD in England indicate a high circulation of B. pertussis in patients with COPD: across the two study timepoints (M12 and M24, spanning a period between June 2012 and June 2014), evidence of exposure to B. pertussis within the last ∼2–3 years was seen in 13.5% of patients (based on anti-PT ≥50 IU/mL) and within the last 12 and 6 months (based on anti-PT ≥70 IU/mL and ≥120 IU/mL) in 5.8% and 2.9% of patients, respectively. Of note, the majority of patients had detectable anti-PT levels albeit at low levels.
While isolation of B. pertussis from culture of nasopharyngeal specimens is the most specific method for confirming pertussis diagnosis, its sensitivity decreases rapidly after 2 weeks of cough onset and is lower in older patients [Citation30,Citation35,Citation42]. Likewise, sensitivity of PCR of nasopharyngeal samples declines after the third week of cough [Citation42]. The risk of false negatives with these methods is therefore high in adults [Citation30,Citation42]. Serology testing based on anti-PT antibodies (at least 2–4 weeks after cough onset and 1 year after the last pertussis vaccination) is therefore commonly used for confirming diagnosis in older children and adults [Citation26,Citation28,Citation29,Citation35,Citation42–44]. Studies in various countries have assessed pertussis seroprevalence in the general population of adults ≥40 years old (albeit not stratified by underlying conditions). The seroprevalence in our study was in line with these published estimates (at the higher end) [Citation45–54]. Comparisons are however complicated by the fact that different assays, thresholds and units of measurement were used across studies, and that studies occurred in different time periods, age groups and settings. Pertussis epidemics are known to occur in cycles with peaks every 2–5 years [Citation4], and estimates are therefore influenced by the local epidemiology at the time a study took place. For instance, in England, a peak in the pertussis incidence was seen in 2012 (with 9367 laboratory-confirmed cases of pertussis, compared to 1051 in 2011 and 4621 in 2013 [Citation40]), which may have been reflected in our estimates.
When comparing our estimate for recent B. pertussis exposure in the AERIS cohort in 2013 (using an anti-PT threshold of 70 IU/mL indicative of B. pertussis infection within the last year) to the reported incidence of clinical pertussis cases in a COPD cohort aged ≥50 years obtained from CPRD-HES in the same calendar year [Citation37], we noted a several 100-fold higher rate of pertussis infections based on seroprevalence. This emphasizes the high circulation of B. pertussis in patients with COPD in England, but also that one should take into account the difficulty in distinguishing increased cough without fever due to pertussis infection from other triggers provoking a worsening of the clinical condition in patients with chronic cough. Although no conclusion can be drawn on the clinical presentation of the pertussis infections detected by seroprevalence screening only, it is likely that some patients would have experienced a pertussis infection as a clinical manifestation or as an exacerbation of their COPD. Yet, at the overall population level, we could not detect an association between the anti-PT levels and the number of acute exacerbations in our study with a limited sample size of 104 patients. This is not unexpected because the mean exacerbation rate in the AERIS study was three exacerbations per patient-year [Citation25], which is much higher than the estimated incidence of pertussis infections. In addition, increased cough was considered a minor symptom for reporting of AECOPD in the study, and increased cough without a major symptom (dyspnea, sputum volume, sputum purulence) would not be regarded an exacerbation. Limitations with our approach to compare pertussis infections based on seroprevalence with reported clinical pertussis cases include differences between the AERIS and the CPRD cohorts in terms of age (40–85-year-olds vs ≥50-year-olds), COPD severity (moderate to very severe with frequent exacerbations vs no selection for COPD severity) and geography (single center vs. the whole of England). In addition, anti-PT serological testing in the AERIS study was conducted in a different laboratory facility compared to English testing facilities, and comparability between these has not been established. Finally, no information on the clinical features or severity of pertussis was available for the AERIS cohort. Nevertheless, the estimated difference in the frequency of infections compared to reported clinical cases in our study is in line with that in previous studies comparing reported incidence rates of pertussis disease with incidences of pertussis infection based on anti-PT seroprevalence, showing differences of several 10-fold to several 1000-fold in the general population of older adults in various countries [Citation1,Citation26]. The main reason for the large discrepancy between notified pertussis disease cases and serologically confirmed infections may be that a substantial part of infections are asymptomatic or present with atypical symptoms [Citation1,Citation26,Citation47,Citation55,Citation56] that may partly overlap with symptoms in patients with chronic respiratory diseases. These infections would be identified in serosurveys but would not all be notified as clinical pertussis disease. In addition, reported cases depend on the level of care seeking [Citation47,Citation55].
Our exploratory analysis of the eosinophilic nature of acute exacerbations in the 14 patients with evidence of exposure to B. pertussis within the last ∼2–3 years showed that more than three quarters of the acute exacerbations in these patients were associated with low blood eosinophil counts (<2%), consistent with bacterial presence [Citation38,Citation41]. Confirmation of B. pertussis at AECOPD was not possible because PCR testing for B. pertussis was not performed on nasopharyngeal swabs in the AERIS study. Analysis of sputum samples from the AERIS cohort showed that approximately half to two thirds of exacerbation samples were positive for bacteria, with non-typeable Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis isolated most commonly [Citation25,Citation57].
The role of B. pertussis in other chronic lung diseases is poorly understood [Citation58,Citation59]. A recent case report described two patients with acute exacerbation of idiopathic pulmonary fibrosis associated with acute pertussis infection [Citation58]. The authors highlighted the challenges of diagnosing pertussis in such patients, as coughing from pertussis may be difficult to distinguish from coughing associated with idiopathic pulmonary fibrosis. These diagnostic challenges in patients with a chronic cough were also raised by Murris et al. who evaluated anti-PT levels in adult cystic fibrosis patients and found 11% (7/64) of patients with anti-PT levels between 35 and 198 IU/mL, of whom four patients had severe lung disease [Citation60]. Additional studies to understand the incidence of pertussis infection and its role in exacerbation of chronic lung disease are warranted.
Our analysis has several limitations. The study was performed in a single center and on a sample of only 104 patients, with moderate to very severe COPD and a history of exacerbations, limiting the generalizability of our results to other settings or other COPD phenotypes. Data would therefore need replication in other geographic areas and other COPD phenotypes to confirm generalizability. There was no control group without COPD to assess whether seroprevalence was higher in patients with compared to without COPD of the same age range, geographical and temporal exposure to B. pertussis.
Despite these limitations, our results, showing a high circulation of B. pertussis in patients with COPD, add to the previously published data of a greater risk of pertussis in patients with underlying conditions such as COPD [Citation20–22]. It is hence expected that these patients may benefit from immunization with a pertussis vaccine, which has been shown to be immunogenic, well tolerated and effective in adults [Citation1,Citation61]. Further research would be needed to understand the true extent of the impact of pertussis infections on AECOPD and confirm the effectiveness of pertussis vaccination in influencing the clinical course in patients with COPD.
Conclusion
Our exploratory analyses suggest a high circulation of B. pertussis in 40–85-year-old patients with moderate to very severe COPD in England between 2012 and 2014. Improved estimates of the incidence of pertussis infections in patients with COPD are needed to understand the extent of the burden of pertussis. Further research is required to better understand the link between pertussis and COPD. More widespread implementation of recommendations for pertussis vaccination in patients with COPD [Citation62,Citation63] to achieve high vaccination coverage may help reduce the health care burden in patients with COPD.
Author contributions
T.M.A. Wilkinson, P. Van den Steen, E. Turriani, N. Meyer, L. Taddei and P. Mukherjee, were involved in the conception and design of the AERIS study and/or the current analyses. T.M.A. Wilkinson, M. Dodet, N. Baudson, L. Taddei and P. Mukherjee were involved in data collection and/or generation. T.M.A. Wilkinson, N. Baudson, M. Dodet and S. Rondini provided material/analysis/reagents/tools. T.M.A. Wilkinson, P. Van den Steen, B. Cheuvart, N. Baudson, M. Dodet, E. Turriani, L. Harrington, N. Meyer, L. Taddei and P. Mukherjee were involved in data analysis and interpretation. All authors critically reviewed drafts of the manuscript and approved the final version.
Acknowledgments
We are grateful to the patients who participated in the study and all study team members for their contributions to the study. We also thank Nicole Guiso for her advice on the analyses. We thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK: Natalie Denef provided medical writing support. Gil Costa provided graphical support and Camille Turlure coordinated manuscript development.
Disclosure statement
T.M.A. Wilkinson reports grants from the GSK group of companies during the conduct of the study; grants from the GSK group of companies, grants and personal fees from AstraZeneca, Synairgen and My mhealth Ltd., outside the submitted work. P. Van den Steen, B. Cheuvart, N. Baudson, M. Dodet, E. Turriani, L. Harrington, N. Meyer, S. Rondini, L. Taddei and P. Mukherjee are employees of the GSK group of companies and declare financial and non-financial relationships and activities. P. Van den Steen, B. Cheuvart, E. Turriani, N. Meyer and P. Mukherjee own (restricted) shares in the GSK group of companies.
Additional information
Funding
References
- Kandeil W, Atanasov P, Avramioti D, et al. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines. 2019;18(5):439–455.
- Kandeil W, van den Ende C, Bunge EM, et al. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev Vaccines. 2020;19(7):621–638.
- Yeung KHT, Duclos P, Nelson EAS, et al. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974–980.
- World Health Organization. Pertussis vaccines: WHO position paper – September 2015. Wkly Epidemiol Rec. 2015;90(35):433–458.
- Jenkins VA, Savic M, Kandeil W. Pertussis in high-risk groups: an overview of the past quarter-century. Hum Vaccin Immunother. 2020;16(11):2609–2617.
- Moore A, Harnden A, Grant CC, et al. Clinically diagnosing pertussis-associated cough in adults and children: CHEST guideline and expert panel report. Chest. 2019;155(1):147–154.
- World Health Organization. Laboratory manual for the diagnosis of whooping cough caused by Bordetella pertussis/Bordetella parapertussis 2014. [cited 2021 Jan 8]. Available from: https://apps.who.int/iris/bitstream/handle/10665/127891/WHO_IVB_14.03_eng.pdf.
- GBD, 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706.
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940.
- Jafarinejad H, Moghoofei M, Mostafaei S, et al. Worldwide prevalence of viral infection in AECOPD patients: a meta-analysis. Microb Pathog. 2017;113:190–196.
- Moghoofei M, Azimzadeh Jamalkandi S, Moein M, et al. Bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Infection. 2020;48(1):19–35.
- Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology. 2016;21(7):1152–1165.
- Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852.
- Dransfield MT, Kunisaki KM, Strand MJ, COPDGene Investigators, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330.
- Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387–395.
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5):1418–1422.
- Soler-Cataluña JJ, Martínez-García MA, Román SP, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931.
- Iheanacho I, Zhang S, King D, et al. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460.
- Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207.
- Buck PO, Meyers JL, Gordon LD, et al. Economic burden of diagnosed pertussis among individuals with asthma or chronic obstructive pulmonary disease in the USA: an analysis of administrative claims. Epidemiol Infect. 2017;145(10):2109–2121.
- Mbayei SA, Faulkner A, Miner C, et al. Severe pertussis infections in the United States, 2011–2015. Clin Infect Dis. 2019;69(2):218–226.
- Blasi F, Bonanni P, Braido F, et al. The unmet need for pertussis prevention in patients with chronic obstructive pulmonary disease in the Italian context. Hum Vaccin Immunother. 2020;16(2):340–348.
- Bonhoeffer J, Bär G, Riffelmann M, et al. The role of Bordetella infections in patients with acute exacerbation of chronic bronchitis. Infection. 2005;33(1):13–17.
- Bourne S, Cohet C, Kim V, et al. Acute exacerbation and respiratory infections in COPD (AERIS): protocol for a prospective, observational cohort study. BMJ Open. 2014;4(3):e004546.
- Wilkinson TMA, Aris E, Bourne S, AERIS Study Group, et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017;72(10):919–927.
- Barkoff AM, Gröndahl-Yli-Hannuksela K, He Q. Seroprevalence studies of pertussis: what have we learned from different immunized populations. Pathog Dis. 2015;73(7):ftv050.
- Guiso N, Berbers G, Fry NK, EU Pertstrain group, et al. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur J Clin Microbiol Infect Dis. 2011;30(3):307–312.
- European Centre for Disease Prevention and Control. Guidance and protocol for the serological diagnosis of human infection with Bordetella pertussis 2012. [cited 2020 Nov 27]. Available from: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/bordetella-pertussis-guidance-protocol-serological-diagnosis.pdf.
- World Health Organization. The immunological basis for immunization series: module 4: pertussis. update 2017 ed. Geneva: World Health Organization; 2017.
- Public Health England. Guidelines for the Public Health Management of Pertussis in England 2018. [cited 2020 Nov 27]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/762766/Guidelines_for_the_Public_Health_management_of_Pertussis_in_England.pdf.
- Mayhew D, Devos N, Lambert C, AERIS Study Group, et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 2018;73(5):422–430. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945&from=EN#page=32
- Rabe KF, Hurd S, Anzueto A, Global Initiative for Chronic Obstructive Lung Disease, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555.
- Public Health England. Pertussis: the green book, chapter 24 2016. [cited 2020 Nov 27]. Available from: https://www.gov.uk/government/publications/pertussis-the-green-book-chapter-24.
- Kovac M, Kostanyan L, Mesaros N, et al. Immunogenicity and safety of a second booster dose of an acellular pertussis vaccine combined with reduced antigen content diphtheria-tetanus toxoids 10 years after a first booster in adolescence: An open, phase III, non-randomized, multi-center study. Hum Vaccin Immunother. 2018;14(8):1977–1986.
- van der Zee A, Schellekens JF, Mooi FR. Laboratory diagnosis of pertussis. Clin Microbiol Rev. 2015;28(4):1005–1026.
- Ausiello CM, Stefanelli P, Carollo M, et al. Pertosse in Italia: ricognizione dei laboratori di diagnosi del Servizio Sanitario Nazionale e valutazione delle tecniche utilizzate (Rapporti ISTISAN 14/3 - Istituto Superiore di Sanita) [Pertussis in Italy: identification of the National Health Service laboratories performing pertussis diagnosis and evaluation of used procedures] 2014. [cited 2020 Nov 27]. Available from: https://www.iss.it/documents/20126/45616/14_3.pdf/.
- Aris E, Akpo EI, Bhavsar A, et al. Late Breaking Abstract - Burden of pertussis in COPD: a retrospective database study in England. Eur Respir J. 2020. 56(suppl 64):2468.
- Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. 2017;50(4):1700853.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671.
- Public Health England. Laboratory confirmed cases of pertussis in England. Annual report for 2019 supplementary data tables 2020. [cited 2020 Nov 27]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881410/Laboratory_confirmed_cases_of_pertussis_in_England_2019.pdf.
- Kolsum U, Donaldson GC, Singh R, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18(1):88.
- Centers for Disease Control and Prevention. Pertussis. Diagnosis confirmation 2019. [cited 2020 Oct 27]. Available from: https://www.cdc.gov/pertussis/clinical/diagnostic-testing/diagnosis-confirmation.html.
- European Union. Commission implementing decision (EU) 2018/945 of 22 June 2018. on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. 3.30 Pertussis 2018 [cited 2020 Oct 27]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945&from=EN#page=32.:32018D0945&from = EN#page=32
- World Health Organization. Vaccine-preventable diseases surveillance standards: Pertussis [cited 2020 Oct 27]. Available from: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_16_Pertussis_R1.pdf?ua=1.
- Ben Fraj I, Smaoui H, Zghal M, et al. Seroprevalence of pertussis among healthcare workers: a cross-sectional study from Tunisia. Vaccine. 2019;37(1):109–112.
- Palazzo R, Carollo M, Fedele G, et al. Evidence of increased circulation of Bordetella pertussis in the Italian adult population from seroprevalence data (2012-2013). J Med Microbiol. 2016;65(7):649–657.
- Rønn PF, Dalby T, Simonsen J, et al. Seroepidemiology of pertussis in a cross-sectional study of an adult general population in Denmark. Epidemiol Infect. 2014;142(4):729–737.
- Wanlapakorn N, Ngaovithunvong V, Thongmee T, et al. Seroprevalence of antibodies to pertussis toxin among different age groups in Thailand after 37 years of universal whole-cell pertussis vaccination. PLoS One. 2016;11(2):e0148338.
- Xu Y, Wang L, Xu J, et al. Seroprevalence of pertussis in China: need to improve vaccination strategies. Hum Vaccin Immunother. 2014;10(1):192–198.
- Zhang Q, Zheng H, Liu M, et al. The seroepidemiology of immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis. 2012;12:138.
- Scott S, van der Sande M, Faye-Joof T, et al. Seroprevalence of pertussis in the Gambia: evidence for continued circulation of bordetella pertussis despite high vaccination rates. Pediatr Infect Dis J. 2015;34(4):333–338.
- Torzsa P, Devadiga R, Tafalla M. Seroprevalence of Bordetella pertussis antibodies in adults in Hungary: results of an epidemiological cross-sectional study. BMC Infect Dis. 2017;17(1):242.
- Garcı́a-Corbeira P, Dal-Ré R, Aguilar L, et al. Seroepidemiology of Bordetella pertussis infections in the Spanish population: a cross-sectional study. Vaccine. 2000;18(21):2173–2176.
- de Greeff SC, de Melker HE, van Gageldonk PG, et al. Seroprevalence of pertussis in The Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS One. 2010;5(12):e14183.
- de Melker HE, Versteegh FGA, Schellekens JFP, et al. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J Infect. 2006;53(2):106–113.
- He H, Yao P, Zhou Y, et al. Is pertussis infection neglected in China? Evidence from a seroepidemiology survey in Zhejiang, an eastern province of China. PLoS One. 2016;11(5):e0155965.
- Wilkinson TMA, Aris E, Bourne SC, et al. Drivers of year-to-year variation in exacerbation frequency of COPD: analysis of the AERIS cohort. ERJ Open Res. 2019;5(1):00248-2018.
- Hirai K, Homma T, Yamaguchi F, et al. Acute exacerbation of idiopathic pulmonary fibrosis induced by pertussis: the first case report. BMC Pulm Med. 2019;19(1):15.
- Bos AC, Beemsterboer P, Wolfs TF, et al. Bordetella species in children with cystic fibrosis: what do we know? The role in acute exacerbations and chronic course. J Cyst Fibros. 2011;10(5):307–312.
- Murris M, Guiso N, Njamkepo E, et al. Whooping cough in cystic fibrosis (CF). Eur Respir J. 2011;38(Suppl 55):p 4563.
- Liu BC, He WQ, Newall AT, et al. Effectiveness of acellular pertussis vaccine in older adults: Nested matched case-control study. Clin Infect Dis. 2020;71(2):340–350.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease – 2021 report [cited 2021 Jan 8]. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
- Centers for Disease Control and Prevention. Vaccine information for adults. Recommended vaccines for adults with health conditions: lung disease including asthma and adult vaccination 2016. [cited 2021 Jan 8]. Available from: https://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/lung-disease.html.